0dc7e8c6d6a006334e8c166e149cb739.ppt
- Количество слайдов: 38
 Genotype 1 HCV in 2016: Clinical Decision Making in a Time of Plenty Ira M. Jacobson, MD Chair, Department of Medicine Mount Sinai Beth Israel Senior Faculty and Vice-Chair, Department of Medicine Icahn School of Medicine at Mount Sinai New York, New York Supported by educational grants from Abb. Vie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck, and Vii. V Healthcare.
Genotype 1 HCV in 2016: Clinical Decision Making in a Time of Plenty Ira M. Jacobson, MD Chair, Department of Medicine Mount Sinai Beth Israel Senior Faculty and Vice-Chair, Department of Medicine Icahn School of Medicine at Mount Sinai New York, New York Supported by educational grants from Abb. Vie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck, and Vii. V Healthcare.
 About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details
About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details
 Disclosures Ira. M. Jacobson, MD, has disclosed that he has received funds for research support from Abb. Vie, Bristol -Myers Squibb, Gilead Sciences, Janssen, and Merck; has served on speaker bureaus for Abb. Vie, Bristol. Myers Squibb, Gilead Sciences, and Janssen; and has received consulting fees from Abb. Vie, Bristol-Myers Squibb, Gilead Sciences, Intercept, Janssen, Merck, and Trek.
Disclosures Ira. M. Jacobson, MD, has disclosed that he has received funds for research support from Abb. Vie, Bristol -Myers Squibb, Gilead Sciences, Janssen, and Merck; has served on speaker bureaus for Abb. Vie, Bristol. Myers Squibb, Gilead Sciences, and Janssen; and has received consulting fees from Abb. Vie, Bristol-Myers Squibb, Gilead Sciences, Intercept, Janssen, Merck, and Trek.
 New Regimens and Data for Genotype 1 HCV Infection AASLD Guidance Updated February 24, 2016 § AASLD guidance stratifies regimens as “recommended” and “alternative” AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
New Regimens and Data for Genotype 1 HCV Infection AASLD Guidance Updated February 24, 2016 § AASLD guidance stratifies regimens as “recommended” and “alternative” AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
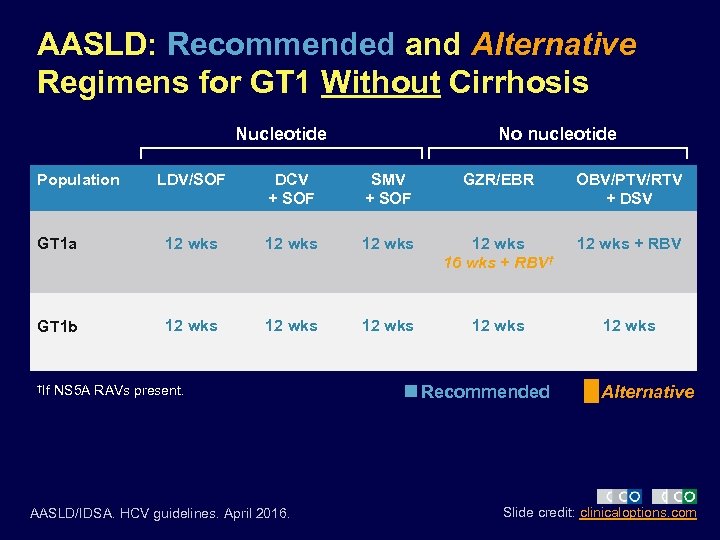 AASLD: Recommended and Alternative Regimens for GT 1 Without Cirrhosis Nucleotide Population No nucleotide LDV/SOF DCV + SOF SMV + SOF GZR/EBR OBV/PTV/RTV + DSV GT 1 a 12 wks 16 wks + RBV† 12 wks + RBV GT 1 b 12 wks 12 wks †If NS 5 A RAVs present. AASLD/IDSA. HCV guidelines. April 2016. Recommended Alternative Slide credit: clinicaloptions. com
AASLD: Recommended and Alternative Regimens for GT 1 Without Cirrhosis Nucleotide Population No nucleotide LDV/SOF DCV + SOF SMV + SOF GZR/EBR OBV/PTV/RTV + DSV GT 1 a 12 wks 16 wks + RBV† 12 wks + RBV GT 1 b 12 wks 12 wks †If NS 5 A RAVs present. AASLD/IDSA. HCV guidelines. April 2016. Recommended Alternative Slide credit: clinicaloptions. com
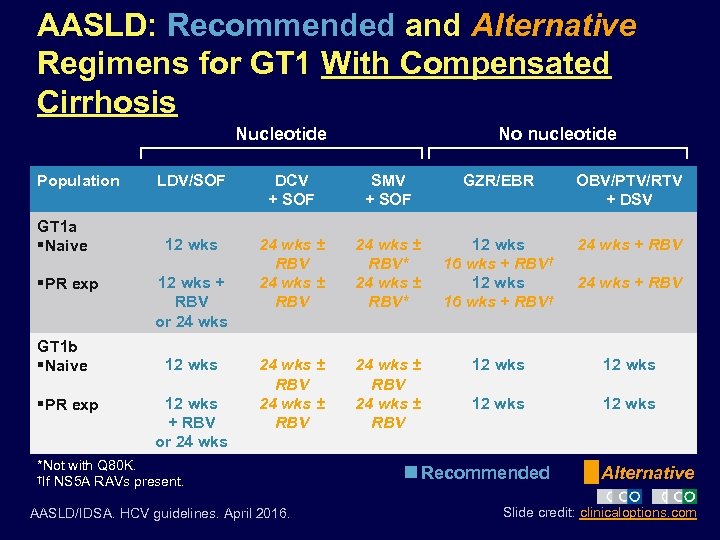 AASLD: Recommended and Alternative Regimens for GT 1 With Compensated Cirrhosis Nucleotide Population GT 1 a §Naive §PR exp GT 1 b §Naive §PR exp No nucleotide LDV/SOF DCV + SOF SMV + SOF GZR/EBR OBV/PTV/RTV + DSV 12 wks 24 wks ± RBV* 12 wks 16 wks + RBV† 24 wks + RBV 24 wks ± RBV 12 wks 12 wks + RBV or 24 wks *Not with Q 80 K. †If NS 5 A RAVs present. AASLD/IDSA. HCV guidelines. April 2016. Recommended 24 wks + RBV Alternative Slide credit: clinicaloptions. com
AASLD: Recommended and Alternative Regimens for GT 1 With Compensated Cirrhosis Nucleotide Population GT 1 a §Naive §PR exp GT 1 b §Naive §PR exp No nucleotide LDV/SOF DCV + SOF SMV + SOF GZR/EBR OBV/PTV/RTV + DSV 12 wks 24 wks ± RBV* 12 wks 16 wks + RBV† 24 wks + RBV 24 wks ± RBV 12 wks 12 wks + RBV or 24 wks *Not with Q 80 K. †If NS 5 A RAVs present. AASLD/IDSA. HCV guidelines. April 2016. Recommended 24 wks + RBV Alternative Slide credit: clinicaloptions. com
 Grazoprevir/Elbasvir: Approved Jan 2016 § Genotype 1 a, with/without compensated cirrhosis, treatment naive or treatment experienced – Without NS 5 A RAVs: GZR/EBR, 12 wks – With NS 5 A RAVs: GZR/EBR + RBV, 16 wks* – RAVs at positions 28, 30, 31, 93 § Genotype 1 b, with/without compensated cirrhosis, treatment naive or treatment experienced – GZR/EBR, 12 wks – RAV testing not indicated *Listed as “alternative” regimen. AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
