f409770efc16afe7d96470b7f1a378b8.ppt
- Количество слайдов: 29

General Chemistry the Central Science Brown & Le. May Chapter One: Introduction, Matter & Measurement

u Molecular Perspective u Classifications of Matter States, Pure Substances, Mixtures, Elements, Compounds u Properties of Matter u Units of Measurement SI Units, Derived Units, Uncertainty, Dimensional Analysis

u Atoms & Molecules -- basic nature of chemistry -necessary to understand the nature of matter art, medicine, agriculture, environment, home u Classifications of Matter -states gas, liquid, solid (H) -pure substance fixed composition/distinct properties (elements or compounds) -elements cannot be decomposed to simpler substances -compounds combination of two or more elements -mixture combination of two or more pure substances (heterogeneous & homogeneous/solutions)

Gas no shape, no volume Liquid volume no shape Solid volume & shape

u Elements -112 elements known -listed on periodic table with symbols (H) -arranged in a particular order such that closely related elements are grouped u Compounds -two or more elements chemically combined -law of constant composition or definite proportions eg. water is always 11% H and 89% O while peroxide is 6% H and 94% O

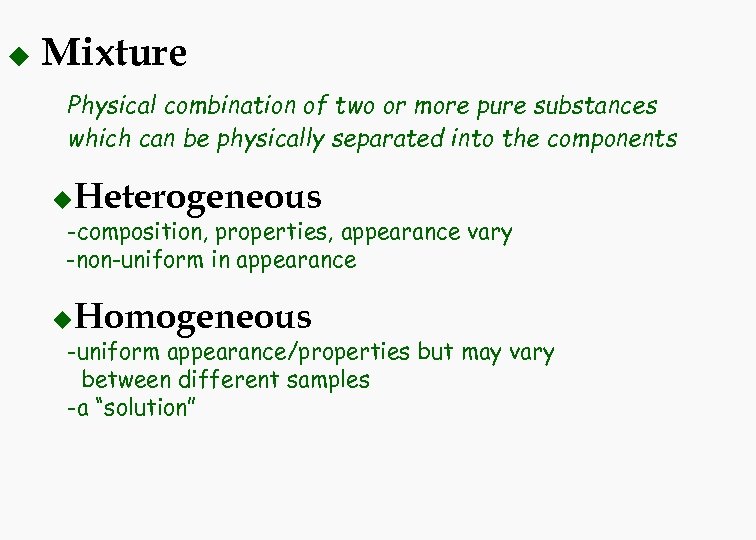
u Mixture Physical combination of two or more pure substances which can be physically separated into the components Heterogeneous u -composition, properties, appearance vary -non-uniform in appearance Homogeneous u -uniform appearance/properties but may vary between different samples -a “solution”

