66b8a5cb97fe519566e14a5636eac98f.ppt
- Количество слайдов: 65
 General Principles of Pathophysiology n. The Cellular Environment n. Fluids & Electrolytes n. Acid-base Balance & Maintenance
General Principles of Pathophysiology n. The Cellular Environment n. Fluids & Electrolytes n. Acid-base Balance & Maintenance
 Topics Describe the distribution of water in the body n Discuss common physiologic electrolytes n Review mechanisms of transport n – osmosis, diffusion, etc Discuss hemostasis & blood types n Discuss concepts of acid-base maintenance n
Topics Describe the distribution of water in the body n Discuss common physiologic electrolytes n Review mechanisms of transport n – osmosis, diffusion, etc Discuss hemostasis & blood types n Discuss concepts of acid-base maintenance n
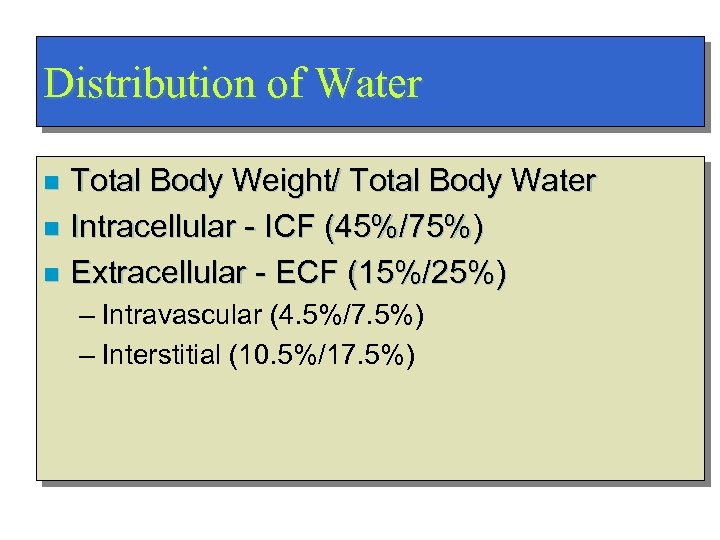 Distribution of Water Total Body Weight/ Total Body Water n Intracellular - ICF (45%/75%) n Extracellular - ECF (15%/25%) n – Intravascular (4. 5%/7. 5%) – Interstitial (10. 5%/17. 5%)
Distribution of Water Total Body Weight/ Total Body Water n Intracellular - ICF (45%/75%) n Extracellular - ECF (15%/25%) n – Intravascular (4. 5%/7. 5%) – Interstitial (10. 5%/17. 5%)
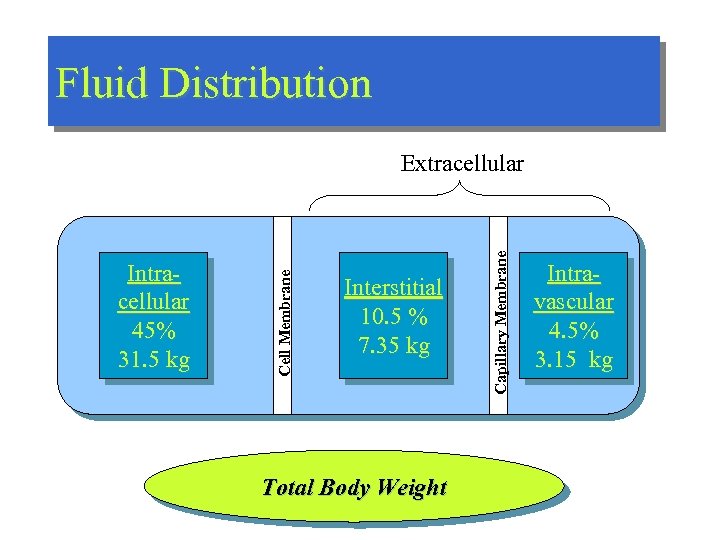 Fluid Distribution Interstitial 10. 5 % 7. 35 kg Total Body Weight Capillary Membrane Intracellular 45% 31. 5 kg Cell Membrane Extracellular Intravascular 4. 5% 3. 15 kg
Fluid Distribution Interstitial 10. 5 % 7. 35 kg Total Body Weight Capillary Membrane Intracellular 45% 31. 5 kg Cell Membrane Extracellular Intravascular 4. 5% 3. 15 kg
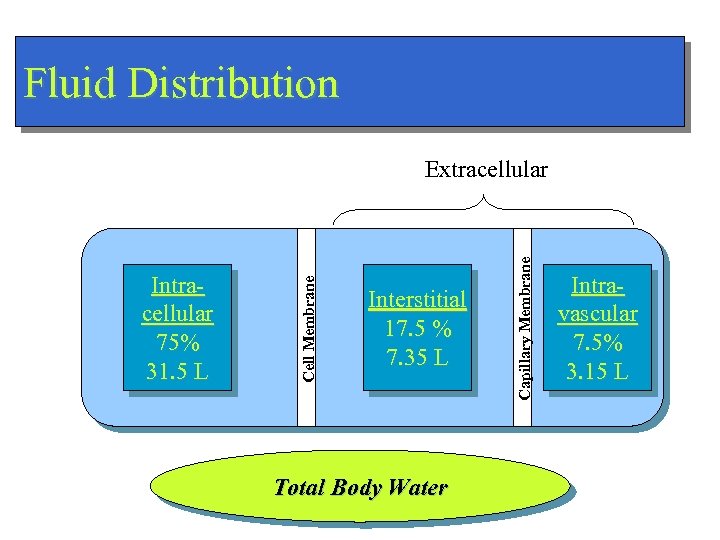 Fluid Distribution Interstitial 17. 5 % 7. 35 L Total Body Water Capillary Membrane Intracellular 75% 31. 5 L Cell Membrane Extracellular Intravascular 7. 5% 3. 15 L
Fluid Distribution Interstitial 17. 5 % 7. 35 L Total Body Water Capillary Membrane Intracellular 75% 31. 5 L Cell Membrane Extracellular Intravascular 7. 5% 3. 15 L
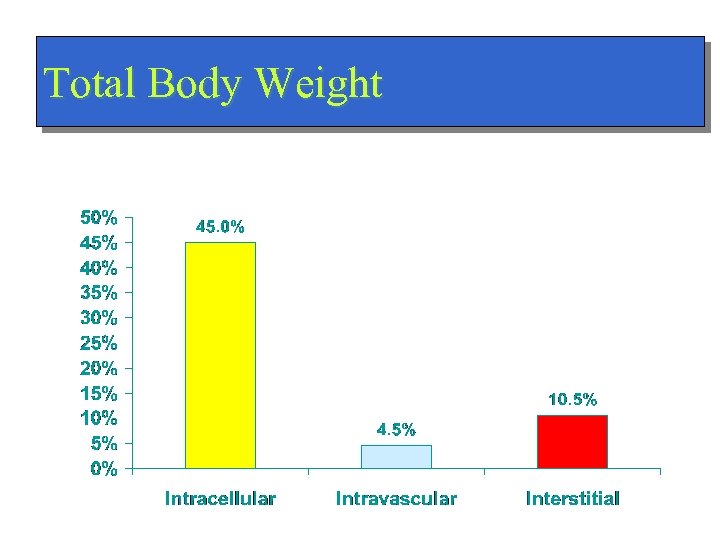 Total Body Weight
Total Body Weight
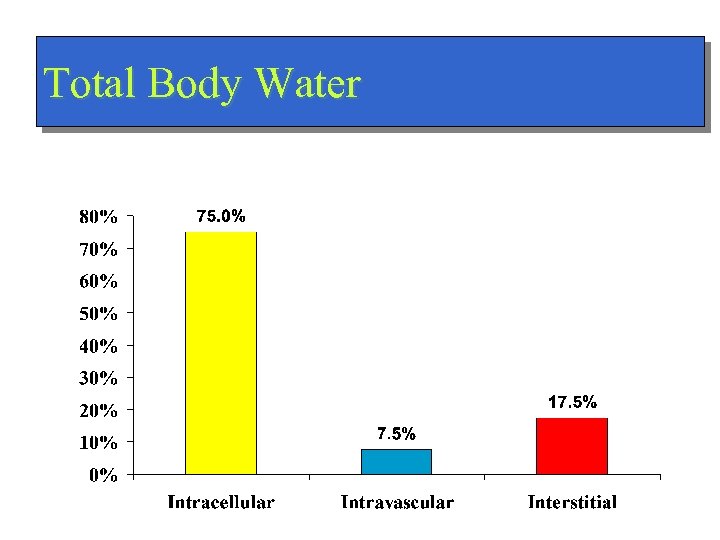 Total Body Water
Total Body Water
 Edema Fluid accumulation in the interstitial compartment n Causes: n – Lymphatic ‘leakage’ – Excessive hydrostatic pressure – Inadequate osmotic pressure
Edema Fluid accumulation in the interstitial compartment n Causes: n – Lymphatic ‘leakage’ – Excessive hydrostatic pressure – Inadequate osmotic pressure
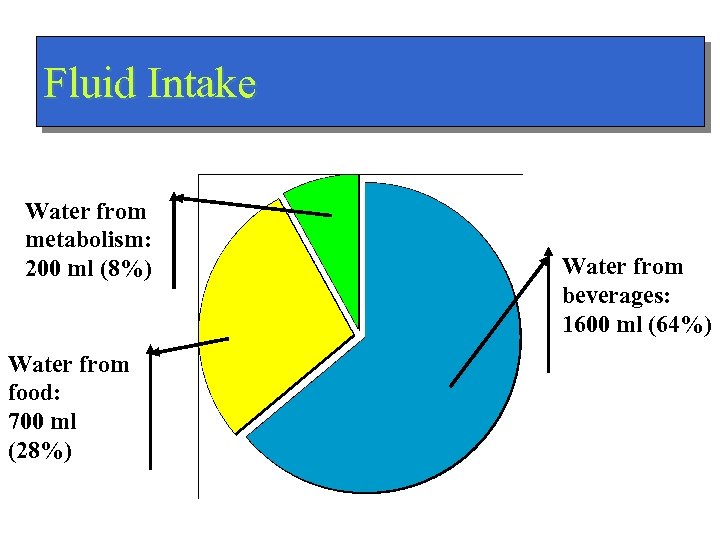 Fluid Intake Water from metabolism: 200 ml (8%) Water from food: 700 ml (28%) Water from beverages: 1600 ml (64%)
Fluid Intake Water from metabolism: 200 ml (8%) Water from food: 700 ml (28%) Water from beverages: 1600 ml (64%)
