824c33d2cb05feeca84c35340cbb352f.ppt
- Количество слайдов: 29
 General Approaches to Polymer Synthesis • 1. Addition Chain Growth Polymerization of Vinyl Monomers Ring Opening Polymerization Heterocylics Metathesis of Cyclic Olefins 2. Condensation Step Growth Polymerization of A-B or AA/BB Monomers 3. Modification of Preformed Polymers Polysaccharides Peptides and Proteins Synthetic Precursors
General Approaches to Polymer Synthesis • 1. Addition Chain Growth Polymerization of Vinyl Monomers Ring Opening Polymerization Heterocylics Metathesis of Cyclic Olefins 2. Condensation Step Growth Polymerization of A-B or AA/BB Monomers 3. Modification of Preformed Polymers Polysaccharides Peptides and Proteins Synthetic Precursors
 Current Strategies in Polymer Synthesis • Objectives: Precise Macromolecular Design • 1 – – . Control of: Molecular Weight Distribution Composition Sequence of repeat units Stereochemistry • 2. Versatility –
Current Strategies in Polymer Synthesis • Objectives: Precise Macromolecular Design • 1 – – . Control of: Molecular Weight Distribution Composition Sequence of repeat units Stereochemistry • 2. Versatility –
 Free Radical Initiated Polymerization • • Classical Free Radical Process Applied to wide range of monomers Broad scope of experimental conditions Molecular weight can be controlled Mw/Mn > 1. 5 2. 0 Statistical compositions and sequences Little stereochemical control
Free Radical Initiated Polymerization • • Classical Free Radical Process Applied to wide range of monomers Broad scope of experimental conditions Molecular weight can be controlled Mw/Mn > 1. 5 2. 0 Statistical compositions and sequences Little stereochemical control