no yes no no yes

u Properties of Matter -Physical Properties -- color, odor, density, b. pt. -Chemical Properties -- chemical changes occur -Intensive Properties -- do not depend upon quantity of material present temp, m. pt. , density -Extensive Properties -- dependent upon the quantity of material present mass, volume u Changes of Matter -Physical -- appearance changes but not composition -Chemical -- transformation into chemically different substance
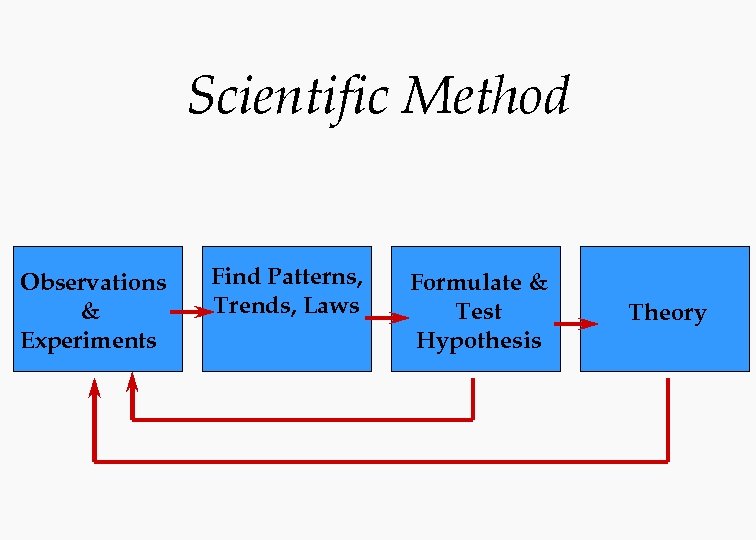
Scientific Method Observations & Experiments Find Patterns, Trends, Laws Formulate & Test Hypothesis Theory
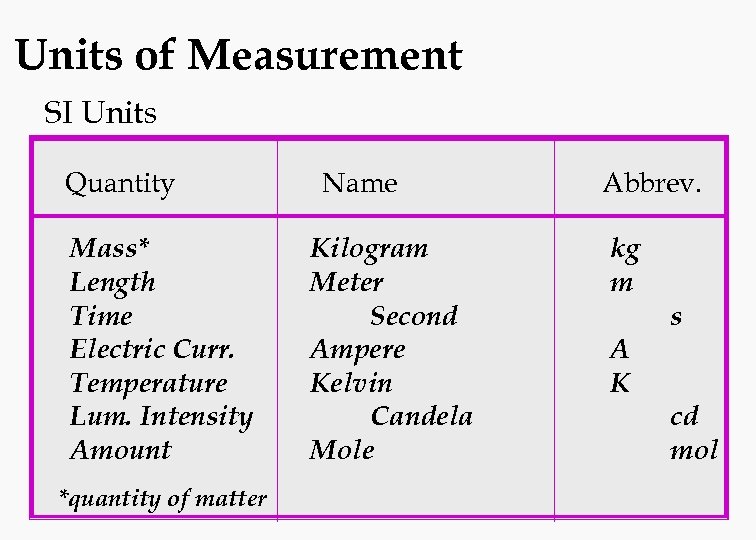
Units of Measurement SI Units Quantity Mass* Length Time Electric Curr. Temperature Lum. Intensity Amount *quantity of matter Name Kilogram Meter Second Ampere Kelvin Candela Mole Abbrev. kg m A K s cd mol
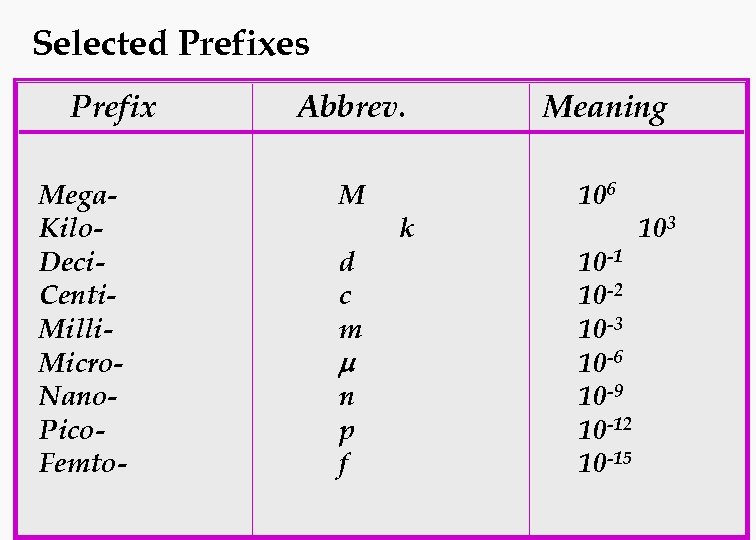
Selected Prefixes Prefix Mega. Kilo. Deci. Centi. Milli. Micro. Nano. Pico. Femto- Abbrev. Meaning M 106 d c m m n p f k 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 10 -15 103
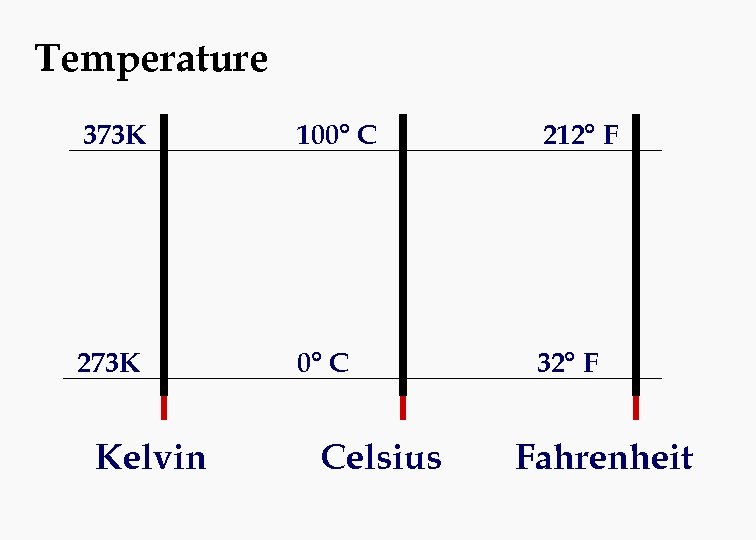
Temperature 373 K 100° C 212° F 273 K 0° C 32° F Kelvin Celsius Fahrenheit