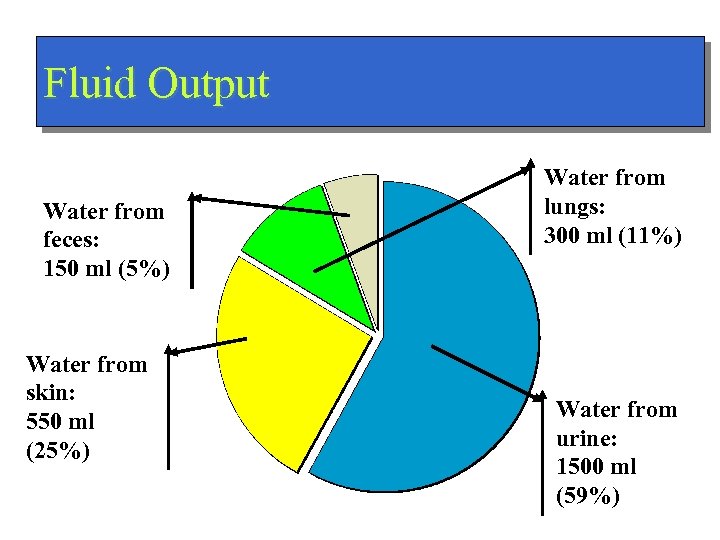 Fluid Output Water from feces: 150 ml (5%) Water from skin: 550 ml (25%) Water from lungs: 300 ml (11%) Water from urine: 1500 ml (59%)
Fluid Output Water from feces: 150 ml (5%) Water from skin: 550 ml (25%) Water from lungs: 300 ml (11%) Water from urine: 1500 ml (59%)
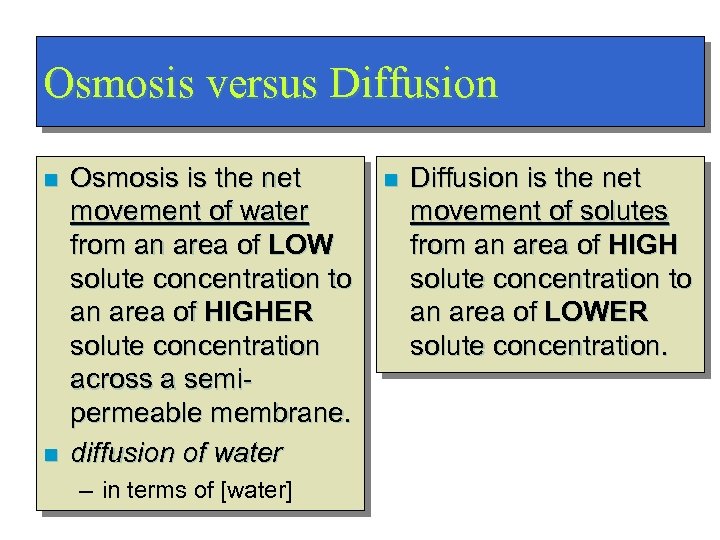 Osmosis versus Diffusion n n Osmosis is the net movement of water from an area of LOW solute concentration to an area of HIGHER solute concentration across a semipermeable membrane. diffusion of water – in terms of [water] n Diffusion is the net movement of solutes from an area of HIGH solute concentration to an area of LOWER solute concentration.
Osmosis versus Diffusion n n Osmosis is the net movement of water from an area of LOW solute concentration to an area of HIGHER solute concentration across a semipermeable membrane. diffusion of water – in terms of [water] n Diffusion is the net movement of solutes from an area of HIGH solute concentration to an area of LOWER solute concentration.
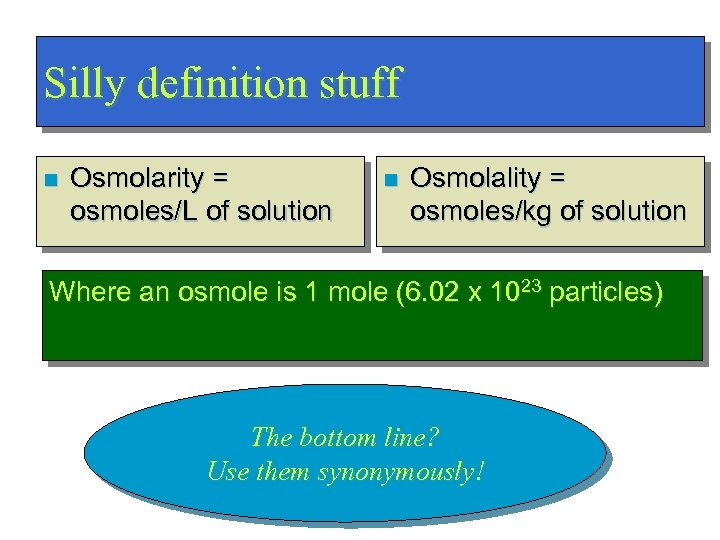 Silly definition stuff n Osmolarity = osmoles/L of solution n Osmolality = osmoles/kg of solution Where an osmole is 1 mole (6. 02 x 1023 particles) The bottom line? Use them synonymously!
Silly definition stuff n Osmolarity = osmoles/L of solution n Osmolality = osmoles/kg of solution Where an osmole is 1 mole (6. 02 x 1023 particles) The bottom line? Use them synonymously!
 Tonicity Isotonic n Hypertonic n Hypotonic n
Tonicity Isotonic n Hypertonic n Hypotonic n
 Isotonic Solutions Same solute concentration as RBC n If injected into vein: no net movement of fluid n Example: 0. 9% sodium chloride solution n – aka Normal Saline
Isotonic Solutions Same solute concentration as RBC n If injected into vein: no net movement of fluid n Example: 0. 9% sodium chloride solution n – aka Normal Saline
 Hypertonic Solutions Higher solute concentration than RBC n If injected into vein: n – Fluid moves INTO veins
Hypertonic Solutions Higher solute concentration than RBC n If injected into vein: n – Fluid moves INTO veins
 Hypotonic Solutions Lower solute concentration than RBC n If injected into vein: n – Fluid moves OUT of veins
Hypotonic Solutions Lower solute concentration than RBC n If injected into vein: n – Fluid moves OUT of veins
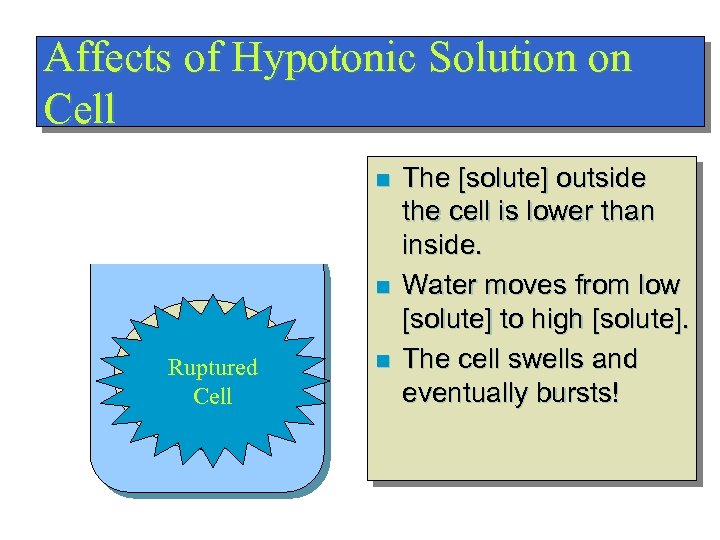 Affects of Hypotonic Solution on Cell n n Swollen Ruptured Swelling Cell n The [solute] outside the cell is lower than inside. Water moves from low [solute] to high [solute]. The cell swells and eventually bursts!