 Anatomy of Addition Polymerizations • Initiation – Generation of active initiator – Reaction with monomer to form growing chains • Propagation – Chain extension by incremental monomer addition • Termination – Conversion of active growing chains to inert polymer • Chain Transfer – Transfer of active growing site by terminating one chain and reinitiating a new chain.
Anatomy of Addition Polymerizations • Initiation – Generation of active initiator – Reaction with monomer to form growing chains • Propagation – Chain extension by incremental monomer addition • Termination – Conversion of active growing chains to inert polymer • Chain Transfer – Transfer of active growing site by terminating one chain and reinitiating a new chain.
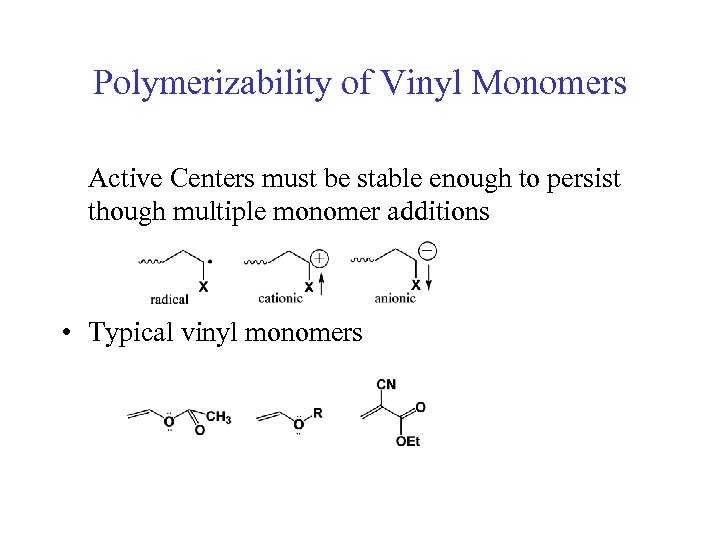 Polymerizability of Vinyl Monomers Active Centers must be stable enough to persist though multiple monomer additions • Typical vinyl monomers
Polymerizability of Vinyl Monomers Active Centers must be stable enough to persist though multiple monomer additions • Typical vinyl monomers
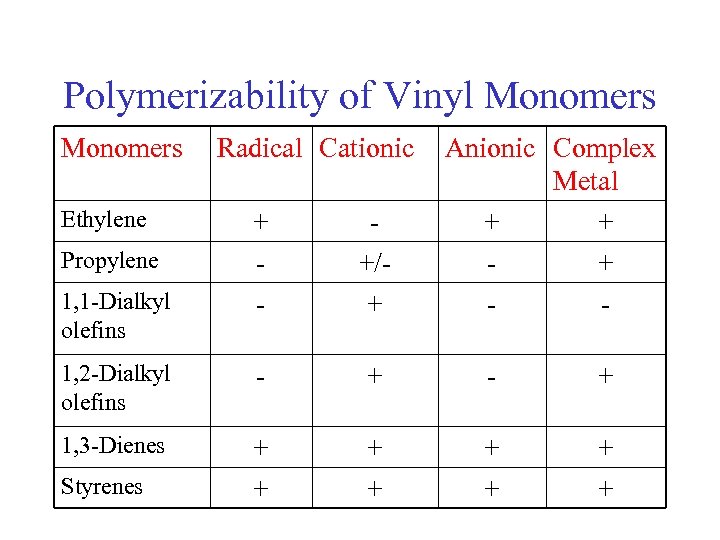 Polymerizability of Vinyl Monomers Ethylene Radical Cationic Anionic Complex Metal + + + - +/+ 1, 2 -Dialkyl olefins - + 1, 3 -Dienes + + + + Propylene 1, 1 -Dialkyl olefins Styrenes
Polymerizability of Vinyl Monomers Ethylene Radical Cationic Anionic Complex Metal + + + - +/+ 1, 2 -Dialkyl olefins - + 1, 3 -Dienes + + + + Propylene 1, 1 -Dialkyl olefins Styrenes
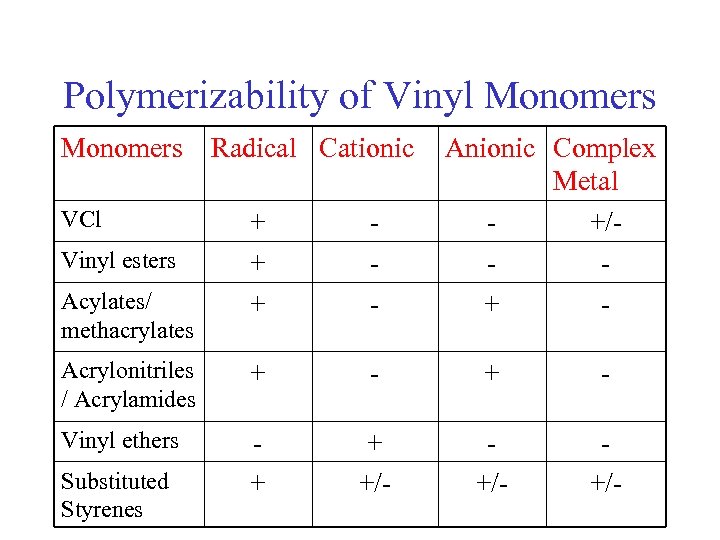 Polymerizability of Vinyl Monomers VCl Radical Cationic Anionic Complex Metal +/+ - + + + - Acrylonitriles / Acrylamides + - Vinyl ethers + + +/- +/- Vinyl esters Acylates/ methacrylates Substituted Styrenes
Polymerizability of Vinyl Monomers VCl Radical Cationic Anionic Complex Metal +/+ - + + + - Acrylonitriles / Acrylamides + - Vinyl ethers + + +/- +/- Vinyl esters Acylates/ methacrylates Substituted Styrenes