Derived Units -- combinations of other units u Volume -SI unit for volume is m 3 -1 L = 1 dm 3 -1 m. L = 1 cm 3 u Density -amount of mass in a unit volume -combination of mass and volume units -typically g/m. L for solids and liquids & g/L for gases
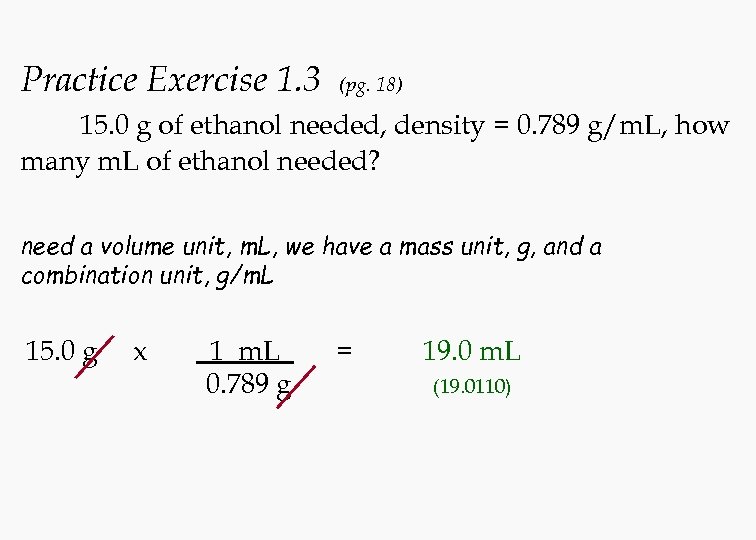
Practice Exercise 1. 3 (pg. 18) 15. 0 g of ethanol needed, density = 0. 789 g/m. L, how many m. L of ethanol needed? need a volume unit, m. L, we have a mass unit, g, and a combination unit, g/m. L 15. 0 g x 1 m. L 0. 789 g = 19. 0 m. L (19. 0110)