Affects of Hypotonic Solution on Cell n n Swollen Ruptured Swelling Cell n The [solute] outside the cell is lower than inside. Water moves from low [solute] to high [solute]. The cell swells and eventually bursts!
![Affects of Hypertonic Solution on Cell n n Shrinking Shrunken Cell n The [solute] Affects of Hypertonic Solution on Cell n n Shrinking Shrunken Cell n The [solute]](https://present5.com/presentation/66b8a5cb97fe519566e14a5636eac98f/image-18.jpg) Affects of Hypertonic Solution on Cell n n Shrinking Shrunken Cell n The [solute] outside the cell is higher than inside. Water moves from low [solute] to high [solute]. The cell shrinks!
Affects of Hypertonic Solution on Cell n n Shrinking Shrunken Cell n The [solute] outside the cell is higher than inside. Water moves from low [solute] to high [solute]. The cell shrinks!
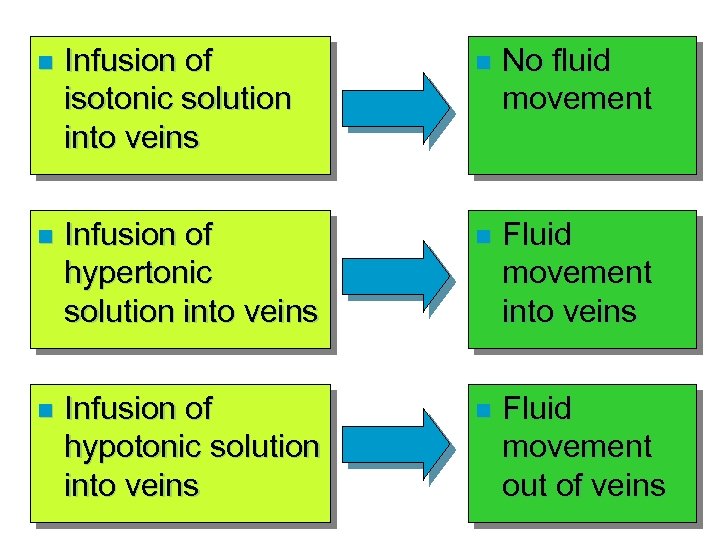 n Infusion of isotonic solution into veins n No fluid movement n Infusion of hypertonic solution into veins n Fluid movement into veins n Infusion of hypotonic solution into veins n Fluid movement out of veins
n Infusion of isotonic solution into veins n No fluid movement n Infusion of hypertonic solution into veins n Fluid movement into veins n Infusion of hypotonic solution into veins n Fluid movement out of veins
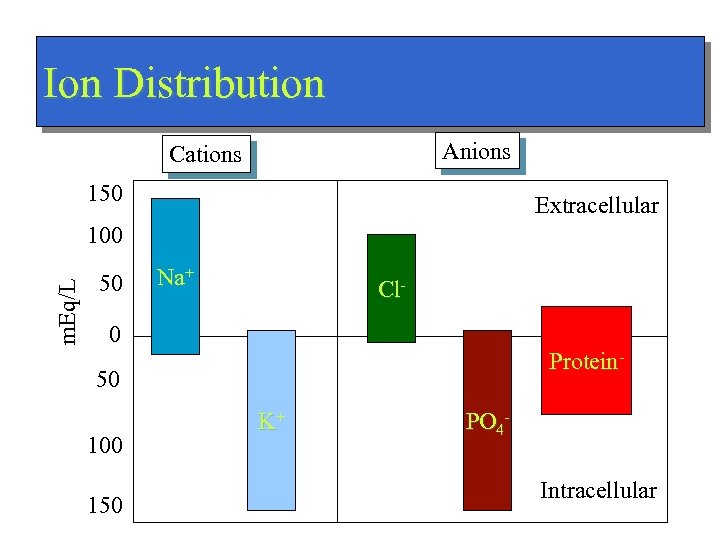 Ion Distribution Anions Cations 150 Extracellular m. Eq/L 100 50 Na+ Cl- 0 Protein- 50 100 150 K+ PO 4 Intracellular
Ion Distribution Anions Cations 150 Extracellular m. Eq/L 100 50 Na+ Cl- 0 Protein- 50 100 150 K+ PO 4 Intracellular
 Example of Role of Electrolytes n Nervous System – Propagation of Action Potential n Cardiovascular System – Cardiac conduction & contraction
Example of Role of Electrolytes n Nervous System – Propagation of Action Potential n Cardiovascular System – Cardiac conduction & contraction
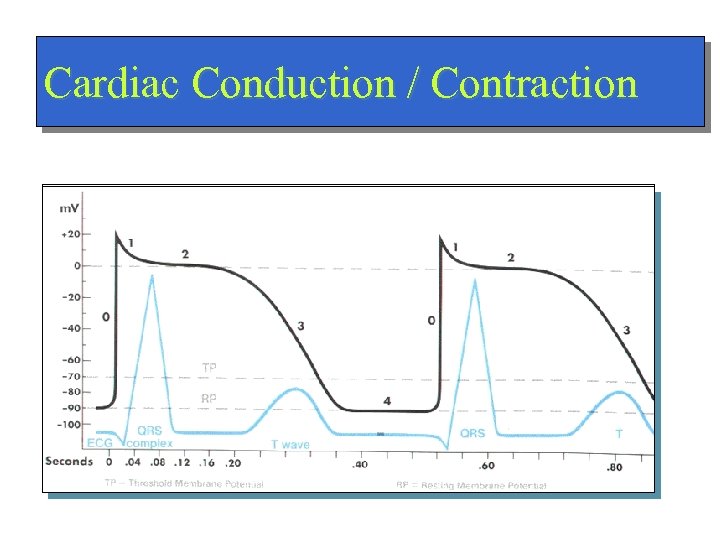 Cardiac Conduction / Contraction
Cardiac Conduction / Contraction
 Composition of Blood 8% of total body weight n Plasma: 55% n – Water: 90% – Solutes: 10% n Formed elements: 45% – Platelets – Erythrocytes
Composition of Blood 8% of total body weight n Plasma: 55% n – Water: 90% – Solutes: 10% n Formed elements: 45% – Platelets – Erythrocytes
 Hematrocrit % of RBC in blood n Normal: n – 37% - 47% (Female) – 40% - 54% (Male)
Hematrocrit % of RBC in blood n Normal: n – 37% - 47% (Female) – 40% - 54% (Male)