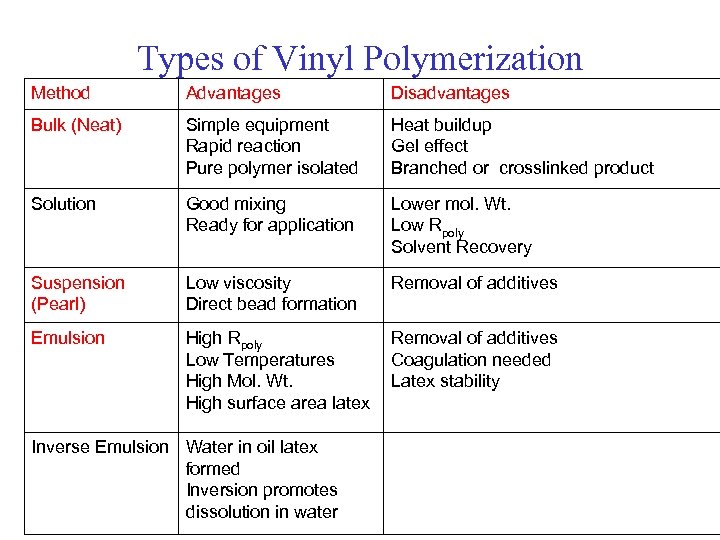 Types of Vinyl Polymerization Method Advantages Disadvantages Bulk (Neat) Simple equipment Rapid reaction Pure polymer isolated Heat buildup Gel effect Branched or crosslinked product Solution Good mixing Ready for application Lower mol. Wt. Low Rpoly Solvent Recovery Suspension (Pearl) Low viscosity Direct bead formation Removal of additives Emulsion High Rpoly Low Temperatures High Mol. Wt. High surface area latex Removal of additives Coagulation needed Latex stability Inverse Emulsion Water in oil latex formed Inversion promotes dissolution in water
Types of Vinyl Polymerization Method Advantages Disadvantages Bulk (Neat) Simple equipment Rapid reaction Pure polymer isolated Heat buildup Gel effect Branched or crosslinked product Solution Good mixing Ready for application Lower mol. Wt. Low Rpoly Solvent Recovery Suspension (Pearl) Low viscosity Direct bead formation Removal of additives Emulsion High Rpoly Low Temperatures High Mol. Wt. High surface area latex Removal of additives Coagulation needed Latex stability Inverse Emulsion Water in oil latex formed Inversion promotes dissolution in water
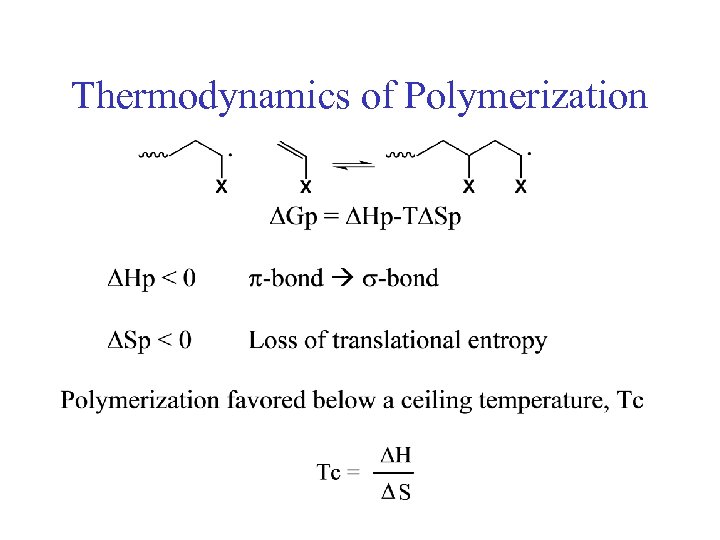 Thermodynamics of Polymerization
Thermodynamics of Polymerization
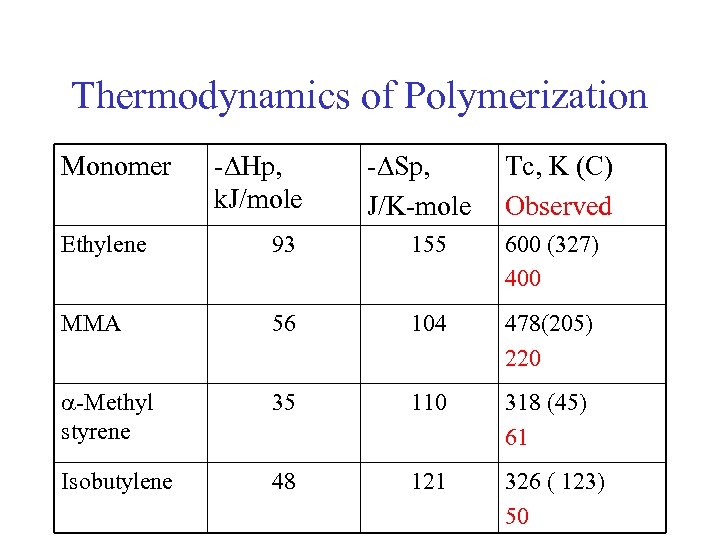 Thermodynamics of Polymerization Monomer -DHp, k. J/mole -DSp, J/K-mole Tc, K (C) Observed Ethylene 93 155 600 (327) 400 MMA 56 104 478(205) 220 a-Methyl styrene 35 110 318 (45) 61 Isobutylene 48 121 326 ( 123) 50
Thermodynamics of Polymerization Monomer -DHp, k. J/mole -DSp, J/K-mole Tc, K (C) Observed Ethylene 93 155 600 (327) 400 MMA 56 104 478(205) 220 a-Methyl styrene 35 110 318 (45) 61 Isobutylene 48 121 326 ( 123) 50
 Suspension Polymerization Equivalent to a "mini-bulk" polymerization Advantages • • Aqueous (hydrocarbon) media provides good heat transfer Good particle size control through agitation and dispersion agents Control of porosity with proper additives and process conditions Product easy to recover and transfer Disadvantages • Suspending Agents contaminate product • Removal of residual monomer necessary