Uncertainty in Measurement u Precision vs Accuracy -Precision -- agreement of measurements -Accuracy -- proximity to true value good precision good accuracy poor precision poor accuracy good precision poor accuracy
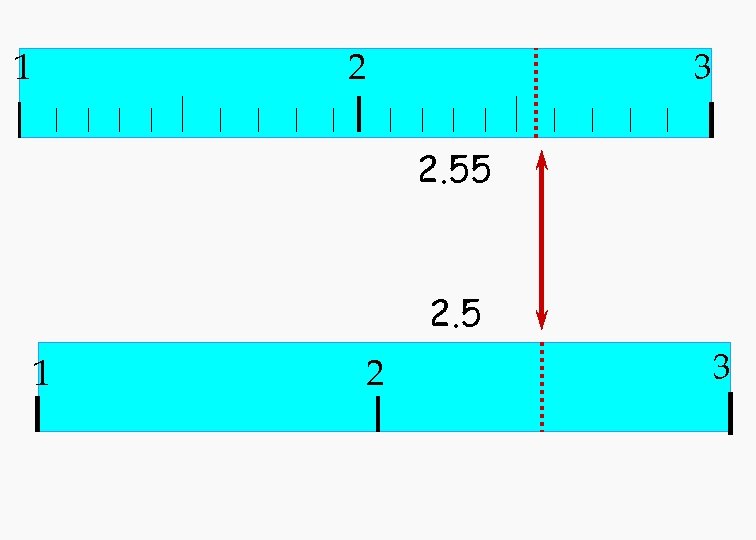
1 2 3 2. 55 2. 5 1 2 3

Significant Figures 1. all non-zero digits are significant 1. 2357 2. zeros between non-zero digits are significant 1. 20054 3. zeros to the left of the first non-zero digit in a number are not significant 0. 0023 4. when a number ends in zeros to the right of decimal point, the zeros are significant 1. 2300 5. when a number ends in zeros which are to the left of the decimal those zeros may or may not be significant 2000 or 2000
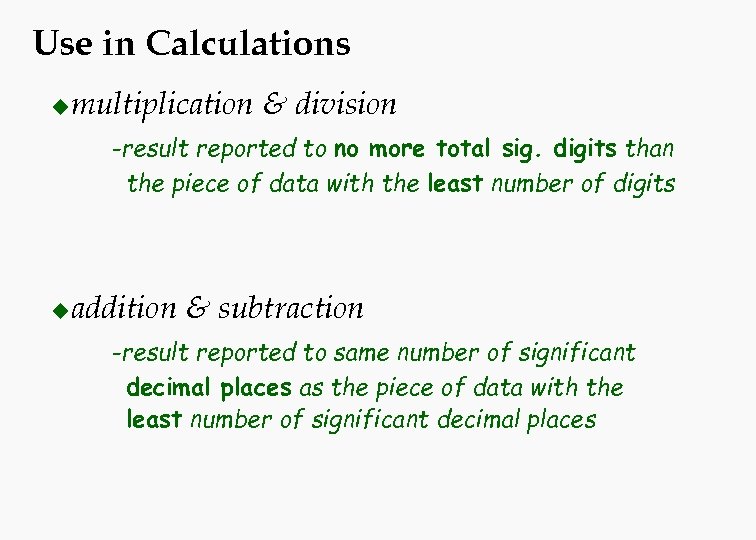
Use in Calculations u multiplication & division -result reported to no more total sig. digits than the piece of data with the least number of digits u addition & subtraction -result reported to same number of significant decimal places as the piece of data with the least number of significant decimal places