 Blood Components Plasma: liquid portion of blood n Contains Proteins n – Albumin (60%) contribute to osmotic pressure – Globulin (36%): lipid transport and antibodies – Fibrinogen (4%): blood clotting
Blood Components Plasma: liquid portion of blood n Contains Proteins n – Albumin (60%) contribute to osmotic pressure – Globulin (36%): lipid transport and antibodies – Fibrinogen (4%): blood clotting
 Blood Components n Formed Elements – Erythrocytes – Leukocytes – Thrombocytes
Blood Components n Formed Elements – Erythrocytes – Leukocytes – Thrombocytes
 Erythrocytes ‘biconcave’ disc n 7 -8 mcm diameter n Packed with hemoglobin n 4. 5 - 6 million RBC/mm 3 (males) n Anucleate n 120 day life span n 2 million replaced per second! n
Erythrocytes ‘biconcave’ disc n 7 -8 mcm diameter n Packed with hemoglobin n 4. 5 - 6 million RBC/mm 3 (males) n Anucleate n 120 day life span n 2 million replaced per second! n
 Leukocytes Most work done in tissues n 5, 000 - 6, 000/mm 3 n – Neutrophils (60 -70%) – Basophils (Mast Cells) (<1%) – Eosinophils (2 -4%) – Lymphocytes (20 -25%) – Monocytes (Macrophages) (3 -8%)
Leukocytes Most work done in tissues n 5, 000 - 6, 000/mm 3 n – Neutrophils (60 -70%) – Basophils (Mast Cells) (<1%) – Eosinophils (2 -4%) – Lymphocytes (20 -25%) – Monocytes (Macrophages) (3 -8%)
 Thrombocytes Platelets n Cell fragments n 250, 000 - 500, 000/mm 3 n Form platelet plugs n
Thrombocytes Platelets n Cell fragments n 250, 000 - 500, 000/mm 3 n Form platelet plugs n
 Hemostasis The stoppage of bleeding. n Three methods n – Vascular constriction – Platelet plug formation – Coagulation
Hemostasis The stoppage of bleeding. n Three methods n – Vascular constriction – Platelet plug formation – Coagulation
 Coagulation Formation of blood clots n Prothrombin activator n Prothrombin Thrombin n Fibrinogen Fibrin n Clot retraction n
Coagulation Formation of blood clots n Prothrombin activator n Prothrombin Thrombin n Fibrinogen Fibrin n Clot retraction n
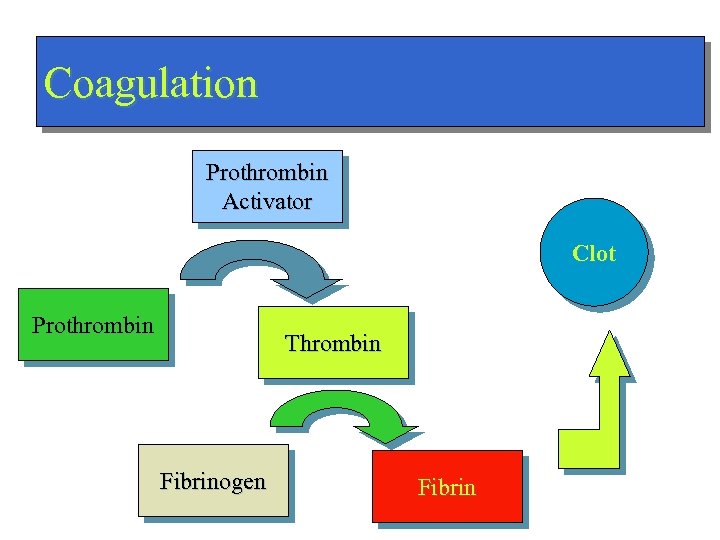 Coagulation Prothrombin Activator Clot Prothrombin Thrombin Fibrinogen Fibrin
Coagulation Prothrombin Activator Clot Prothrombin Thrombin Fibrinogen Fibrin
 Fibrinolysis Plasminogen n tissue plasminogen activator (t. PA) n Plasmin n
Fibrinolysis Plasminogen n tissue plasminogen activator (t. PA) n Plasmin n
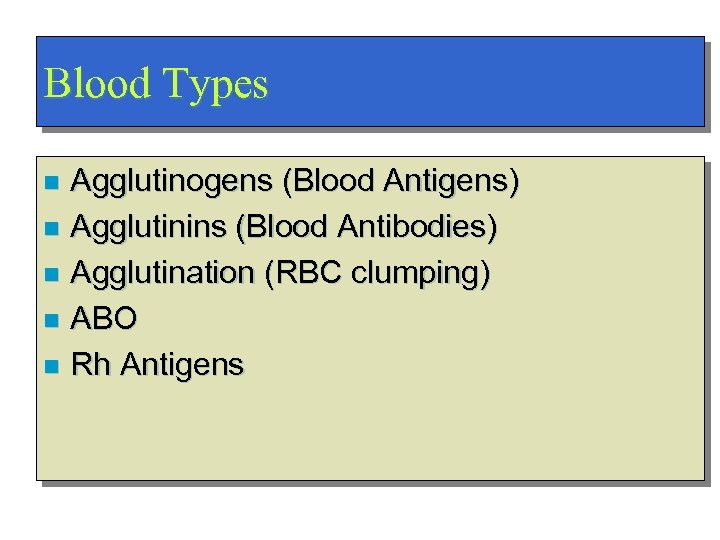 Blood Types Agglutinogens (Blood Antigens) n Agglutinins (Blood Antibodies) n Agglutination (RBC clumping) n ABO n Rh Antigens n
Blood Types Agglutinogens (Blood Antigens) n Agglutinins (Blood Antibodies) n Agglutination (RBC clumping) n ABO n Rh Antigens n