Grazoprevir/Elbasvir: Approved Jan 2016 § Genotype 1 a, with/without compensated cirrhosis, treatment naive or treatment experienced – Without NS 5 A RAVs: GZR/EBR, 12 wks – With NS 5 A RAVs: GZR/EBR + RBV, 16 wks* – RAVs at positions 28, 30, 31, 93 § Genotype 1 b, with/without compensated cirrhosis, treatment naive or treatment experienced – GZR/EBR, 12 wks – RAV testing not indicated *Listed as “alternative” regimen. AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
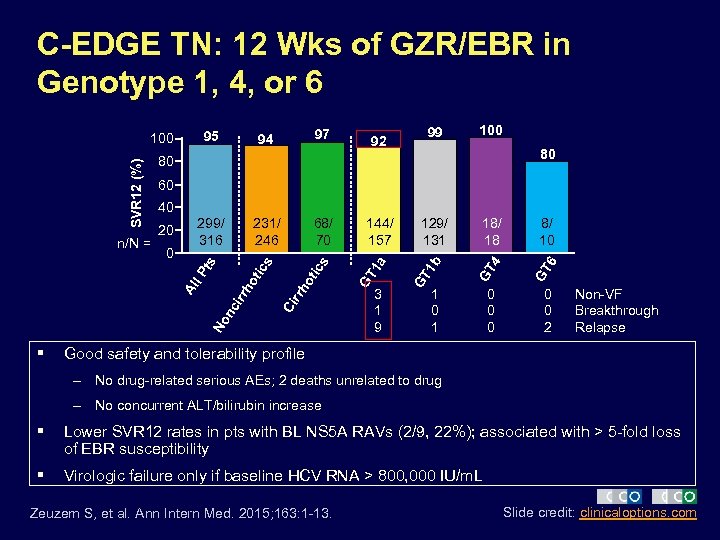 C-EDGE TN: 12 Wks of GZR/EBR in Genotype 1, 4, or 6 97 231/ 246 68/ 70 99 100 80 60 nc ir 3 1 9 18/ 18 8/ 10 GT 4 GT 6 1 b 129/ 131 GT 144/ 157 GT 1 a ot No Ci rrh s rh o l P 0 299/ 316 tic 20 ics 40 Al § 92 80 ts SVR 12 (%) n/N = 94 95 100 1 0 0 0 2 Non-VF Breakthrough Relapse Good safety and tolerability profile – No drug-related serious AEs; 2 deaths unrelated to drug – No concurrent ALT/bilirubin increase § Lower SVR 12 rates in pts with BL NS 5 A RAVs (2/9, 22%); associated with > 5 -fold loss of EBR susceptibility § Virologic failure only if baseline HCV RNA > 800, 000 IU/m. L Zeuzem S, et al. Ann Intern Med. 2015; 163: 1 -13. Slide credit: clinicaloptions. com
C-EDGE TN: 12 Wks of GZR/EBR in Genotype 1, 4, or 6 97 231/ 246 68/ 70 99 100 80 60 nc ir 3 1 9 18/ 18 8/ 10 GT 4 GT 6 1 b 129/ 131 GT 144/ 157 GT 1 a ot No Ci rrh s rh o l P 0 299/ 316 tic 20 ics 40 Al § 92 80 ts SVR 12 (%) n/N = 94 95 100 1 0 0 0 2 Non-VF Breakthrough Relapse Good safety and tolerability profile – No drug-related serious AEs; 2 deaths unrelated to drug – No concurrent ALT/bilirubin increase § Lower SVR 12 rates in pts with BL NS 5 A RAVs (2/9, 22%); associated with > 5 -fold loss of EBR susceptibility § Virologic failure only if baseline HCV RNA > 800, 000 IU/m. L Zeuzem S, et al. Ann Intern Med. 2015; 163: 1 -13. Slide credit: clinicaloptions. com
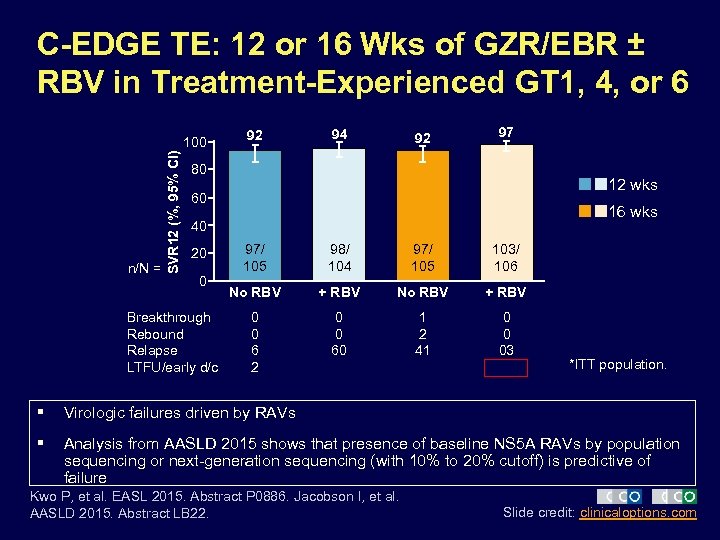 C-EDGE TE: 12 or 16 Wks of GZR/EBR ± RBV in Treatment-Experienced GT 1, 4, or 6 n/N = SVR 12 (%, 95% CI) 100 92 94 92 97 80 12 wks 60 16 wks 40 20 0 Breakthrough Rebound Relapse LTFU/early d/c 97/ 105 98/ 104 97/ 105 103/ 106 No RBV + RBV 0 0 6 2 0 0 60 1 2 41 0 0 03 *ITT population. § Virologic failures driven by RAVs § Analysis from AASLD 2015 shows that presence of baseline NS 5 A RAVs by population sequencing or next-generation sequencing (with 10% to 20% cutoff) is predictive of failure Kwo P, et al. EASL 2015. Abstract P 0886. Jacobson I, et al. AASLD 2015. Abstract LB 22. Slide credit: clinicaloptions. com
C-EDGE TE: 12 or 16 Wks of GZR/EBR ± RBV in Treatment-Experienced GT 1, 4, or 6 n/N = SVR 12 (%, 95% CI) 100 92 94 92 97 80 12 wks 60 16 wks 40 20 0 Breakthrough Rebound Relapse LTFU/early d/c 97/ 105 98/ 104 97/ 105 103/ 106 No RBV + RBV 0 0 6 2 0 0 60 1 2 41 0 0 03 *ITT population. § Virologic failures driven by RAVs § Analysis from AASLD 2015 shows that presence of baseline NS 5 A RAVs by population sequencing or next-generation sequencing (with 10% to 20% cutoff) is predictive of failure Kwo P, et al. EASL 2015. Abstract P 0886. Jacobson I, et al. AASLD 2015. Abstract LB 22. Slide credit: clinicaloptions. com
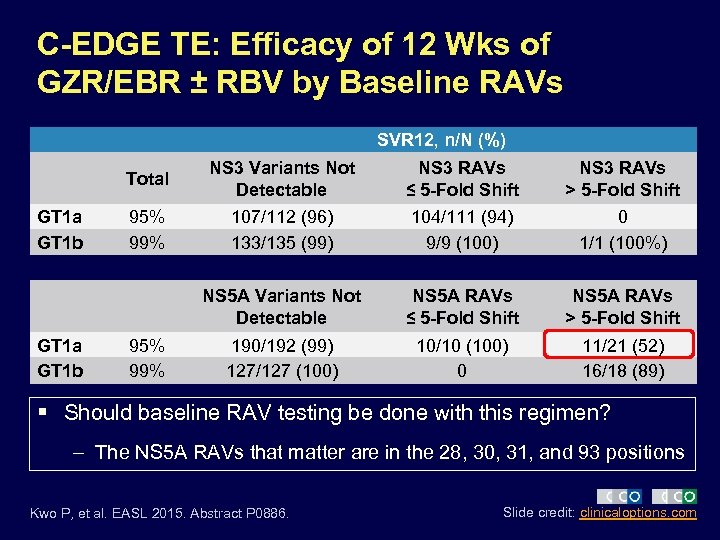 C-EDGE TE: Efficacy of 12 Wks of GZR/EBR ± RBV by Baseline RAVs SVR 12, n/N (%) Total GT 1 a GT 1 b NS 3 RAVs ≤ 5 -Fold Shift NS 3 RAVs > 5 -Fold Shift 95% 99% 107/112 (96) 133/135 (99) 104/111 (94) 9/9 (100) 0 1/1 (100%) NS 5 A Variants Not Detectable GT 1 a GT 1 b NS 3 Variants Not Detectable NS 5 A RAVs ≤ 5 -Fold Shift NS 5 A RAVs > 5 -Fold Shift 190/192 (99) 127/127 (100) 10/10 (100) 0 11/21 (52) 16/18 (89) 95% 99% § Should baseline RAV testing be done with this regimen? – The NS 5 A RAVs that matter are in the 28, 30, 31, and 93 positions Kwo P, et al. EASL 2015. Abstract P 0886. Slide credit: clinicaloptions. com