Suspension Polymerization Equivalent to a "mini-bulk" polymerization Advantages • • Aqueous (hydrocarbon) media provides good heat transfer Good particle size control through agitation and dispersion agents Control of porosity with proper additives and process conditions Product easy to recover and transfer Disadvantages • Suspending Agents contaminate product • Removal of residual monomer necessary
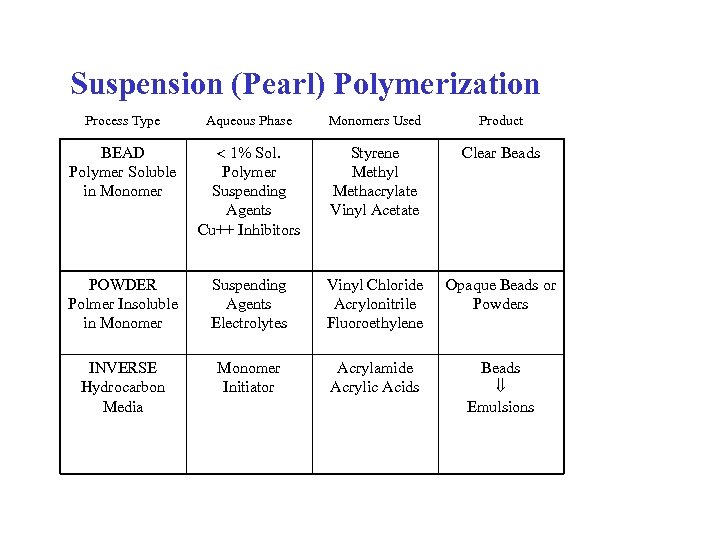 Suspension (Pearl) Polymerization Process Type Aqueous Phase Monomers Used Product BEAD Polymer Soluble in Monomer 1% Sol. Polymer Suspending Agents Cu++ Inhibitors Styrene Methyl Methacrylate Vinyl Acetate Clear Beads POWDER Polmer Insoluble in Monomer Suspending Agents Electrolytes Vinyl Chloride Acrylonitrile Fluoroethylene Opaque Beads or Powders INVERSE Hydrocarbon Media Monomer Initiator Acrylamide Acrylic Acids Beads Emulsions
Suspension (Pearl) Polymerization Process Type Aqueous Phase Monomers Used Product BEAD Polymer Soluble in Monomer 1% Sol. Polymer Suspending Agents Cu++ Inhibitors Styrene Methyl Methacrylate Vinyl Acetate Clear Beads POWDER Polmer Insoluble in Monomer Suspending Agents Electrolytes Vinyl Chloride Acrylonitrile Fluoroethylene Opaque Beads or Powders INVERSE Hydrocarbon Media Monomer Initiator Acrylamide Acrylic Acids Beads Emulsions
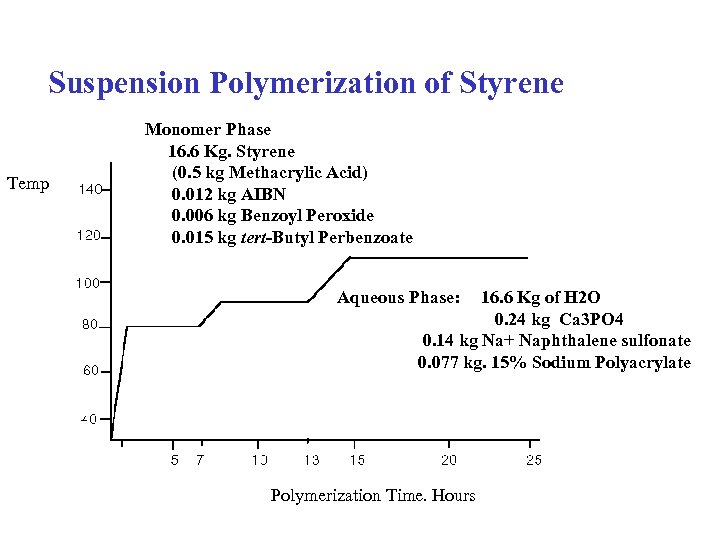 Suspension Polymerization of Styrene Temp Monomer Phase 16. 6 Kg. Styrene (0. 5 kg Methacrylic Acid) 0. 012 kg AIBN 0. 006 kg Benzoyl Peroxide 0. 015 kg tert-Butyl Perbenzoate Aqueous Phase: 16. 6 Kg of H 2 O 0. 24 kg Ca 3 PO 4 0. 14 kg Na+ Naphthalene sulfonate 0. 077 kg. 15% Sodium Polyacrylate Polymerization Time. Hours