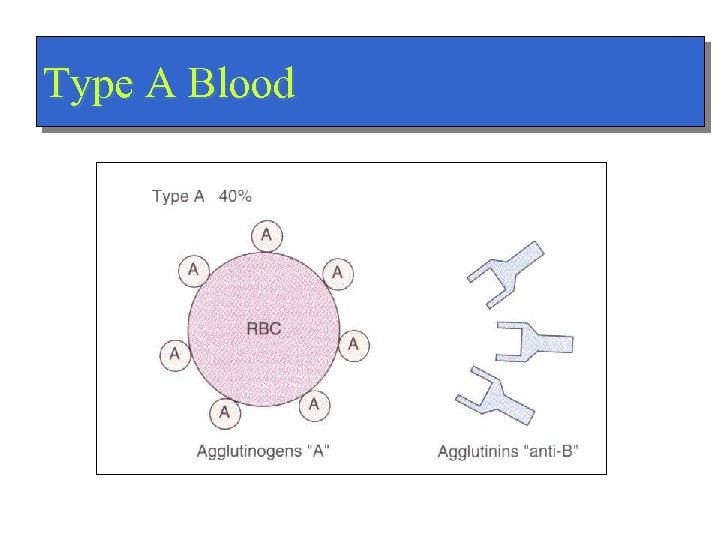 Type A Blood
Type A Blood
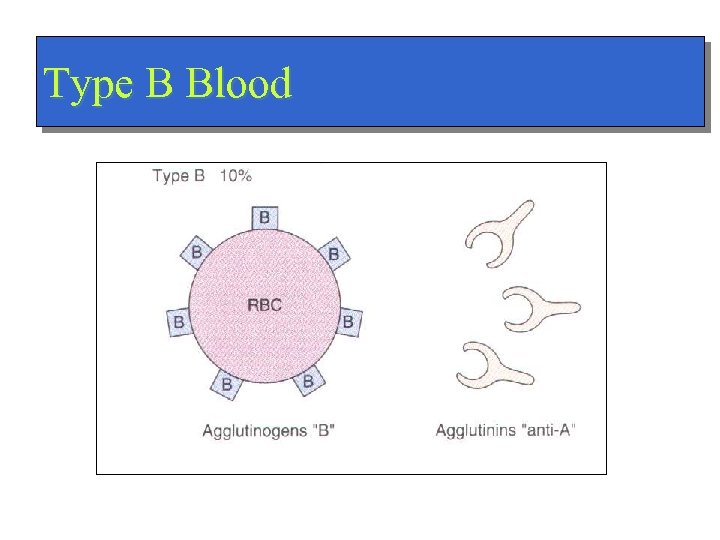 Type B Blood
Type B Blood
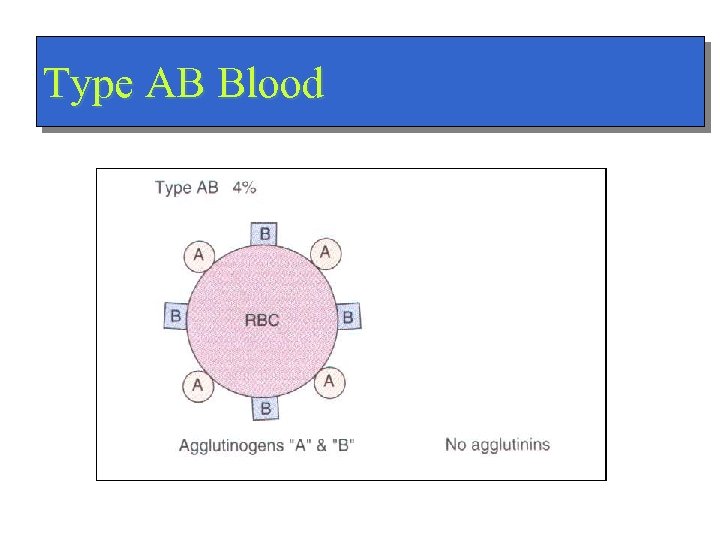 Type AB Blood
Type AB Blood
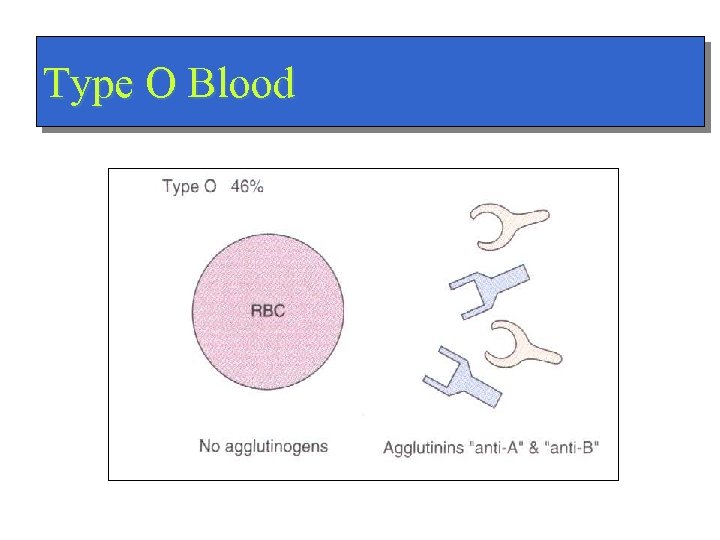 Type O Blood
Type O Blood
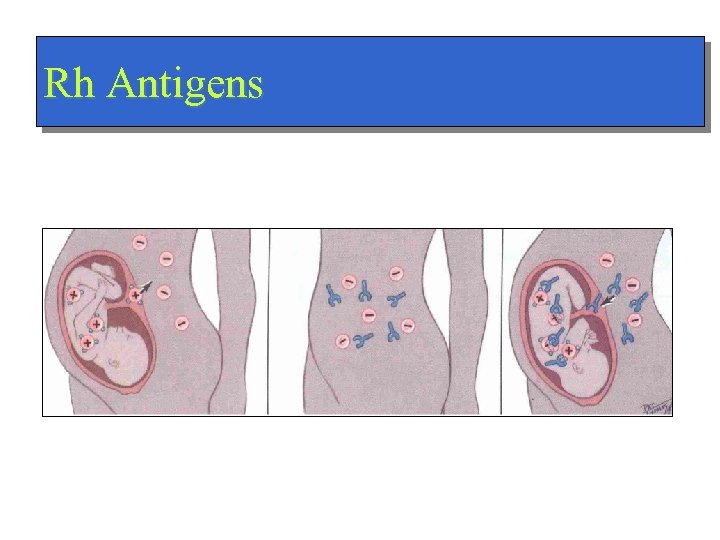 Rh Antigens
Rh Antigens
![Bottom line of Acid-Base n Regulation of [H+] – normally about 1/3. 5 million Bottom line of Acid-Base n Regulation of [H+] – normally about 1/3. 5 million](https://present5.com/presentation/66b8a5cb97fe519566e14a5636eac98f/image-40.jpg) Bottom line of Acid-Base n Regulation of [H+] – normally about 1/3. 5 million that of [Na+] – 0. 00004 m. Eq/L (4 x 10 -8 Eq/L) n Dependent upon – Kidneys – Chemical Buffers n Precise regulation necessary for peak enzyme activity
Bottom line of Acid-Base n Regulation of [H+] – normally about 1/3. 5 million that of [Na+] – 0. 00004 m. Eq/L (4 x 10 -8 Eq/L) n Dependent upon – Kidneys – Chemical Buffers n Precise regulation necessary for peak enzyme activity
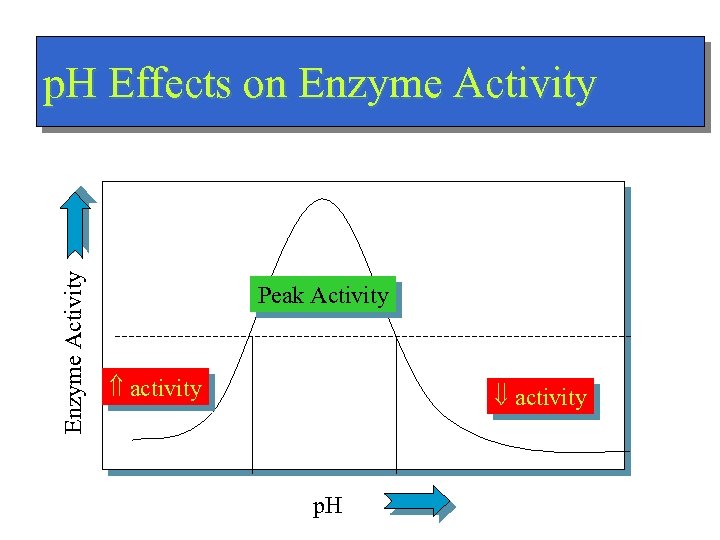 Enzyme Activity p. H Effects on Enzyme Activity Peak Activity activity p. H
Enzyme Activity p. H Effects on Enzyme Activity Peak Activity activity p. H
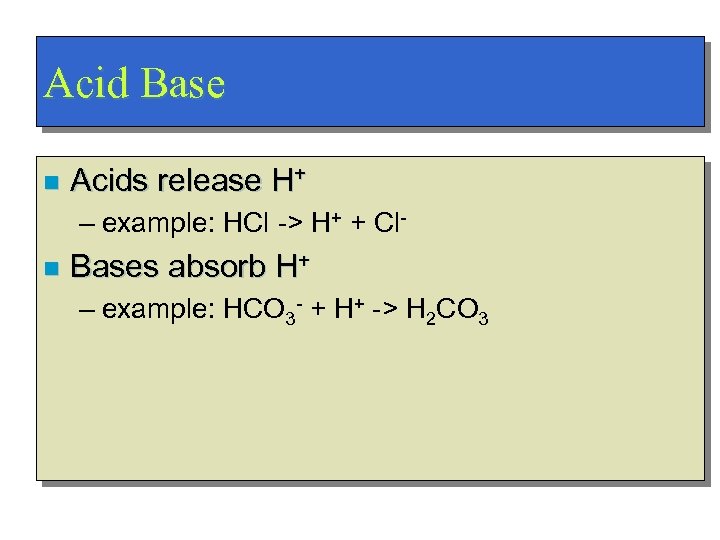 Acid Base n Acids release H+ – example: HCl -> H+ + Cl- n Bases absorb H+ – example: HCO 3 - + H+ -> H 2 CO 3
Acid Base n Acids release H+ – example: HCl -> H+ + Cl- n Bases absorb H+ – example: HCO 3 - + H+ -> H 2 CO 3
![p. H is logarithmic p. H = log 1/[H+] n = - log 0. p. H is logarithmic p. H = log 1/[H+] n = - log 0.](https://present5.com/presentation/66b8a5cb97fe519566e14a5636eac98f/image-43.jpg) p. H is logarithmic p. H = log 1/[H+] n = - log 0. 00000004 Eq/L n p. H = 7. 4 n n Think of p. H as ‘power of [H+]
p. H is logarithmic p. H = log 1/[H+] n = - log 0. 00000004 Eq/L n p. H = 7. 4 n n Think of p. H as ‘power of [H+]
![p. H is Logarithmic p. H is inversely related to [H+] Small p. H p. H is Logarithmic p. H is inversely related to [H+] Small p. H](https://present5.com/presentation/66b8a5cb97fe519566e14a5636eac98f/image-44.jpg) p. H is Logarithmic p. H is inversely related to [H+] Small p. H mean large [H+] as [H+] p. H & as [H+] p. H 7. 4 = 0. 00000004 p. H 7. 1 = 0. 00000008 (it doubled!)
p. H is Logarithmic p. H is inversely related to [H+] Small p. H mean large [H+] as [H+] p. H & as [H+] p. H 7. 4 = 0. 00000004 p. H 7. 1 = 0. 00000008 (it doubled!)