C-EDGE TE: Efficacy of 12 Wks of GZR/EBR ± RBV by Baseline RAVs SVR 12, n/N (%) Total GT 1 a GT 1 b NS 3 RAVs ≤ 5 -Fold Shift NS 3 RAVs > 5 -Fold Shift 95% 99% 107/112 (96) 133/135 (99) 104/111 (94) 9/9 (100) 0 1/1 (100%) NS 5 A Variants Not Detectable GT 1 a GT 1 b NS 3 Variants Not Detectable NS 5 A RAVs ≤ 5 -Fold Shift NS 5 A RAVs > 5 -Fold Shift 190/192 (99) 127/127 (100) 10/10 (100) 0 11/21 (52) 16/18 (89) 95% 99% § Should baseline RAV testing be done with this regimen? – The NS 5 A RAVs that matter are in the 28, 30, 31, and 93 positions Kwo P, et al. EASL 2015. Abstract P 0886. Slide credit: clinicaloptions. com
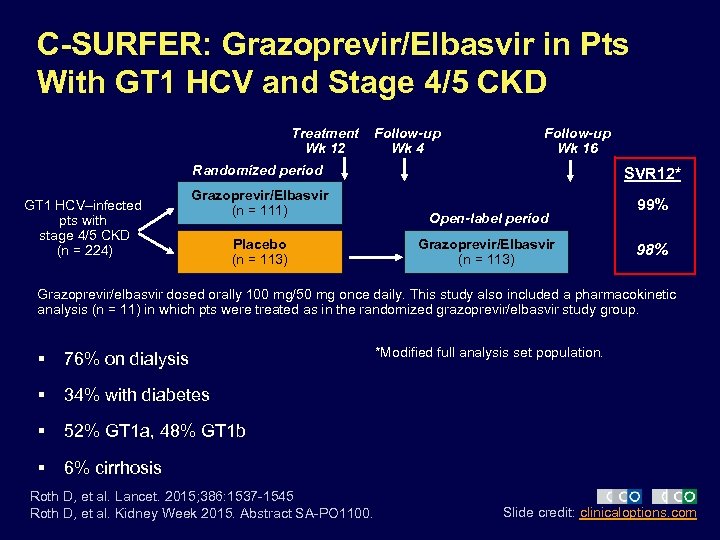 C-SURFER: Grazoprevir/Elbasvir in Pts With GT 1 HCV and Stage 4/5 CKD Treatment Wk 12 Follow-up Wk 4 Follow-up Wk 16 Randomized period GT 1 HCV–infected pts with stage 4/5 CKD (n = 224) Grazoprevir/Elbasvir (n = 111) Placebo (n = 113) SVR 12* Open-label period Grazoprevir/Elbasvir (n = 113) 99% 98% Grazoprevir/elbasvir dosed orally 100 mg/50 mg once daily. This study also included a pharmacokinetic analysis (n = 11) in which pts were treated as in the randomized grazoprevir/elbasvir study group. § 76% on dialysis § 34% with diabetes § 52% GT 1 a, 48% GT 1 b § *Modified full analysis set population. 6% cirrhosis Roth D, et al. Lancet. 2015; 386: 1537 -1545 Roth D, et al. Kidney Week 2015. Abstract SA-PO 1100. Slide credit: clinicaloptions. com
C-SURFER: Grazoprevir/Elbasvir in Pts With GT 1 HCV and Stage 4/5 CKD Treatment Wk 12 Follow-up Wk 4 Follow-up Wk 16 Randomized period GT 1 HCV–infected pts with stage 4/5 CKD (n = 224) Grazoprevir/Elbasvir (n = 111) Placebo (n = 113) SVR 12* Open-label period Grazoprevir/Elbasvir (n = 113) 99% 98% Grazoprevir/elbasvir dosed orally 100 mg/50 mg once daily. This study also included a pharmacokinetic analysis (n = 11) in which pts were treated as in the randomized grazoprevir/elbasvir study group. § 76% on dialysis § 34% with diabetes § 52% GT 1 a, 48% GT 1 b § *Modified full analysis set population. 6% cirrhosis Roth D, et al. Lancet. 2015; 386: 1537 -1545 Roth D, et al. Kidney Week 2015. Abstract SA-PO 1100. Slide credit: clinicaloptions. com
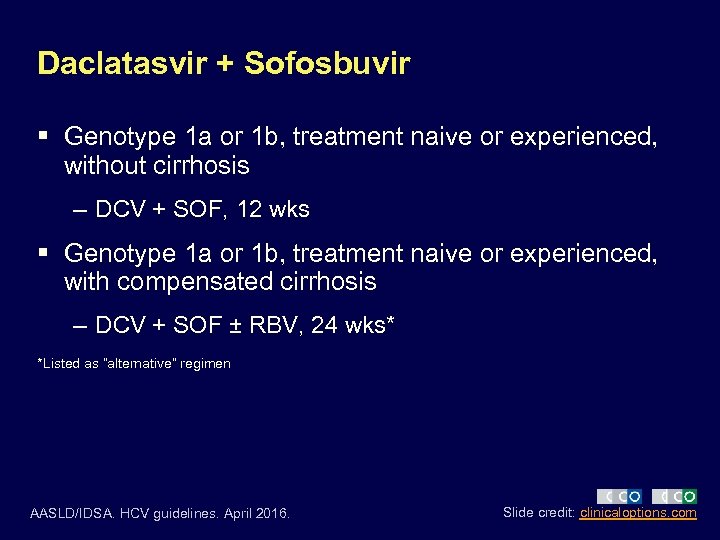 Daclatasvir + Sofosbuvir § Genotype 1 a or 1 b, treatment naive or experienced, without cirrhosis – DCV + SOF, 12 wks § Genotype 1 a or 1 b, treatment naive or experienced, with compensated cirrhosis – DCV + SOF ± RBV, 24 wks* *Listed as “alternative” regimen AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
Daclatasvir + Sofosbuvir § Genotype 1 a or 1 b, treatment naive or experienced, without cirrhosis – DCV + SOF, 12 wks § Genotype 1 a or 1 b, treatment naive or experienced, with compensated cirrhosis – DCV + SOF ± RBV, 24 wks* *Listed as “alternative” regimen AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
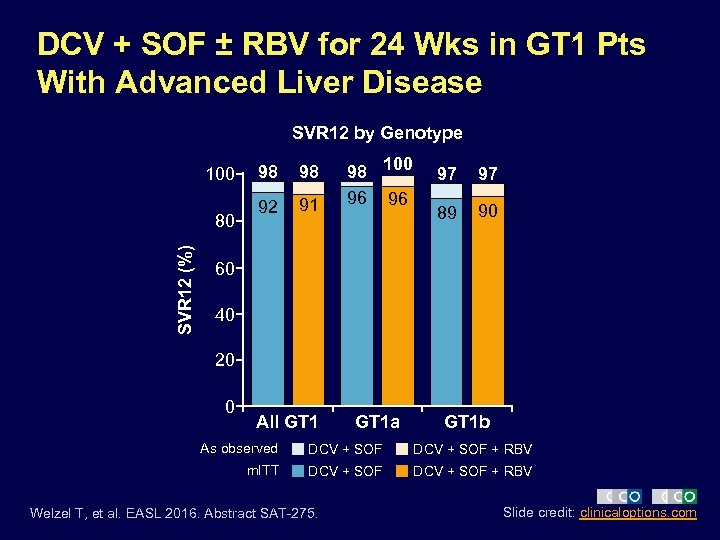 DCV + SOF ± RBV for 24 Wks in GT 1 Pts With Advanced Liver Disease SVR 12 by Genotype 100 SVR 12 (%) 80 98 98 92 91 98 100 96 96 97 97 89 90 60 40 20 0 All GT 1 a GT 1 b As observed DCV + SOF + RBV m. ITT DCV + SOF + RBV Welzel T, et al. EASL 2016. Abstract SAT-275. Slide credit: clinicaloptions. com
DCV + SOF ± RBV for 24 Wks in GT 1 Pts With Advanced Liver Disease SVR 12 by Genotype 100 SVR 12 (%) 80 98 98 92 91 98 100 96 96 97 97 89 90 60 40 20 0 All GT 1 a GT 1 b As observed DCV + SOF + RBV m. ITT DCV + SOF + RBV Welzel T, et al. EASL 2016. Abstract SAT-275. Slide credit: clinicaloptions. com
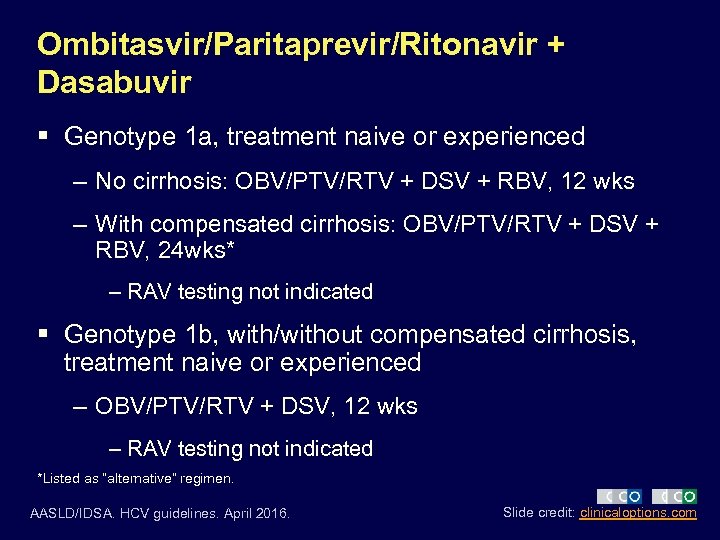 Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir § Genotype 1 a, treatment naive or experienced – No cirrhosis: OBV/PTV/RTV + DSV + RBV, 12 wks – With compensated cirrhosis: OBV/PTV/RTV + DSV + RBV, 24 wks* – RAV testing not indicated § Genotype 1 b, with/without compensated cirrhosis, treatment naive or experienced – OBV/PTV/RTV + DSV, 12 wks – RAV testing not indicated *Listed as “alternative” regimen. AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir § Genotype 1 a, treatment naive or experienced – No cirrhosis: OBV/PTV/RTV + DSV + RBV, 12 wks – With compensated cirrhosis: OBV/PTV/RTV + DSV + RBV, 24 wks* – RAV testing not indicated § Genotype 1 b, with/without compensated cirrhosis, treatment naive or experienced – OBV/PTV/RTV + DSV, 12 wks – RAV testing not indicated *Listed as “alternative” regimen. AASLD/IDSA. HCV guidelines. April 2016. Slide credit: clinicaloptions. com