Suspension Polymerization of Styrene Temp Monomer Phase 16. 6 Kg. Styrene (0. 5 kg Methacrylic Acid) 0. 012 kg AIBN 0. 006 kg Benzoyl Peroxide 0. 015 kg tert-Butyl Perbenzoate Aqueous Phase: 16. 6 Kg of H 2 O 0. 24 kg Ca 3 PO 4 0. 14 kg Na+ Naphthalene sulfonate 0. 077 kg. 15% Sodium Polyacrylate Polymerization Time. Hours
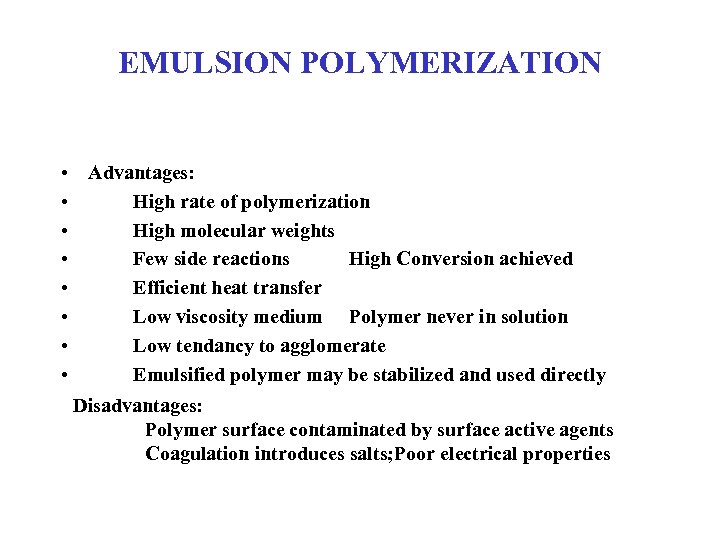 EMULSION POLYMERIZATION • Advantages: • High rate of polymerization • High molecular weights • Few side reactions High Conversion achieved • Efficient heat transfer • Low viscosity medium Polymer never in solution • Low tendancy to agglomerate • Emulsified polymer may be stabilized and used directly Disadvantages: Polymer surface contaminated by surface active agents Coagulation introduces salts; Poor electrical properties
EMULSION POLYMERIZATION • Advantages: • High rate of polymerization • High molecular weights • Few side reactions High Conversion achieved • Efficient heat transfer • Low viscosity medium Polymer never in solution • Low tendancy to agglomerate • Emulsified polymer may be stabilized and used directly Disadvantages: Polymer surface contaminated by surface active agents Coagulation introduces salts; Poor electrical properties
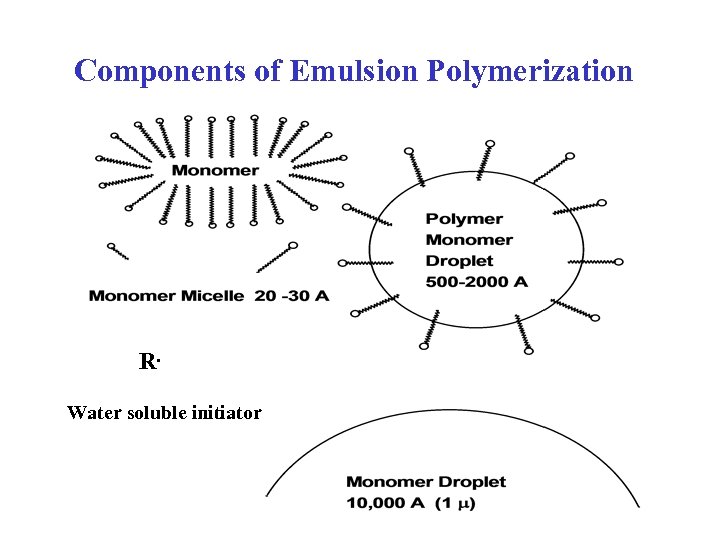 Components of Emulsion Polymerization R. Water soluble initiator
Components of Emulsion Polymerization R. Water soluble initiator
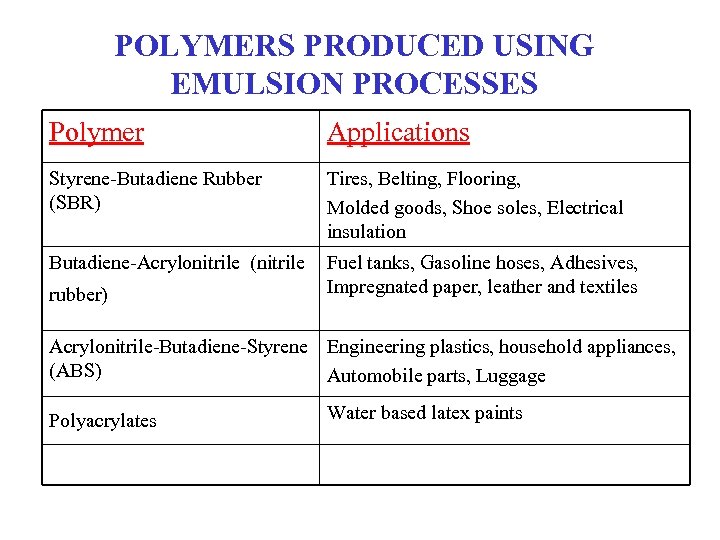 POLYMERS PRODUCED USING EMULSION PROCESSES Polymer Applications Styrene-Butadiene Rubber (SBR) Tires, Belting, Flooring, Molded goods, Shoe soles, Electrical insulation Butadiene-Acrylonitrile (nitrile Fuel tanks, Gasoline hoses, Adhesives, Impregnated paper, leather and textiles rubber) Acrylonitrile-Butadiene-Styrene Engineering plastics, household appliances, (ABS) Automobile parts, Luggage Polyacrylates Water based latex paints