 Buffers Resist p. H Changes Weak acid & conjugate base pair n H 2 CO 3 HCO 3 + H+ n Conjugate Acid conjugate base + acid n
Buffers Resist p. H Changes Weak acid & conjugate base pair n H 2 CO 3 HCO 3 + H+ n Conjugate Acid conjugate base + acid n
![Henderson-Hasselbalch Equation n p. H = p. Ka + log [base]/[acid] – Ex: • Henderson-Hasselbalch Equation n p. H = p. Ka + log [base]/[acid] – Ex: •](https://present5.com/presentation/66b8a5cb97fe519566e14a5636eac98f/image-46.jpg) Henderson-Hasselbalch Equation n p. H = p. Ka + log [base]/[acid] – Ex: • = 6. 1 + log 20/1 • = 6. 1 + 1. 3 • = 7. 4 n Key ratio is base: acid – HCO 3 - : CO 2 (standing in for H 2 CO 3)
Henderson-Hasselbalch Equation n p. H = p. Ka + log [base]/[acid] – Ex: • = 6. 1 + log 20/1 • = 6. 1 + 1. 3 • = 7. 4 n Key ratio is base: acid – HCO 3 - : CO 2 (standing in for H 2 CO 3)
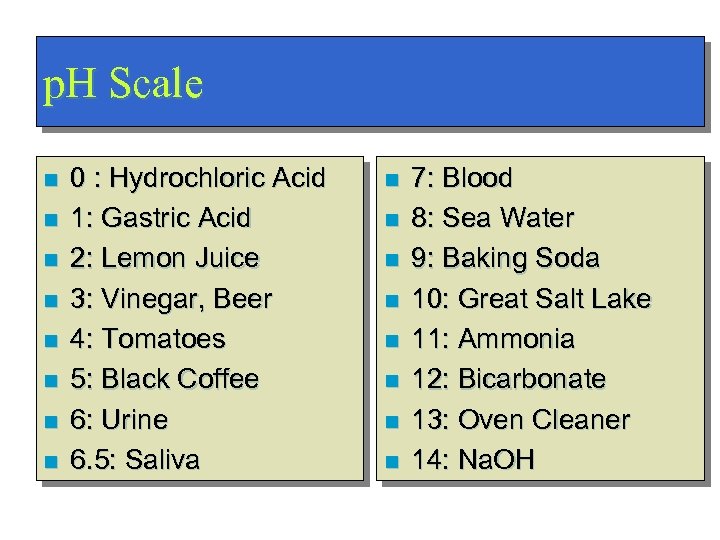 p. H Scale n n n n 0 : Hydrochloric Acid 1: Gastric Acid 2: Lemon Juice 3: Vinegar, Beer 4: Tomatoes 5: Black Coffee 6: Urine 6. 5: Saliva n n n n 7: Blood 8: Sea Water 9: Baking Soda 10: Great Salt Lake 11: Ammonia 12: Bicarbonate 13: Oven Cleaner 14: Na. OH
p. H Scale n n n n 0 : Hydrochloric Acid 1: Gastric Acid 2: Lemon Juice 3: Vinegar, Beer 4: Tomatoes 5: Black Coffee 6: Urine 6. 5: Saliva n n n n 7: Blood 8: Sea Water 9: Baking Soda 10: Great Salt Lake 11: Ammonia 12: Bicarbonate 13: Oven Cleaner 14: Na. OH
 Acid Base Compensation Buffer System n Respiratory System n Renal System n
Acid Base Compensation Buffer System n Respiratory System n Renal System n
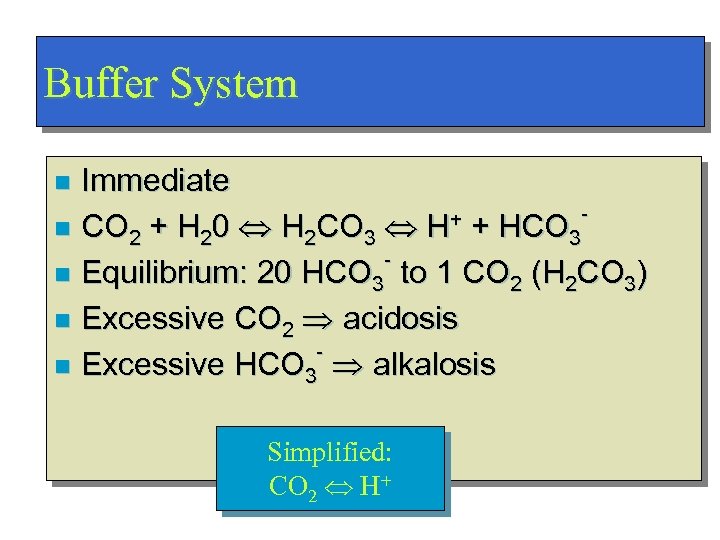 Buffer System Immediate + + HCO n CO 2 + H 20 H 2 CO 3 H 3 n Equilibrium: 20 HCO 3 to 1 CO 2 (H 2 CO 3) n Excessive CO 2 acidosis n Excessive HCO 3 alkalosis n Simplified: CO 2 H+