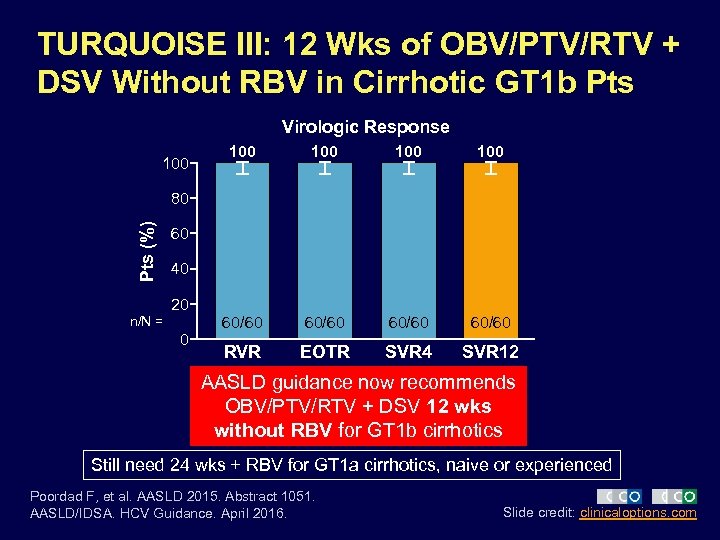 TURQUOISE III: 12 Wks of OBV/PTV/RTV + DSV Without RBV in Cirrhotic GT 1 b Pts Virologic Response 100 100 100 60/60 RVR EOTR SVR 4 SVR 12 Pts (%) 80 60 40 20 n/N = 0 AASLD guidance now recommends OBV/PTV/RTV + DSV 12 wks without RBV for GT 1 b cirrhotics Still need 24 wks + RBV for GT 1 a cirrhotics, naive or experienced Poordad F, et al. AASLD 2015. Abstract 1051. AASLD/IDSA. HCV Guidance. April 2016. Slide credit: clinicaloptions. com
TURQUOISE III: 12 Wks of OBV/PTV/RTV + DSV Without RBV in Cirrhotic GT 1 b Pts Virologic Response 100 100 100 60/60 RVR EOTR SVR 4 SVR 12 Pts (%) 80 60 40 20 n/N = 0 AASLD guidance now recommends OBV/PTV/RTV + DSV 12 wks without RBV for GT 1 b cirrhotics Still need 24 wks + RBV for GT 1 a cirrhotics, naive or experienced Poordad F, et al. AASLD 2015. Abstract 1051. AASLD/IDSA. HCV Guidance. April 2016. Slide credit: clinicaloptions. com
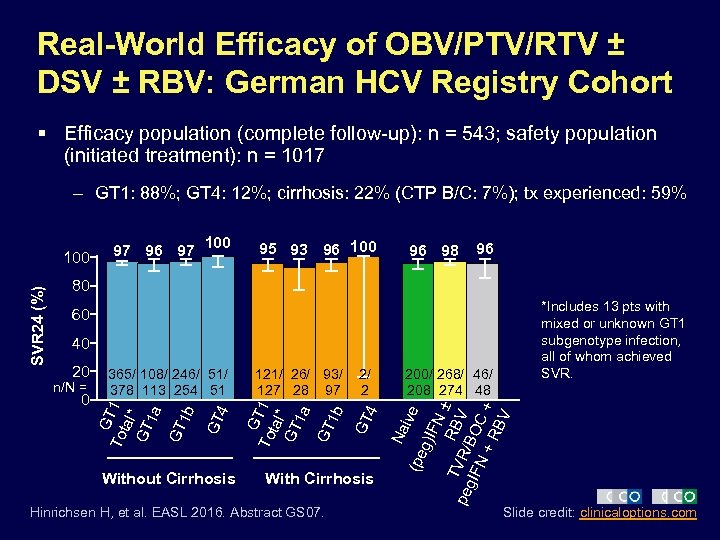 Real-World Efficacy of OBV/PTV/RTV ± DSV ± RBV: German HCV Registry Cohort § Efficacy population (complete follow-up): n = 543; safety population (initiated treatment): n = 1017 – GT 1: 88%; GT 4: 12%; cirrhosis: 22% (CTP B/C: 7%); tx experienced: 59% 97 96 97 100 95 93 96 100 96 98 96 80 *Includes 13 pts with mixed or unknown GT 1 subgenotype infection, all of whom achieved SVR. 60 Hinrichsen H, et al. EASL 2016. Abstract GS 07. g)I ive FN ± TV RBV peg R/B IFN OC +R + BV With Cirrhosis 200/ 268/ 46/ 208 274 48 Na 4 2/ 2 (pe Without Cirrhosis 121/ 26/ 93/ 127 28 97 GT 0 4 n/N = 365/ 108/ 246/ 51/ 378 113 254 51 GT 20 G To T 1 tal* GT 1 a GT 1 b 40 G To T 1 tal* GT 1 a GT 1 b SVR 24 (%) 100 Slide credit: clinicaloptions. com
Real-World Efficacy of OBV/PTV/RTV ± DSV ± RBV: German HCV Registry Cohort § Efficacy population (complete follow-up): n = 543; safety population (initiated treatment): n = 1017 – GT 1: 88%; GT 4: 12%; cirrhosis: 22% (CTP B/C: 7%); tx experienced: 59% 97 96 97 100 95 93 96 100 96 98 96 80 *Includes 13 pts with mixed or unknown GT 1 subgenotype infection, all of whom achieved SVR. 60 Hinrichsen H, et al. EASL 2016. Abstract GS 07. g)I ive FN ± TV RBV peg R/B IFN OC +R + BV With Cirrhosis 200/ 268/ 46/ 208 274 48 Na 4 2/ 2 (pe Without Cirrhosis 121/ 26/ 93/ 127 28 97 GT 0 4 n/N = 365/ 108/ 246/ 51/ 378 113 254 51 GT 20 G To T 1 tal* GT 1 a GT 1 b 40 G To T 1 tal* GT 1 a GT 1 b SVR 24 (%) 100 Slide credit: clinicaloptions. com
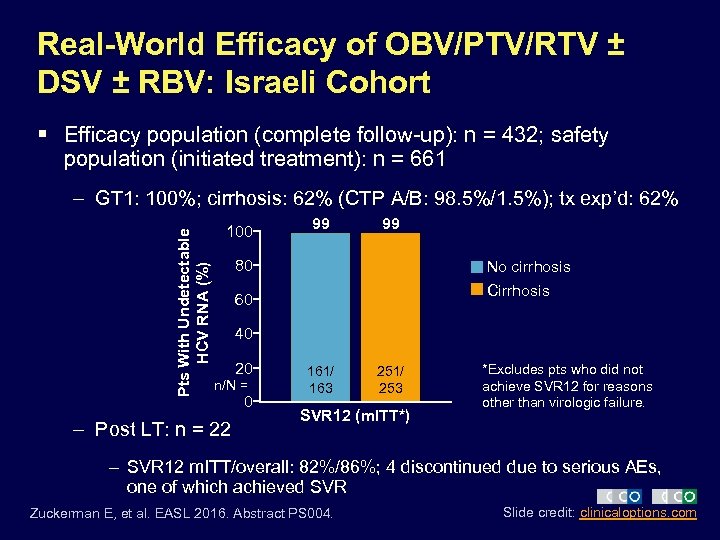 Real-World Efficacy of OBV/PTV/RTV ± DSV ± RBV: Israeli Cohort § Efficacy population (complete follow-up): n = 432; safety population (initiated treatment): n = 661 Pts With Undetectable HCV RNA (%) – GT 1: 100%; cirrhosis: 62% (CTP A/B: 98. 5%/1. 5%); tx exp’d: 62% 100 99 99 80 No cirrhosis Cirrhosis 60 40 20 n/N = – Post LT: n = 22 0 161/ 163 251/ 253 SVR 12 (m. ITT*) *Excludes pts who did not achieve SVR 12 for reasons other than virologic failure. – SVR 12 m. ITT/overall: 82%/86%; 4 discontinued due to serious AEs, one of which achieved SVR Zuckerman E, et al. EASL 2016. Abstract PS 004. Slide credit: clinicaloptions. com
Real-World Efficacy of OBV/PTV/RTV ± DSV ± RBV: Israeli Cohort § Efficacy population (complete follow-up): n = 432; safety population (initiated treatment): n = 661 Pts With Undetectable HCV RNA (%) – GT 1: 100%; cirrhosis: 62% (CTP A/B: 98. 5%/1. 5%); tx exp’d: 62% 100 99 99 80 No cirrhosis Cirrhosis 60 40 20 n/N = – Post LT: n = 22 0 161/ 163 251/ 253 SVR 12 (m. ITT*) *Excludes pts who did not achieve SVR 12 for reasons other than virologic failure. – SVR 12 m. ITT/overall: 82%/86%; 4 discontinued due to serious AEs, one of which achieved SVR Zuckerman E, et al. EASL 2016. Abstract PS 004. Slide credit: clinicaloptions. com
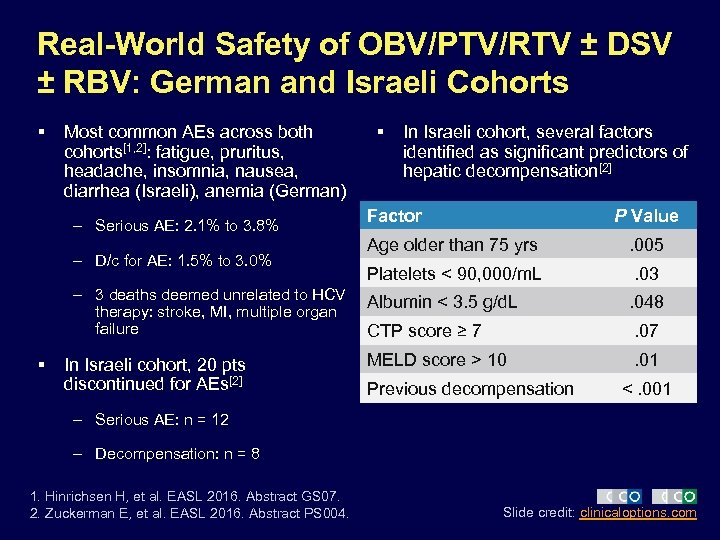 Real-World Safety of OBV/PTV/RTV ± DSV ± RBV: German and Israeli Cohorts § Most common AEs across both cohorts[1, 2]: fatigue, pruritus, headache, insomnia, nausea, diarrhea (Israeli), anemia (German) – Serious AE: 2. 1% to 3. 8% – D/c for AE: 1. 5% to 3. 0% – 3 deaths deemed unrelated to HCV therapy: stroke, MI, multiple organ failure § In Israeli cohort, 20 pts discontinued for AEs[2] § In Israeli cohort, several factors identified as significant predictors of hepatic decompensation[2] Factor P Value Age older than 75 yrs . 005 Platelets < 90, 000/m. L . 03 Albumin < 3. 5 g/d. L . 048 CTP score ≥ 7 . 07 MELD score > 10 . 01 Previous decompensation <. 001 – Serious AE: n = 12 – Decompensation: n = 8 1. Hinrichsen H, et al. EASL 2016. Abstract GS 07. 2. Zuckerman E, et al. EASL 2016. Abstract PS 004. Slide credit: clinicaloptions. com