POLYMERS PRODUCED USING EMULSION PROCESSES Polymer Applications Styrene-Butadiene Rubber (SBR) Tires, Belting, Flooring, Molded goods, Shoe soles, Electrical insulation Butadiene-Acrylonitrile (nitrile Fuel tanks, Gasoline hoses, Adhesives, Impregnated paper, leather and textiles rubber) Acrylonitrile-Butadiene-Styrene Engineering plastics, household appliances, (ABS) Automobile parts, Luggage Polyacrylates Water based latex paints
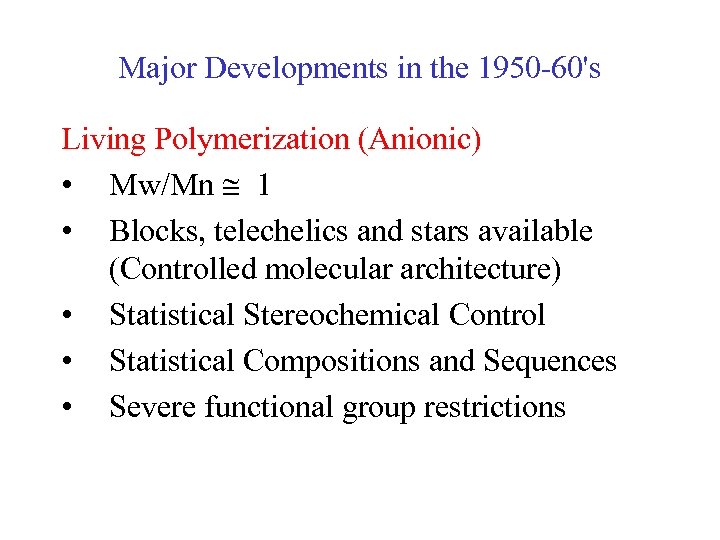 Major Developments in the 1950 -60's Living Polymerization (Anionic) • Mw/Mn 1 • Blocks, telechelics and stars available (Controlled molecular architecture) • Statistical Stereochemical Control • Statistical Compositions and Sequences • Severe functional group restrictions
Major Developments in the 1950 -60's Living Polymerization (Anionic) • Mw/Mn 1 • Blocks, telechelics and stars available (Controlled molecular architecture) • Statistical Stereochemical Control • Statistical Compositions and Sequences • Severe functional group restrictions
 Ziegler-Natta (Metal-Coordinated) Polymerization • • Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i. e. olefins • SINGLE SITE CATALYSTS
Ziegler-Natta (Metal-Coordinated) Polymerization • • Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i. e. olefins • SINGLE SITE CATALYSTS
 Polyolefins • Polypropylene (1954) • • • PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
Polyolefins • Polypropylene (1954) • • • PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
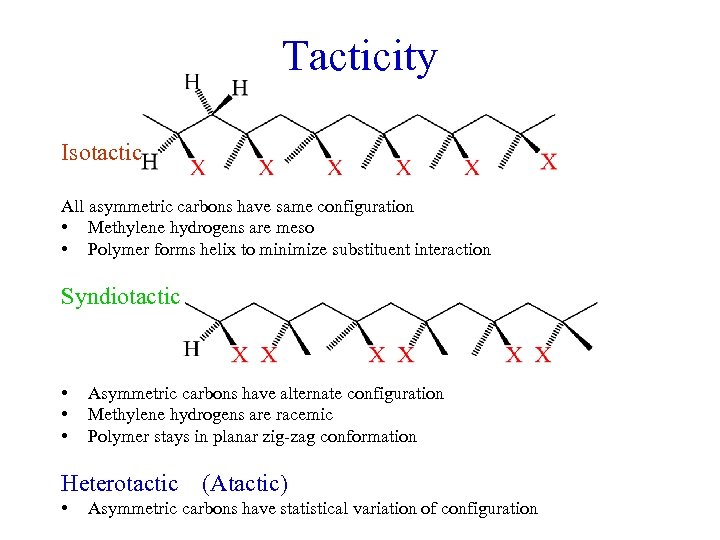 Tacticity Isotactic All asymmetric carbons have same configuration • Methylene hydrogens are meso • Polymer forms helix to minimize substituent interaction Syndiotactic • • • Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic • (Atactic) Asymmetric carbons have statistical variation of configuration