Buffer System Immediate + + HCO n CO 2 + H 20 H 2 CO 3 H 3 n Equilibrium: 20 HCO 3 to 1 CO 2 (H 2 CO 3) n Excessive CO 2 acidosis n Excessive HCO 3 alkalosis n Simplified: CO 2 H+
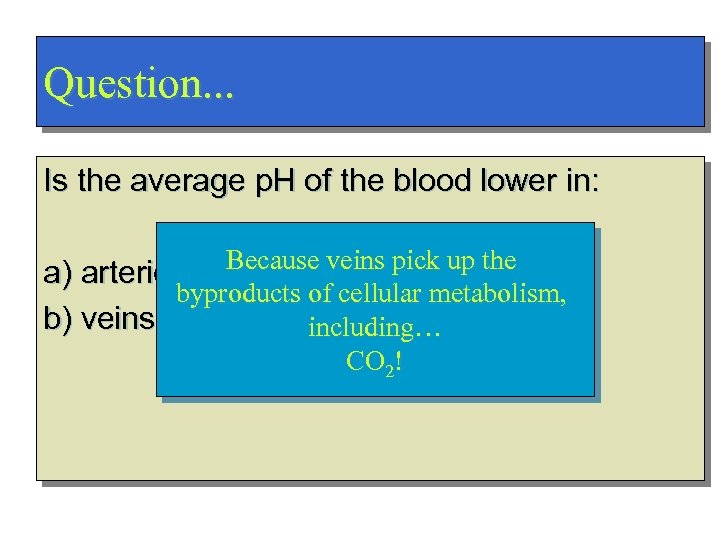 Question. . . Is the average p. H of the blood lower in: a) arteries Because veins pick up the Veins! byproducts of cellular metabolism, b) veins including… Why? CO 2!
Question. . . Is the average p. H of the blood lower in: a) arteries Because veins pick up the Veins! byproducts of cellular metabolism, b) veins including… Why? CO 2!
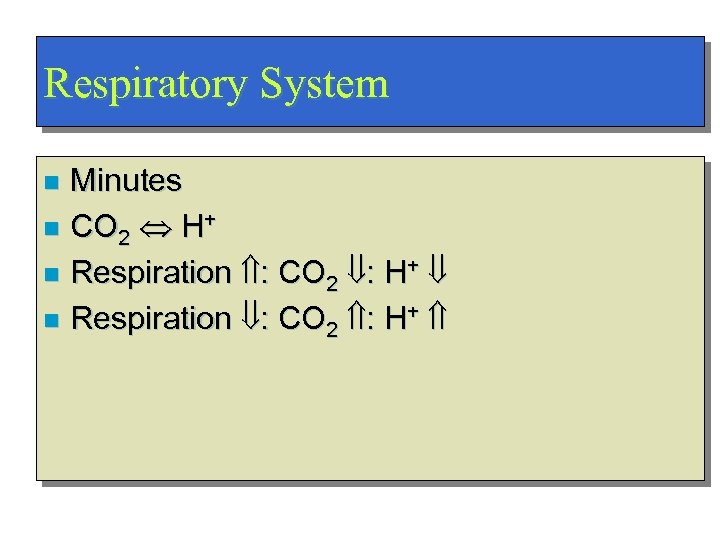 Respiratory System Minutes n CO 2 H+ n Respiration : CO 2 : H+ n
Respiratory System Minutes n CO 2 H+ n Respiration : CO 2 : H+ n
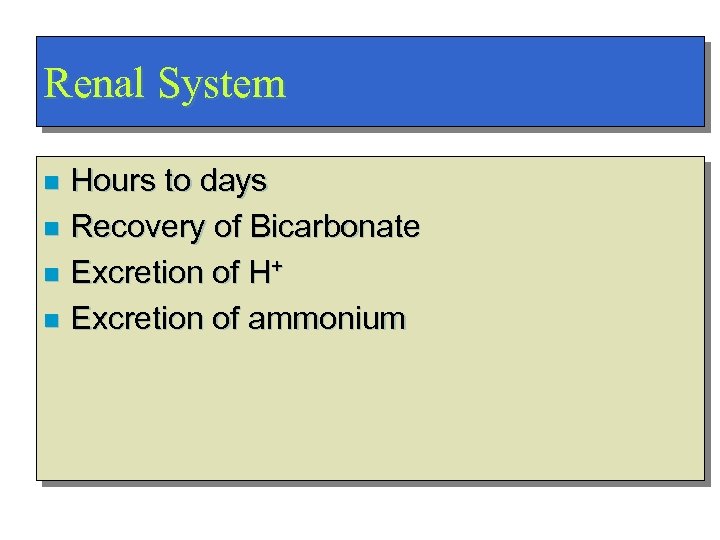 Renal System Hours to days n Recovery of Bicarbonate n Excretion of H+ n Excretion of ammonium n
Renal System Hours to days n Recovery of Bicarbonate n Excretion of H+ n Excretion of ammonium n
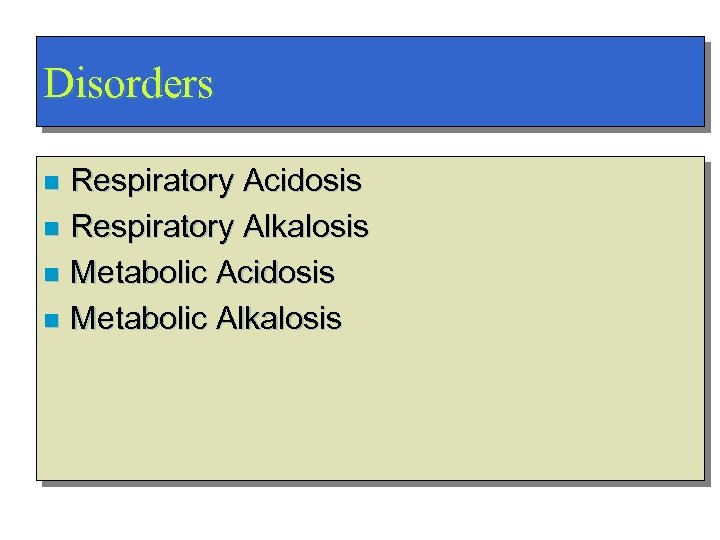 Disorders Respiratory Acidosis n Respiratory Alkalosis n Metabolic Acidosis n Metabolic Alkalosis n
Disorders Respiratory Acidosis n Respiratory Alkalosis n Metabolic Acidosis n Metabolic Alkalosis n
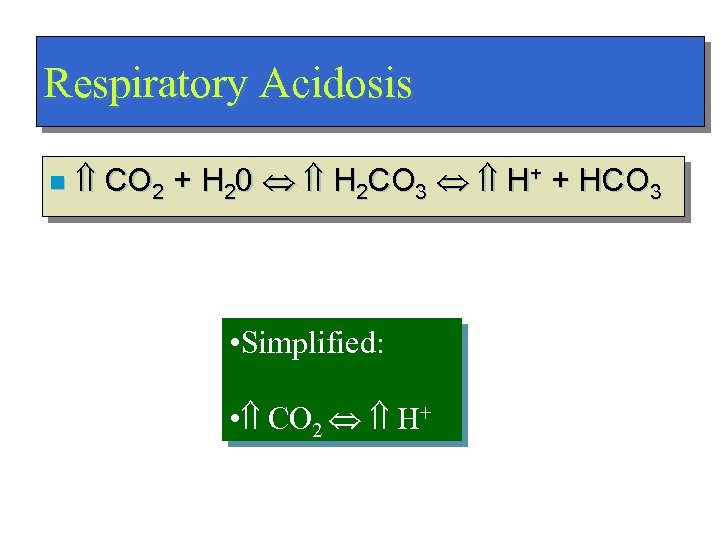 Respiratory Acidosis n CO 2 + H 20 H 2 CO 3 H+ + HCO 3 • Simplified: • CO 2 H+
Respiratory Acidosis n CO 2 + H 20 H 2 CO 3 H+ + HCO 3 • Simplified: • CO 2 H+
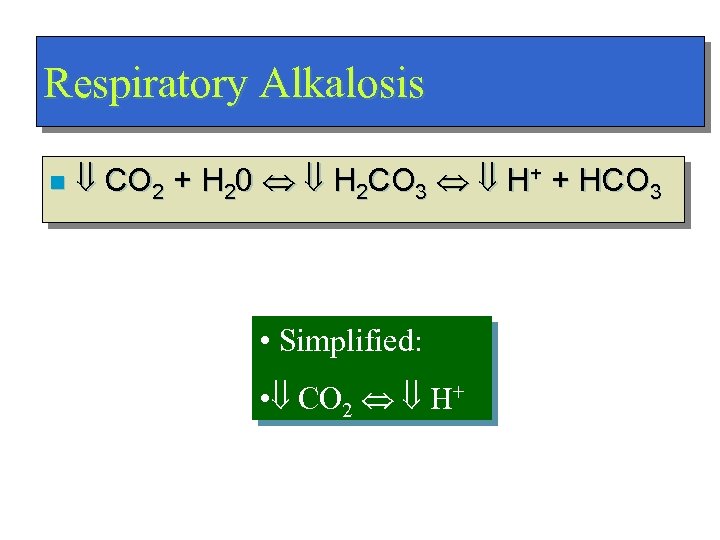 Respiratory Alkalosis n CO 2 + H 20 H 2 CO 3 H+ + HCO 3 • Simplified: • CO 2 H+
Respiratory Alkalosis n CO 2 + H 20 H 2 CO 3 H+ + HCO 3 • Simplified: • CO 2 H+
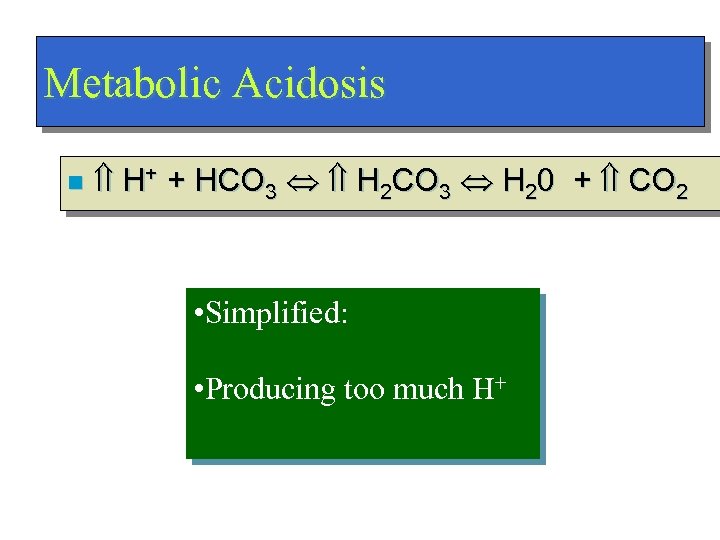 Metabolic Acidosis n H+ + HCO 3 H 2 CO 3 H 20 + CO 2 • Simplified: • Producing too much H+
Metabolic Acidosis n H+ + HCO 3 H 2 CO 3 H 20 + CO 2 • Simplified: • Producing too much H+