Real-World Safety of OBV/PTV/RTV ± DSV ± RBV: German and Israeli Cohorts § Most common AEs across both cohorts[1, 2]: fatigue, pruritus, headache, insomnia, nausea, diarrhea (Israeli), anemia (German) – Serious AE: 2. 1% to 3. 8% – D/c for AE: 1. 5% to 3. 0% – 3 deaths deemed unrelated to HCV therapy: stroke, MI, multiple organ failure § In Israeli cohort, 20 pts discontinued for AEs[2] § In Israeli cohort, several factors identified as significant predictors of hepatic decompensation[2] Factor P Value Age older than 75 yrs . 005 Platelets < 90, 000/m. L . 03 Albumin < 3. 5 g/d. L . 048 CTP score ≥ 7 . 07 MELD score > 10 . 01 Previous decompensation <. 001 – Serious AE: n = 12 – Decompensation: n = 8 1. Hinrichsen H, et al. EASL 2016. Abstract GS 07. 2. Zuckerman E, et al. EASL 2016. Abstract PS 004. Slide credit: clinicaloptions. com
 Ledipasvir/Sofosbuvir for GT 1 Tx-Naive Noncirrhotics With HCV RNA < 6 M IU/m. L: Are 8 wks sufficient? Or are 12 wks better? § Established by retrospective analysis of ION-3 § Many clinicians were initially uncomfortable § What do “real-world” data show? Slide credit: clinicaloptions. com
Ledipasvir/Sofosbuvir for GT 1 Tx-Naive Noncirrhotics With HCV RNA < 6 M IU/m. L: Are 8 wks sufficient? Or are 12 wks better? § Established by retrospective analysis of ION-3 § Many clinicians were initially uncomfortable § What do “real-world” data show? Slide credit: clinicaloptions. com
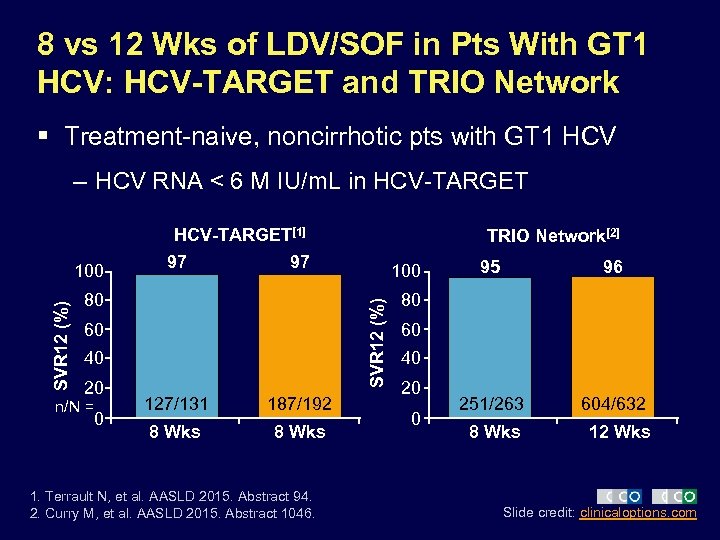 8 vs 12 Wks of LDV/SOF in Pts With GT 1 HCV: HCV-TARGET and TRIO Network § Treatment-naive, noncirrhotic pts with GT 1 HCV – HCV RNA < 6 M IU/m. L in HCV-TARGET[1] 97 97 80 60 40 20 n/N = 0 100 SVR 12 (%) 100 TRIO Network[2] 127/131 187/192 8 Wks 1. Terrault N, et al. AASLD 2015. Abstract 94. 2. Curry M, et al. AASLD 2015. Abstract 1046. 95 96 251/263 604/632 80 60 40 20 0 8 Wks 12 Wks Slide credit: clinicaloptions. com
8 vs 12 Wks of LDV/SOF in Pts With GT 1 HCV: HCV-TARGET and TRIO Network § Treatment-naive, noncirrhotic pts with GT 1 HCV – HCV RNA < 6 M IU/m. L in HCV-TARGET[1] 97 97 80 60 40 20 n/N = 0 100 SVR 12 (%) 100 TRIO Network[2] 127/131 187/192 8 Wks 1. Terrault N, et al. AASLD 2015. Abstract 94. 2. Curry M, et al. AASLD 2015. Abstract 1046. 95 96 251/263 604/632 80 60 40 20 0 8 Wks 12 Wks Slide credit: clinicaloptions. com
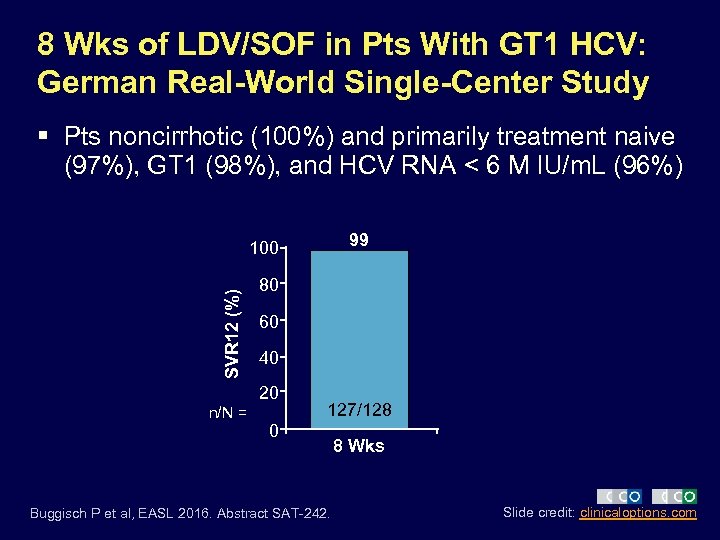 8 Wks of LDV/SOF in Pts With GT 1 HCV: German Real-World Single-Center Study § Pts noncirrhotic (100%) and primarily treatment naive (97%), GT 1 (98%), and HCV RNA < 6 M IU/m. L (96%) 99 SVR 12 (%) 100 80 60 40 20 n/N = 127/128 0 Buggisch P et al, EASL 2016. Abstract SAT-242. 8 Wks Slide credit: clinicaloptions. com
8 Wks of LDV/SOF in Pts With GT 1 HCV: German Real-World Single-Center Study § Pts noncirrhotic (100%) and primarily treatment naive (97%), GT 1 (98%), and HCV RNA < 6 M IU/m. L (96%) 99 SVR 12 (%) 100 80 60 40 20 n/N = 127/128 0 Buggisch P et al, EASL 2016. Abstract SAT-242. 8 Wks Slide credit: clinicaloptions. com
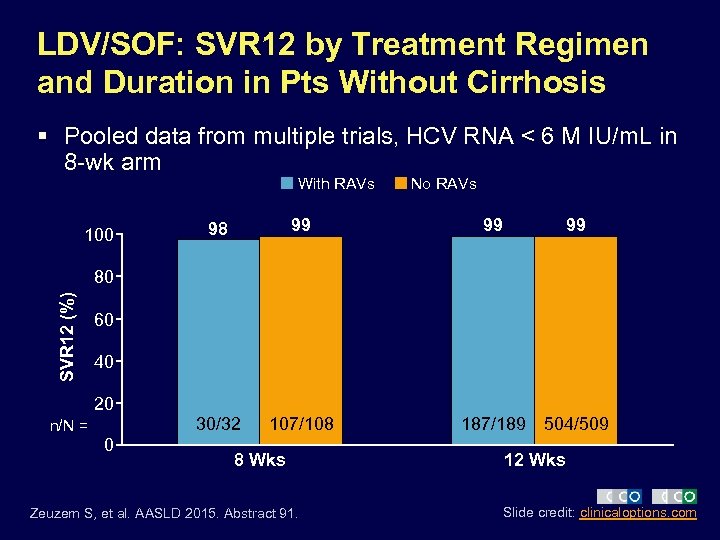 LDV/SOF: SVR 12 by Treatment Regimen and Duration in Pts Without Cirrhosis § Pooled data from multiple trials, HCV RNA < 6 M IU/m. L in 8 -wk arm With RAVs 98 99 30/32 100 107/108 No RAVs 99 99 SVR 12 (%) 80 60 40 20 n/N = 0 8 Wks Zeuzem S, et al. AASLD 2015. Abstract 91. 187/189 504/509 12 Wks Slide credit: clinicaloptions. com
LDV/SOF: SVR 12 by Treatment Regimen and Duration in Pts Without Cirrhosis § Pooled data from multiple trials, HCV RNA < 6 M IU/m. L in 8 -wk arm With RAVs 98 99 30/32 100 107/108 No RAVs 99 99 SVR 12 (%) 80 60 40 20 n/N = 0 8 Wks Zeuzem S, et al. AASLD 2015. Abstract 91. 187/189 504/509 12 Wks Slide credit: clinicaloptions. com
 PPIs and Ledipasvir: Does Acid Suppression Matter?
PPIs and Ledipasvir: Does Acid Suppression Matter?