Tacticity Isotactic All asymmetric carbons have same configuration • Methylene hydrogens are meso • Polymer forms helix to minimize substituent interaction Syndiotactic • • • Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic • (Atactic) Asymmetric carbons have statistical variation of configuration
 Additional Developments in the 1980's • "Immortal" Polymerization (Cationic) – – – Mw/Mn 1. 05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions
Additional Developments in the 1980's • "Immortal" Polymerization (Cationic) – – – Mw/Mn 1. 05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions
 Free Radical Initiated Polymerization • • Controlled Free Radical Polymerization Broad range of monomers available Accurate control of molecular weight Mw/Mn 1. 05 --Almost monodisperse Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences
Free Radical Initiated Polymerization • • Controlled Free Radical Polymerization Broad range of monomers available Accurate control of molecular weight Mw/Mn 1. 05 --Almost monodisperse Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences
 Genetic Approaches via Modified Microorganisms • • • Monodisperse in MW Monodisperse in Composition Sequentially Uniform Stereochemically Pure Diverse set of functional groups possible through synthesis of novel amino acids
Genetic Approaches via Modified Microorganisms • • • Monodisperse in MW Monodisperse in Composition Sequentially Uniform Stereochemically Pure Diverse set of functional groups possible through synthesis of novel amino acids
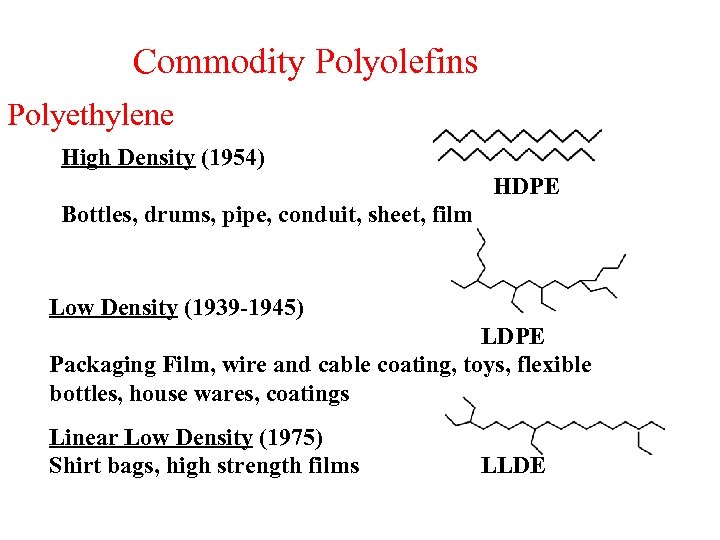 Commodity Polyolefins Polyethylene High Density (1954) HDPE Bottles, drums, pipe, conduit, sheet, film Low Density (1939 -1945) LDPE Packaging Film, wire and cable coating, toys, flexible bottles, house wares, coatings Linear Low Density (1975) Shirt bags, high strength films LLDE