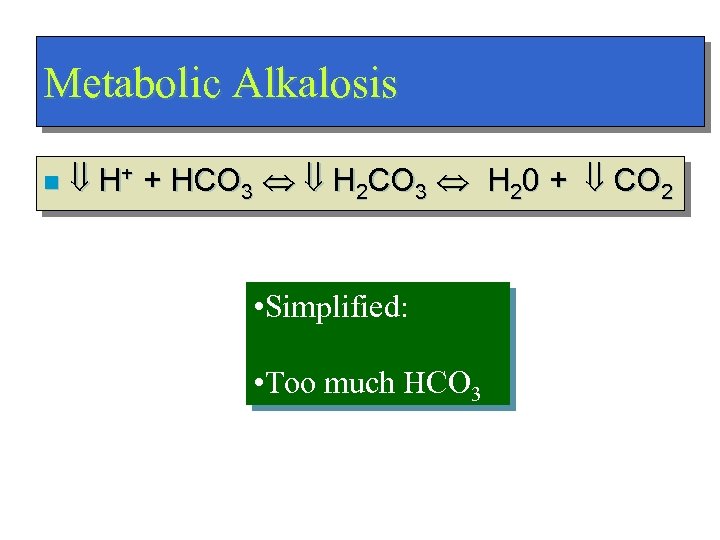 Metabolic Alkalosis n H+ + HCO 3 H 2 CO 3 H 20 + CO 2 • Simplified: • Too much HCO 3
Metabolic Alkalosis n H+ + HCO 3 H 2 CO 3 H 20 + CO 2 • Simplified: • Too much HCO 3
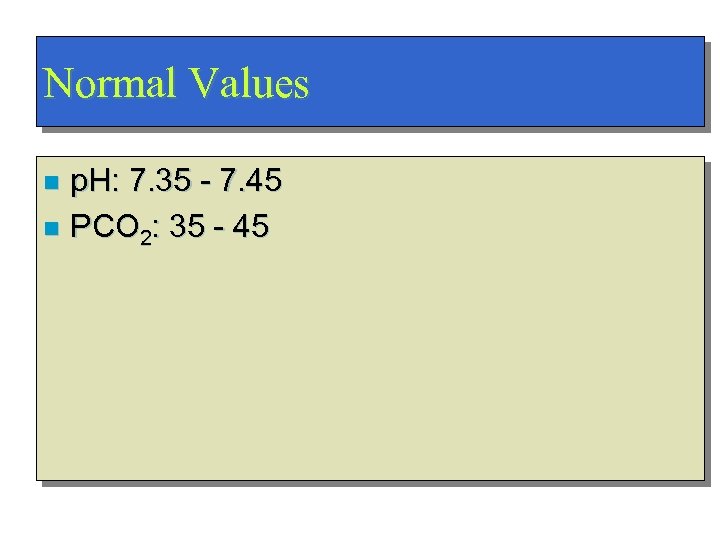 Normal Values p. H: 7. 35 - 7. 45 n PCO 2: 35 - 45 n
Normal Values p. H: 7. 35 - 7. 45 n PCO 2: 35 - 45 n
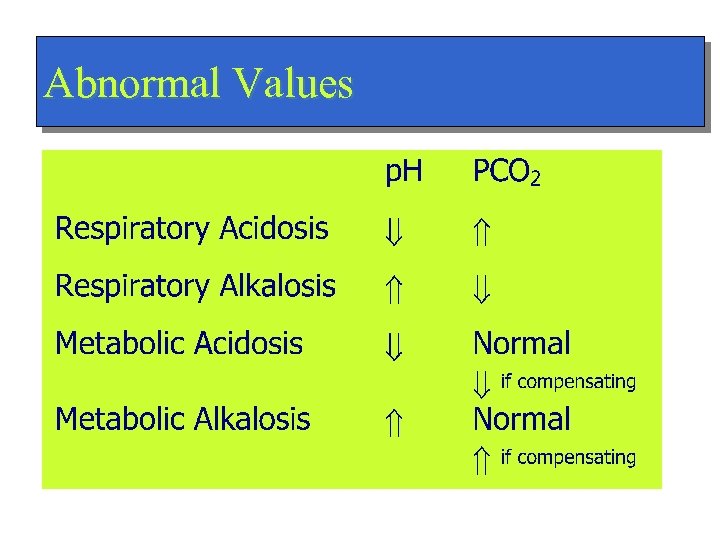 Abnormal Values
Abnormal Values
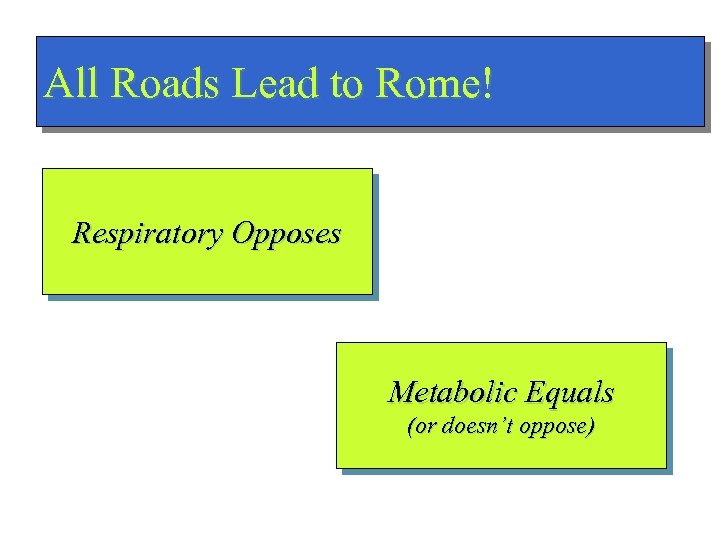 All Roads Lead to Rome! Respiratory Opposes Metabolic Equals (or doesn’t oppose)
All Roads Lead to Rome! Respiratory Opposes Metabolic Equals (or doesn’t oppose)
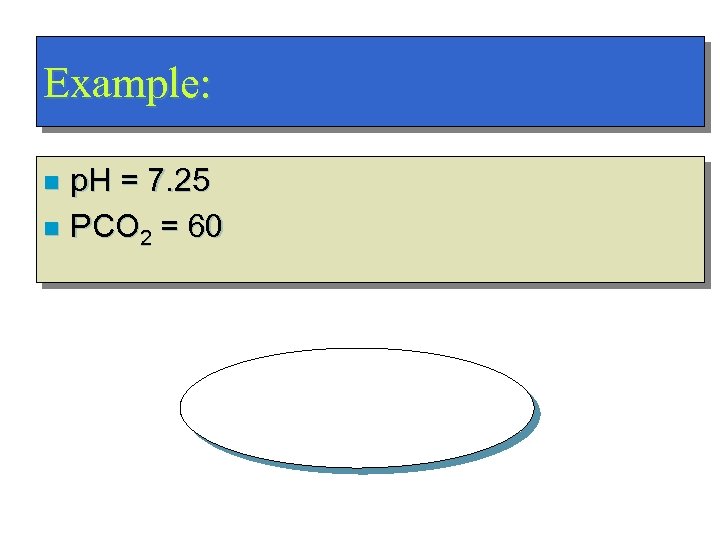 Example: p. H = 7. 25 n PCO 2 = 60 n Respiratory Acidosis!
Example: p. H = 7. 25 n PCO 2 = 60 n Respiratory Acidosis!
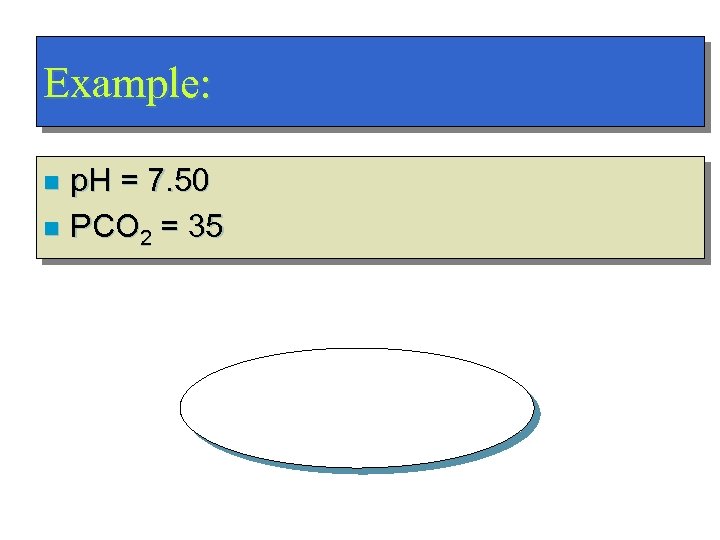 Example: p. H = 7. 50 n PCO 2 = 35 n Metabolic Alkalosis!
Example: p. H = 7. 50 n PCO 2 = 35 n Metabolic Alkalosis!
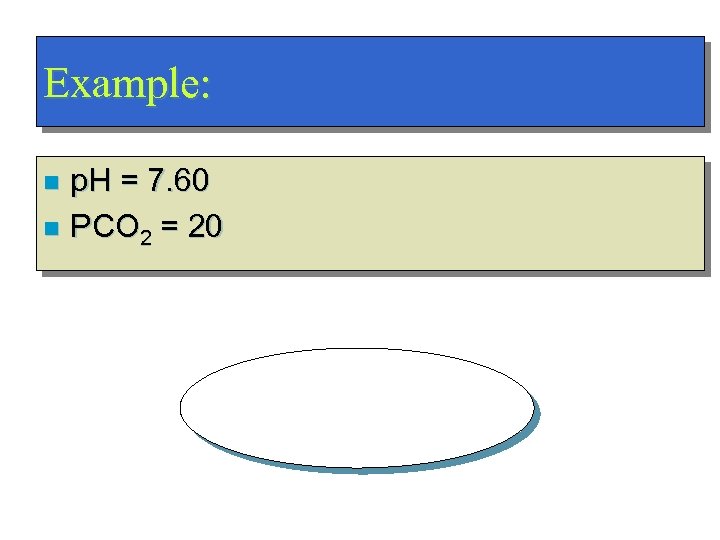 Example: p. H = 7. 60 n PCO 2 = 20 n Respiratory Alkalosis!
Example: p. H = 7. 60 n PCO 2 = 20 n Respiratory Alkalosis!
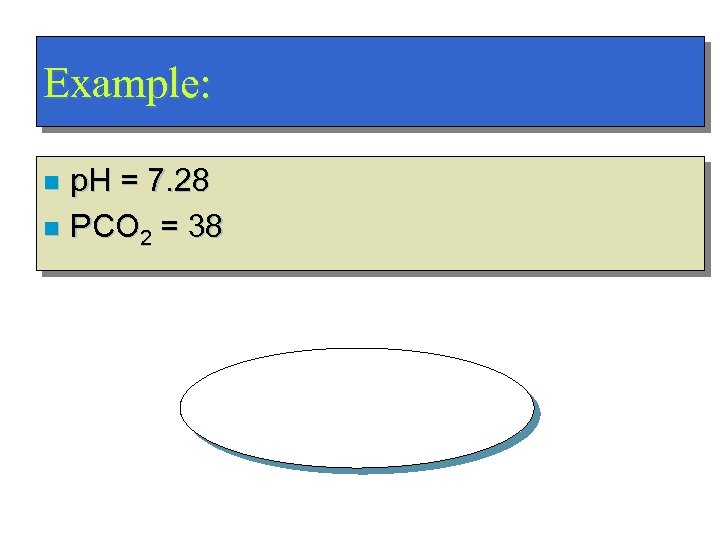 Example: p. H = 7. 28 n PCO 2 = 38 n Metabolic Acidosis!
Example: p. H = 7. 28 n PCO 2 = 38 n Metabolic Acidosis!
 Resources n A Continuing Education article on Acid. Base disturbances is available on our web site at: n http: //www. templejc. edu/ems/resource. htm n A great online tutorial at: n http: //www. tmc. tulane. edu/departments/anesthesi ology/acid. html
Resources n A Continuing Education article on Acid. Base disturbances is available on our web site at: n http: //www. templejc. edu/ems/resource. htm n A great online tutorial at: n http: //www. tmc. tulane. edu/departments/anesthesi ology/acid. html