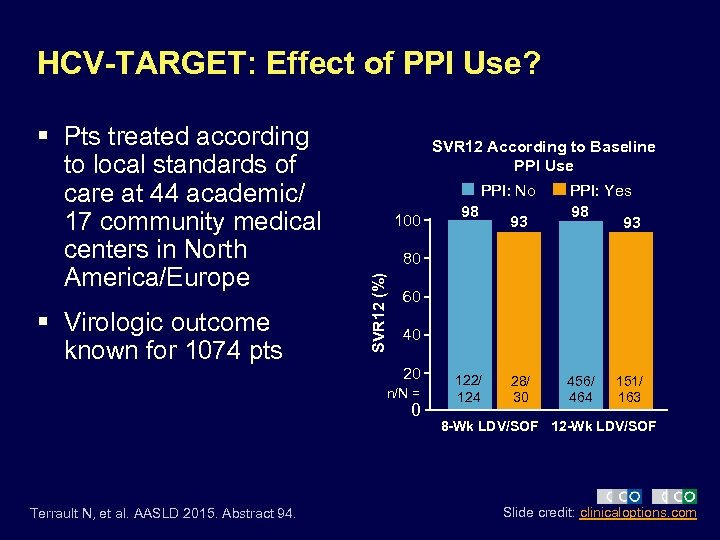 HCV-TARGET: Effect of PPI Use? § Virologic outcome known for 1074 pts SVR 12 According to Baseline PPI Use PPI: No 100 93 28/ 30 456/ 464 60 40 20 n/N = 0 Terrault N, et al. AASLD 2015. Abstract 94. 98 PPI: Yes 98 93 80 SVR 12 (%) § Pts treated according to local standards of care at 44 academic/ 17 community medical centers in North America/Europe 122/ 124 151/ 163 8 -Wk LDV/SOF 12 -Wk LDV/SOF Slide credit: clinicaloptions. com
HCV-TARGET: Effect of PPI Use? § Virologic outcome known for 1074 pts SVR 12 According to Baseline PPI Use PPI: No 100 93 28/ 30 456/ 464 60 40 20 n/N = 0 Terrault N, et al. AASLD 2015. Abstract 94. 98 PPI: Yes 98 93 80 SVR 12 (%) § Pts treated according to local standards of care at 44 academic/ 17 community medical centers in North America/Europe 122/ 124 151/ 163 8 -Wk LDV/SOF 12 -Wk LDV/SOF Slide credit: clinicaloptions. com
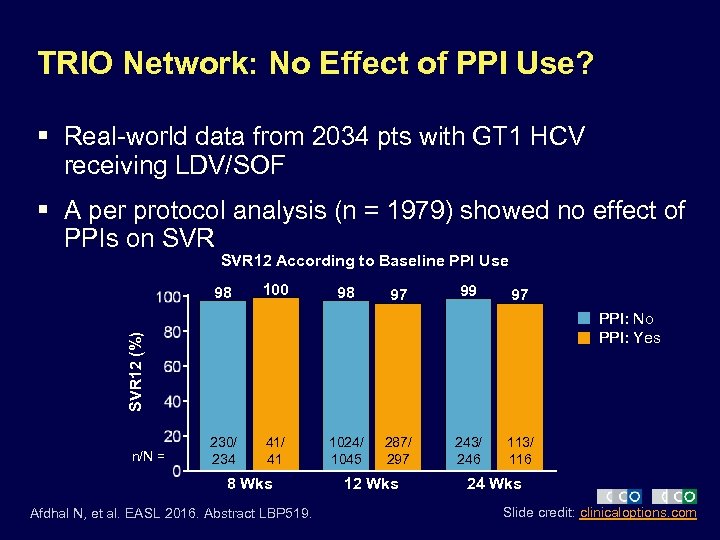 TRIO Network: No Effect of PPI Use? § Real-world data from 2034 pts with GT 1 HCV receiving LDV/SOF § A per protocol analysis (n = 1979) showed no effect of PPIs on SVR 12 According to Baseline PPI Use 98 100 98 97 99 97 SVR 12 (%) PPI: No PPI: Yes n/N = 230/ 234 41/ 41 8 Wks Afdhal N, et al. EASL 2016. Abstract LBP 519. 1024/ 1045 287/ 297 12 Wks 243/ 246 113/ 116 24 Wks Slide credit: clinicaloptions. com
TRIO Network: No Effect of PPI Use? § Real-world data from 2034 pts with GT 1 HCV receiving LDV/SOF § A per protocol analysis (n = 1979) showed no effect of PPIs on SVR 12 According to Baseline PPI Use 98 100 98 97 99 97 SVR 12 (%) PPI: No PPI: Yes n/N = 230/ 234 41/ 41 8 Wks Afdhal N, et al. EASL 2016. Abstract LBP 519. 1024/ 1045 287/ 297 12 Wks 243/ 246 113/ 116 24 Wks Slide credit: clinicaloptions. com
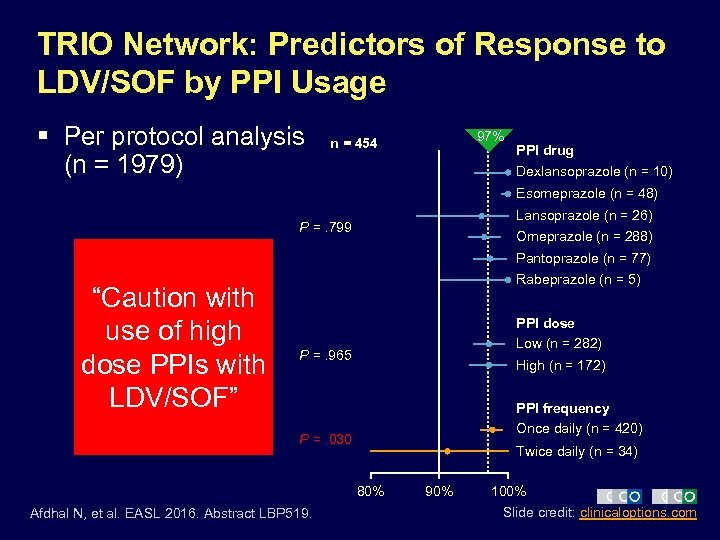 TRIO Network: Predictors of Response to LDV/SOF by PPI Usage § Per protocol analysis (n = 1979) 97% n = 454 PPI drug Dexlansoprazole (n = 10) Esomeprazole (n = 48) Lansoprazole (n = 26) P =. 799 Omeprazole (n = 288) Pantoprazole (n = 77) “Caution with use of high dose PPIs with LDV/SOF” Rabeprazole (n = 5) PPI dose Low (n = 282) P =. 965 High (n = 172) PPI frequency Once daily (n = 420) P =. 030 Twice daily (n = 34) 80% Afdhal N, et al. EASL 2016. Abstract LBP 519. 90% 100% Slide credit: clinicaloptions. com
TRIO Network: Predictors of Response to LDV/SOF by PPI Usage § Per protocol analysis (n = 1979) 97% n = 454 PPI drug Dexlansoprazole (n = 10) Esomeprazole (n = 48) Lansoprazole (n = 26) P =. 799 Omeprazole (n = 288) Pantoprazole (n = 77) “Caution with use of high dose PPIs with LDV/SOF” Rabeprazole (n = 5) PPI dose Low (n = 282) P =. 965 High (n = 172) PPI frequency Once daily (n = 420) P =. 030 Twice daily (n = 34) 80% Afdhal N, et al. EASL 2016. Abstract LBP 519. 90% 100% Slide credit: clinicaloptions. com
 New Regimens
New Regimens
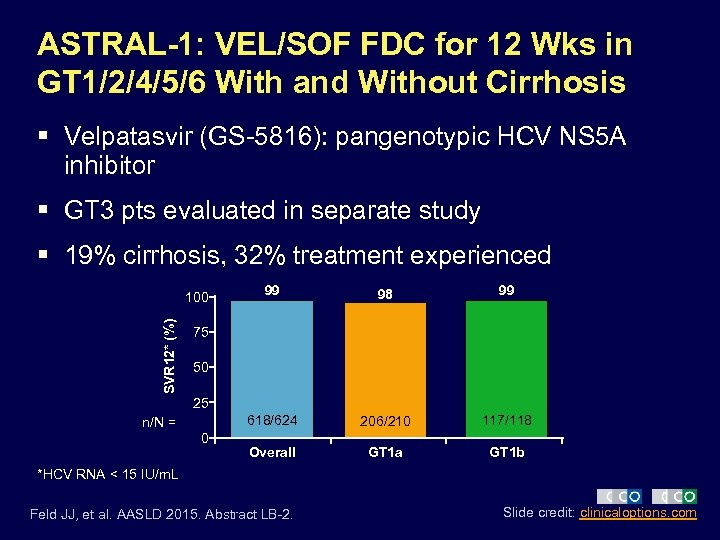 ASTRAL-1: VEL/SOF FDC for 12 Wks in GT 1/2/4/5/6 With and Without Cirrhosis § Velpatasvir (GS-5816): pangenotypic HCV NS 5 A inhibitor § GT 3 pts evaluated in separate study § 19% cirrhosis, 32% treatment experienced SVR 12* (%) 99 98 99 618/624 100 206/210 117/118 Overall GT 1 a GT 1 b 75 50 25 n/N = 0 *HCV RNA < 15 IU/m. L Feld JJ, et al. AASLD 2015. Abstract LB-2. Slide credit: clinicaloptions. com
ASTRAL-1: VEL/SOF FDC for 12 Wks in GT 1/2/4/5/6 With and Without Cirrhosis § Velpatasvir (GS-5816): pangenotypic HCV NS 5 A inhibitor § GT 3 pts evaluated in separate study § 19% cirrhosis, 32% treatment experienced SVR 12* (%) 99 98 99 618/624 100 206/210 117/118 Overall GT 1 a GT 1 b 75 50 25 n/N = 0 *HCV RNA < 15 IU/m. L Feld JJ, et al. AASLD 2015. Abstract LB-2. Slide credit: clinicaloptions. com
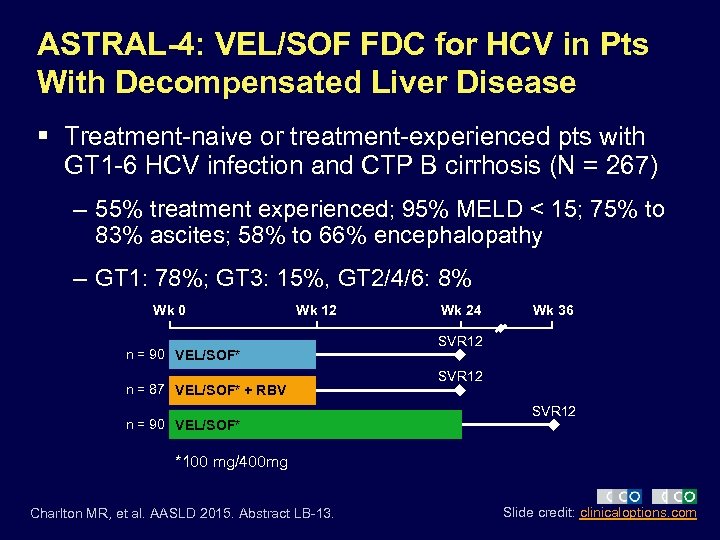 ASTRAL-4: VEL/SOF FDC for HCV in Pts With Decompensated Liver Disease § Treatment-naive or treatment-experienced pts with GT 1 -6 HCV infection and CTP B cirrhosis (N = 267) – 55% treatment experienced; 95% MELD < 15; 75% to 83% ascites; 58% to 66% encephalopathy – GT 1: 78%; GT 3: 15%, GT 2/4/6: 8% Wk 0 Wk 12 n = 90 VEL/SOF* n = 87 VEL/SOF* + RBV n = 90 VEL/SOF* Wk 24 Wk 36 SVR 12 *100 mg/400 mg Charlton MR, et al. AASLD 2015. Abstract LB-13. Slide credit: clinicaloptions. com