Use in Calculations u multiplication & division -result reported to no more total sig. digits than the piece of data with the least number of digits (1. 27)(2. 3) = 2. 921 u 2. 9 addition & subtraction -result reported to same number of significant decimal places as the piece of data with the least number of significant decimal places 1. 0 + 2. 15 + 3. 468 = 6. 618 6. 6
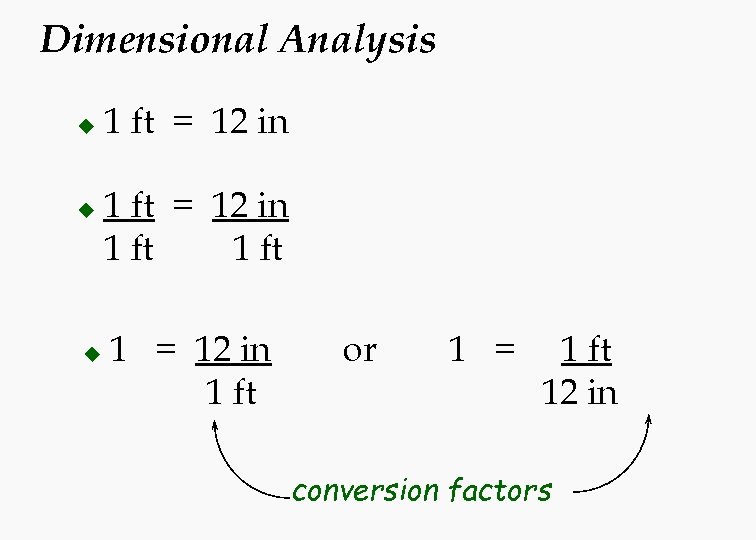
Dimensional Analysis u u u 1 ft = 12 in 1 ft 1 = 12 in 1 ft or 1 = 1 ft 12 in conversion factors
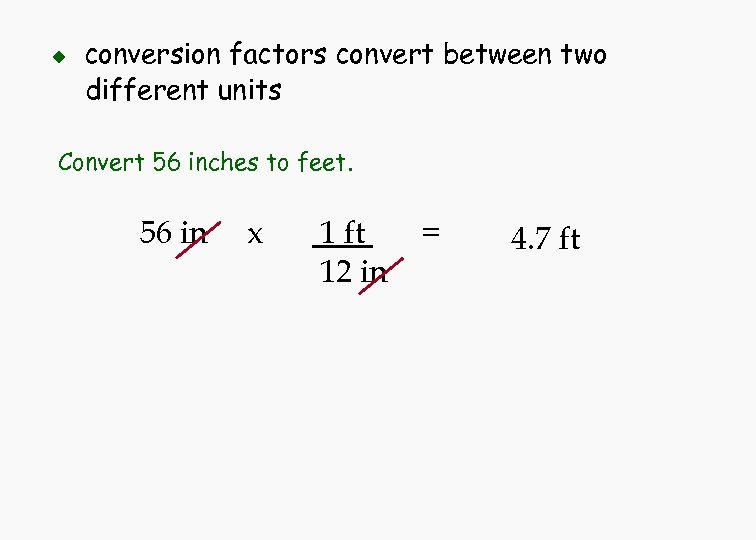
u conversion factors convert between two different units Convert 56 inches to feet. 56 in x 1 ft 12 in = 4. 7 ft
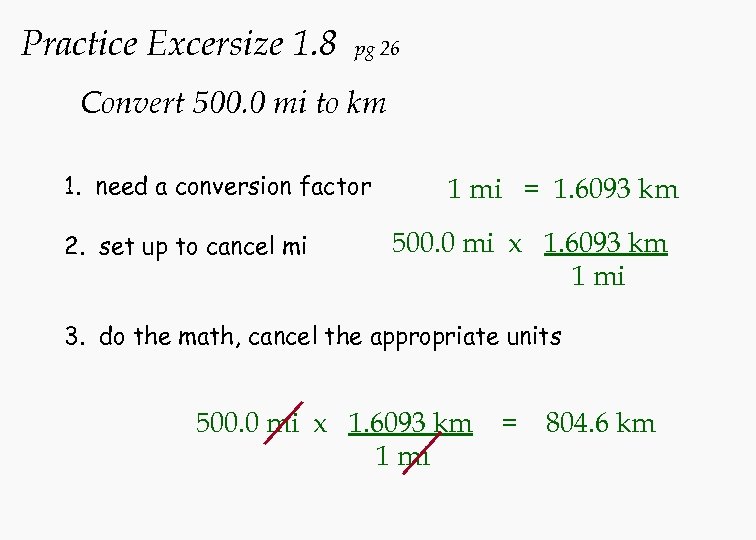
Practice Excersize 1. 8 pg 26 Convert 500. 0 mi to km 1. need a conversion factor 2. set up to cancel mi 1 mi = 1. 6093 km 500. 0 mi x 1. 6093 km 1 mi 3. do the math, cancel the appropriate units 500. 0 mi x 1. 6093 km 1 mi = 804. 6 km
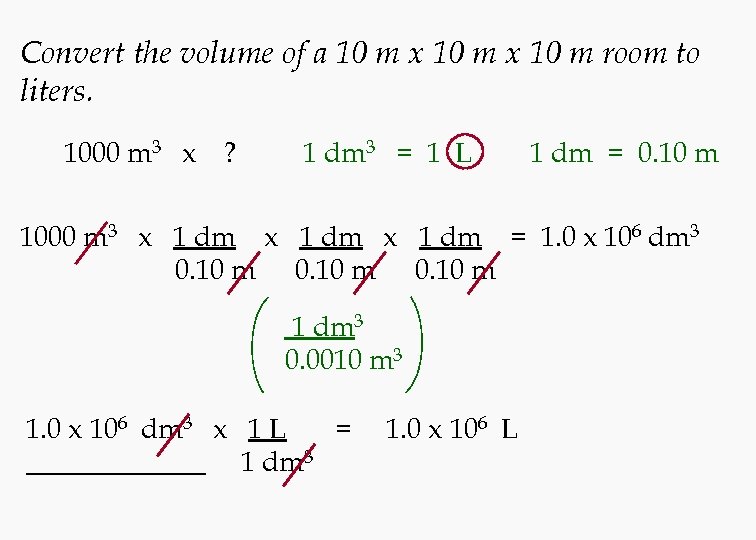
Convert the volume of a 10 m x 10 m room to liters. 1000 m 3 x ? 1 dm 3 = 1 L 1 dm = 0. 10 m 1000 m 3 x 1 dm = 1. 0 x 106 dm 3 0. 10 m 1 dm 3 0. 0010 m 3 1. 0 x 106 dm 3 x 1 L = 1 dm 3 1. 0 x 106 L