ASTRAL-4: VEL/SOF FDC for HCV in Pts With Decompensated Liver Disease § Treatment-naive or treatment-experienced pts with GT 1 -6 HCV infection and CTP B cirrhosis (N = 267) – 55% treatment experienced; 95% MELD < 15; 75% to 83% ascites; 58% to 66% encephalopathy – GT 1: 78%; GT 3: 15%, GT 2/4/6: 8% Wk 0 Wk 12 n = 90 VEL/SOF* n = 87 VEL/SOF* + RBV n = 90 VEL/SOF* Wk 24 Wk 36 SVR 12 *100 mg/400 mg Charlton MR, et al. AASLD 2015. Abstract LB-13. Slide credit: clinicaloptions. com
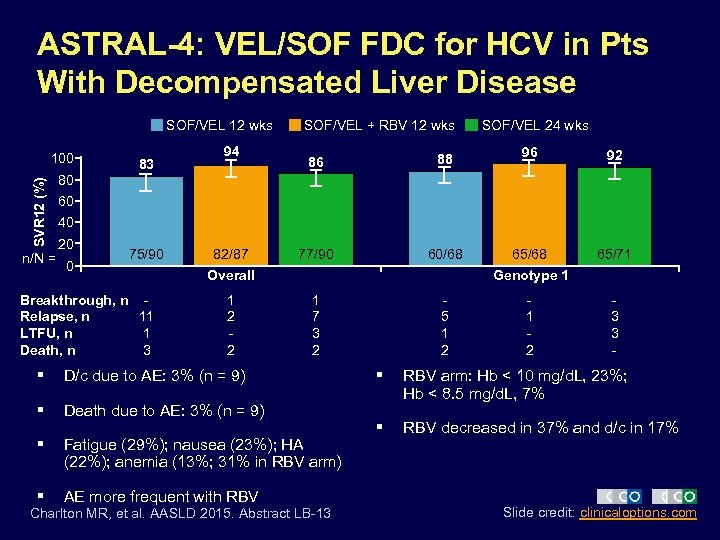 ASTRAL-4: VEL/SOF FDC for HCV in Pts With Decompensated Liver Disease SOF/VEL 12 wks SVR 12 (%) 100 n/N = 83 94 SOF/VEL + RBV 12 wks 86 88 77/90 60/68 SOF/VEL 24 wks 96 92 65/68 65/71 80 60 40 20 0 75/90 Breakthrough, n Relapse, n 11 LTFU, n 1 Death, n 3 82/87 Overall 1 2 2 Genotype 1 1 7 3 2 § D/c due to AE: 3% (n = 9) § Death due to AE: 3% (n = 9) § Fatigue (29%); nausea (23%); HA (22%); anemia (13%; 31% in RBV arm) § AE more frequent with RBV Charlton MR, et al. AASLD 2015. Abstract LB-13 5 1 2 3 3 - § RBV arm: Hb < 10 mg/d. L, 23%; Hb < 8. 5 mg/d. L, 7% § RBV decreased in 37% and d/c in 17% Slide credit: clinicaloptions. com
ASTRAL-4: VEL/SOF FDC for HCV in Pts With Decompensated Liver Disease SOF/VEL 12 wks SVR 12 (%) 100 n/N = 83 94 SOF/VEL + RBV 12 wks 86 88 77/90 60/68 SOF/VEL 24 wks 96 92 65/68 65/71 80 60 40 20 0 75/90 Breakthrough, n Relapse, n 11 LTFU, n 1 Death, n 3 82/87 Overall 1 2 2 Genotype 1 1 7 3 2 § D/c due to AE: 3% (n = 9) § Death due to AE: 3% (n = 9) § Fatigue (29%); nausea (23%); HA (22%); anemia (13%; 31% in RBV arm) § AE more frequent with RBV Charlton MR, et al. AASLD 2015. Abstract LB-13 5 1 2 3 3 - § RBV arm: Hb < 10 mg/d. L, 23%; Hb < 8. 5 mg/d. L, 7% § RBV decreased in 37% and d/c in 17% Slide credit: clinicaloptions. com
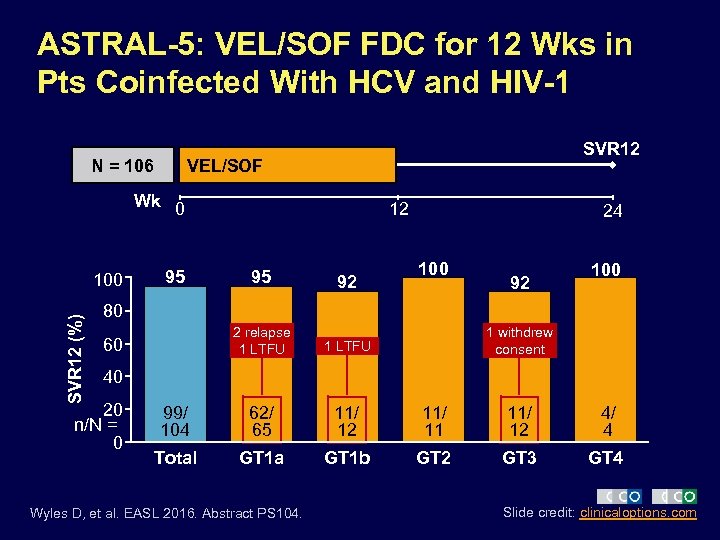 ASTRAL-5: VEL/SOF FDC for 12 Wks in Pts Coinfected With HCV and HIV-1 N = 106 SVR 12 VEL/SOF Wk 0 SVR 12 (%) 100 95 12 24 100 95 92 2 relapse 1 LTFU 99/ 104 62/ 65 11/ 12 11/ 11 11/ 12 4/ 4 Total GT 1 a GT 1 b GT 2 GT 3 GT 4 92 80 60 1 withdrew consent 40 20 n/N = 0 Wyles D, et al. EASL 2016. Abstract PS 104. Slide credit: clinicaloptions. com
ASTRAL-5: VEL/SOF FDC for 12 Wks in Pts Coinfected With HCV and HIV-1 N = 106 SVR 12 VEL/SOF Wk 0 SVR 12 (%) 100 95 12 24 100 95 92 2 relapse 1 LTFU 99/ 104 62/ 65 11/ 12 11/ 11 11/ 12 4/ 4 Total GT 1 a GT 1 b GT 2 GT 3 GT 4 92 80 60 1 withdrew consent 40 20 n/N = 0 Wyles D, et al. EASL 2016. Abstract PS 104. Slide credit: clinicaloptions. com
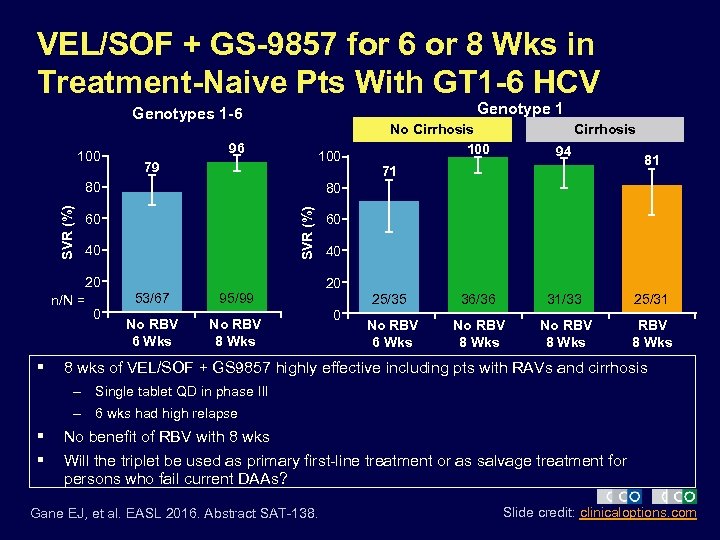 VEL/SOF + GS-9857 for 6 or 8 Wks in Treatment-Naive Pts With GT 1 -6 HCV Genotype 1 Genotypes 1 -6 100 96 100 79 60 40 20 n/N = § 53/67 0 Cirrhosis 94 71 81 80 SVR (%) 80 No Cirrhosis 100 95/99 No RBV 6 Wks No RBV 8 Wks 60 40 20 25/35 0 36/36 31/33 25/31 No RBV 6 Wks No RBV 8 Wks 8 wks of VEL/SOF + GS 9857 highly effective including pts with RAVs and cirrhosis – Single tablet QD in phase III – 6 wks had high relapse § § No benefit of RBV with 8 wks Will the triplet be used as primary first-line treatment or as salvage treatment for persons who fail current DAAs? Gane EJ, et al. EASL 2016. Abstract SAT-138. Slide credit: clinicaloptions. com
VEL/SOF + GS-9857 for 6 or 8 Wks in Treatment-Naive Pts With GT 1 -6 HCV Genotype 1 Genotypes 1 -6 100 96 100 79 60 40 20 n/N = § 53/67 0 Cirrhosis 94 71 81 80 SVR (%) 80 No Cirrhosis 100 95/99 No RBV 6 Wks No RBV 8 Wks 60 40 20 25/35 0 36/36 31/33 25/31 No RBV 6 Wks No RBV 8 Wks 8 wks of VEL/SOF + GS 9857 highly effective including pts with RAVs and cirrhosis – Single tablet QD in phase III – 6 wks had high relapse § § No benefit of RBV with 8 wks Will the triplet be used as primary first-line treatment or as salvage treatment for persons who fail current DAAs? Gane EJ, et al. EASL 2016. Abstract SAT-138. Slide credit: clinicaloptions. com
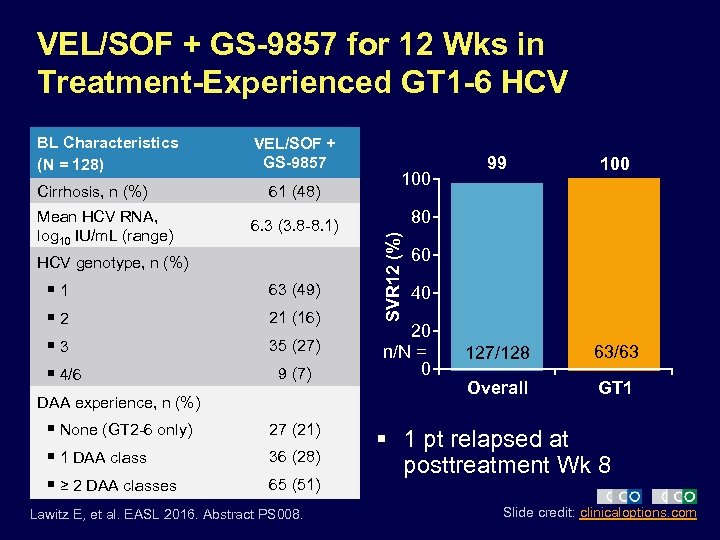 VEL/SOF + GS-9857 for 12 Wks in Treatment-Experienced GT 1 -6 HCV Cirrhosis, n (%) Mean HCV RNA, log 10 IU/m. L (range) VEL/SOF + GS-9857 61 (48) 6. 3 (3. 8 -8. 1) HCV genotype, n (%) § 1 § 2 § 3 § 4/6 63 (49) 21 (16) 35 (27) 9 (7) DAA experience, n (%) § None (GT 2 -6 only) § 1 DAA class § ≥ 2 DAA classes 27 (21) 36 (28) 65 (51) Lawitz E, et al. EASL 2016. Abstract PS 008. 100 99 100 127/128 63/63 Overall GT 1 80 SVR 12 (%) BL Characteristics (N = 128) 60 40 20 n/N = 0 § 1 pt relapsed at posttreatment Wk 8 Slide credit: clinicaloptions. com
VEL/SOF + GS-9857 for 12 Wks in Treatment-Experienced GT 1 -6 HCV Cirrhosis, n (%) Mean HCV RNA, log 10 IU/m. L (range) VEL/SOF + GS-9857 61 (48) 6. 3 (3. 8 -8. 1) HCV genotype, n (%) § 1 § 2 § 3 § 4/6 63 (49) 21 (16) 35 (27) 9 (7) DAA experience, n (%) § None (GT 2 -6 only) § 1 DAA class § ≥ 2 DAA classes 27 (21) 36 (28) 65 (51) Lawitz E, et al. EASL 2016. Abstract PS 008. 100 99 100 127/128 63/63 Overall GT 1 80 SVR 12 (%) BL Characteristics (N = 128) 60 40 20 n/N = 0 § 1 pt relapsed at posttreatment Wk 8 Slide credit: clinicaloptions. com
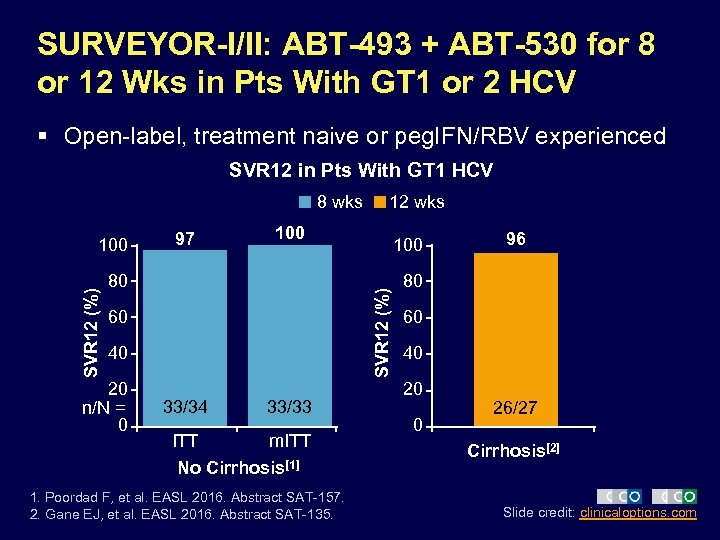 SURVEYOR-I/II: ABT-493 + ABT-530 for 8 or 12 Wks in Pts With GT 1 or 2 HCV § Open-label, treatment naive or peg. IFN/RBV experienced SVR 12 in Pts With GT 1 HCV 8 wks 97 100 80 60 40 20 n/N = 0 100 SVR 12 (%) 100 12 wks 33/34 33/33 ITT m. ITT No Cirrhosis[1] 1. Poordad F, et al. EASL 2016. Abstract SAT-157. 2. Gane EJ, et al. EASL 2016. Abstract SAT-135. 96 80 60 40 20 0 26/27 Cirrhosis[2] Slide credit: clinicaloptions. com
SURVEYOR-I/II: ABT-493 + ABT-530 for 8 or 12 Wks in Pts With GT 1 or 2 HCV § Open-label, treatment naive or peg. IFN/RBV experienced SVR 12 in Pts With GT 1 HCV 8 wks 97 100 80 60 40 20 n/N = 0 100 SVR 12 (%) 100 12 wks 33/34 33/33 ITT m. ITT No Cirrhosis[1] 1. Poordad F, et al. EASL 2016. Abstract SAT-157. 2. Gane EJ, et al. EASL 2016. Abstract SAT-135. 96 80 60 40 20 0 26/27 Cirrhosis[2] Slide credit: clinicaloptions. com
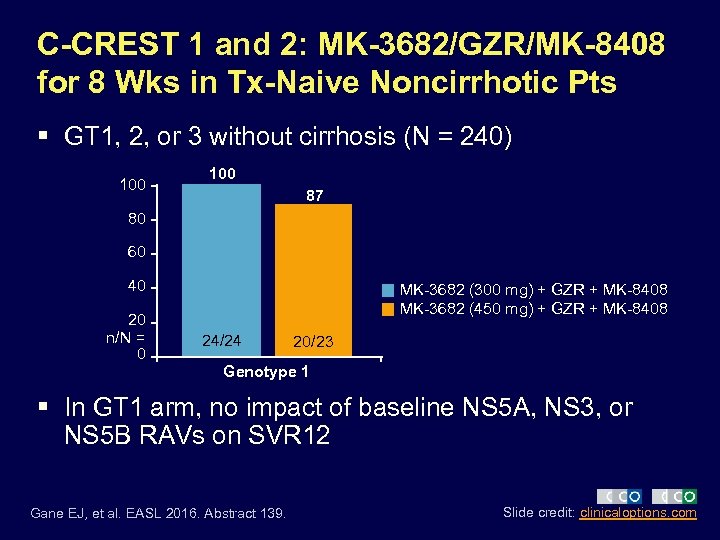 C-CREST 1 and 2: MK-3682/GZR/MK-8408 for 8 Wks in Tx-Naive Noncirrhotic Pts § GT 1, 2, or 3 without cirrhosis (N = 240) 100 87 80 60 40 20 n/N = 0 MK-3682 (300 mg) + GZR + MK-8408 MK-3682 (450 mg) + GZR + MK-8408 24/24 20/23 Genotype 1 § In GT 1 arm, no impact of baseline NS 5 A, NS 3, or NS 5 B RAVs on SVR 12 Gane EJ, et al. EASL 2016. Abstract 139. Slide credit: clinicaloptions. com
C-CREST 1 and 2: MK-3682/GZR/MK-8408 for 8 Wks in Tx-Naive Noncirrhotic Pts § GT 1, 2, or 3 without cirrhosis (N = 240) 100 87 80 60 40 20 n/N = 0 MK-3682 (300 mg) + GZR + MK-8408 MK-3682 (450 mg) + GZR + MK-8408 24/24 20/23 Genotype 1 § In GT 1 arm, no impact of baseline NS 5 A, NS 3, or NS 5 B RAVs on SVR 12 Gane EJ, et al. EASL 2016. Abstract 139. Slide credit: clinicaloptions. com
 Conclusions § 5 highly effective regimens approved for GT 1 HCV – Newest is GZR/EBR, which requires RAV testing in GT 1 a § Need for extended therapy and/or RBV in cirrhotics depending on regimen, GT 1 subtype, and prior treatment status § Real-world data reflect efficacy in clinical trials § Data support 8 wks of LDV/SOF in GT 1 treatment-naive noncirrhotics with HCV RNA < 6 M IU/m. L § High-dose PPIs should be avoided with LDV (same likely to be the case with VEL based on clinical trial designs) Slide credit: clinicaloptions. com
Conclusions § 5 highly effective regimens approved for GT 1 HCV – Newest is GZR/EBR, which requires RAV testing in GT 1 a § Need for extended therapy and/or RBV in cirrhotics depending on regimen, GT 1 subtype, and prior treatment status § Real-world data reflect efficacy in clinical trials § Data support 8 wks of LDV/SOF in GT 1 treatment-naive noncirrhotics with HCV RNA < 6 M IU/m. L § High-dose PPIs should be avoided with LDV (same likely to be the case with VEL based on clinical trial designs) Slide credit: clinicaloptions. com
 New Regimens in Development § Promising regimens – VEL/SOF (likely to be approved in June 2016) – Second-generation 2 -drug regimen of ABT-493/ ABT-530 – Triplet regimens of PI/NS 5 A/Nuc § New regimens may offer 8 -week option for noncirrhotics § High SVR rates in DAA failures Slide credit: clinicaloptions. com
New Regimens in Development § Promising regimens – VEL/SOF (likely to be approved in June 2016) – Second-generation 2 -drug regimen of ABT-493/ ABT-530 – Triplet regimens of PI/NS 5 A/Nuc § New regimens may offer 8 -week option for noncirrhotics § High SVR rates in DAA failures Slide credit: clinicaloptions. com
 Go Online for More CCO Coverage of HIV! Multimedia modules featuring video of expert faculty discussions of controversies and challenging cases Downloadable slidesets for your own study or presentations clinicaloptions. com/hcv
Go Online for More CCO Coverage of HIV! Multimedia modules featuring video of expert faculty discussions of controversies and challenging cases Downloadable slidesets for your own study or presentations clinicaloptions. com/hcv