Problems u u If I weigh 52. 3 kg, how many grams do I weigh? If a tennis ball is served at 140 mph, what is its speed in m/s? You have a 40 lb baggage limit, the scale reads 17. 97 kg. Are you over or under? Convert 2. 5 L to cm 3

Problems u u Glycerol is a viscous, sweet-tasting liquid. In an experiment, a 25. 0 m. L sample was found to weigh 31. 6 g. What is the density of glycerol? (2. 60) Diethyl ether has a density of 0. 713 g/m. L. What volume of diethyl ether is needed to equal 28. 0 g? (2. 64)

Problems u u The density of the sulfuric acid solution of a fully charged lead storage battery is about 1. 28 g/m. L. What is the mass of the solution whose volume is 1. 50 L? (2. 89) The density of red blood cells is about 1. 093 g/cm 3, somewhat higher than that of blood plasma. What is the mass of cells whose volume is 5. 67 cm 3? (2. 88)

Problems u u To identify a metal, an experimenter placed a 32. 6 g sample in a grad. cylinder. He then poured in 25. 0 m. L of water, covering the metal and bringing the liquid level in the cyliner to 28. 7 m. L. What is the density of the metal? (2. 62) You are buying carpet @ $15. 00/sq. yd for a 1500 sq. ft house. How much will it cost?

Problems u u If liquid nitrogen has a boiling point of 77 K, and the temperature is -300°F, will it be a solid, liquid or gas? A 175 lb patient is to undergo surgery and will be given an anesthetic intravenously. The safe dosage of anesthetic is 12 mg/kg of body weight. Determine the maximum dose of anesthetic that should be used.
f409770efc16afe7d96470b7f1a378b8.ppt