Commodity Polyolefins Polyethylene High Density (1954) HDPE Bottles, drums, pipe, conduit, sheet, film Low Density (1939 -1945) LDPE Packaging Film, wire and cable coating, toys, flexible bottles, house wares, coatings Linear Low Density (1975) Shirt bags, high strength films LLDE
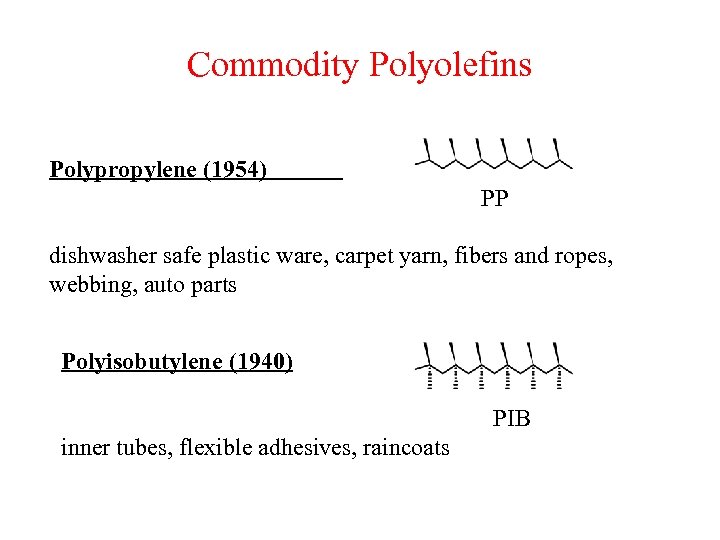 Commodity Polyolefins Polypropylene (1954) PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts Polyisobutylene (1940) PIB inner tubes, flexible adhesives, raincoats
Commodity Polyolefins Polypropylene (1954) PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts Polyisobutylene (1940) PIB inner tubes, flexible adhesives, raincoats
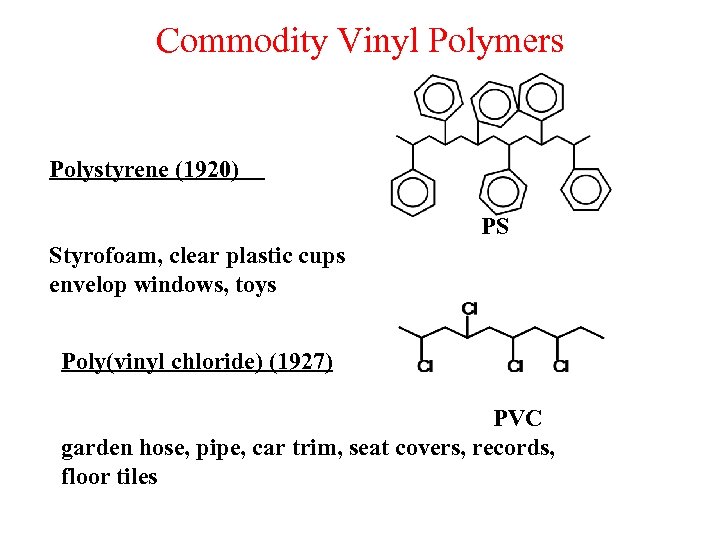 Commodity Vinyl Polymers Polystyrene (1920) PS Styrofoam, clear plastic cups envelop windows, toys Poly(vinyl chloride) (1927) PVC garden hose, pipe, car trim, seat covers, records, floor tiles
Commodity Vinyl Polymers Polystyrene (1920) PS Styrofoam, clear plastic cups envelop windows, toys Poly(vinyl chloride) (1927) PVC garden hose, pipe, car trim, seat covers, records, floor tiles
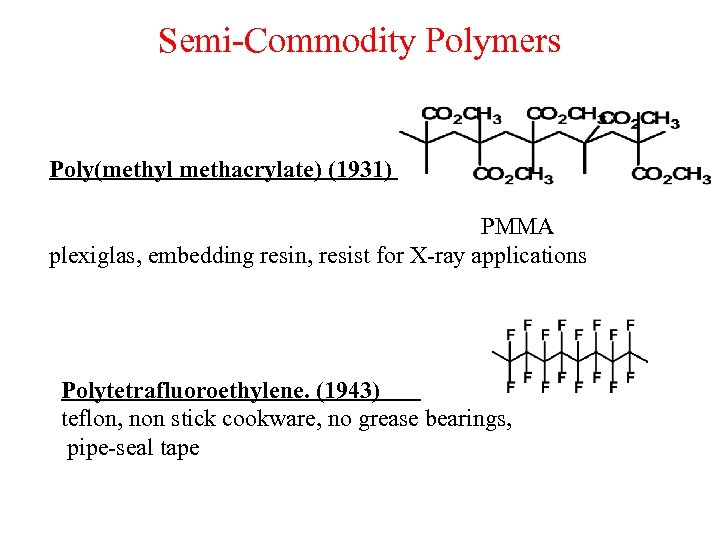 Semi-Commodity Polymers Poly(methyl methacrylate) (1931) PMMA plexiglas, embedding resin, resist for X-ray applications Polytetrafluoroethylene. (1943) teflon, non stick cookware, no grease bearings, pipe-seal tape
Semi-Commodity Polymers Poly(methyl methacrylate) (1931) PMMA plexiglas, embedding resin, resist for X-ray applications Polytetrafluoroethylene. (1943) teflon, non stick cookware, no grease bearings, pipe-seal tape
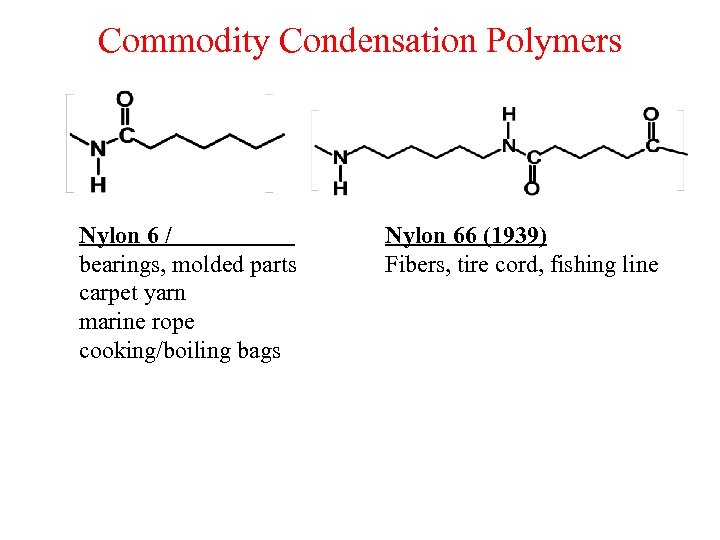 Commodity Condensation Polymers Nylon 6 / bearings, molded parts carpet yarn marine rope cooking/boiling bags Nylon 66 (1939) Fibers, tire cord, fishing line
Commodity Condensation Polymers Nylon 6 / bearings, molded parts carpet yarn marine rope cooking/boiling bags Nylon 66 (1939) Fibers, tire cord, fishing line
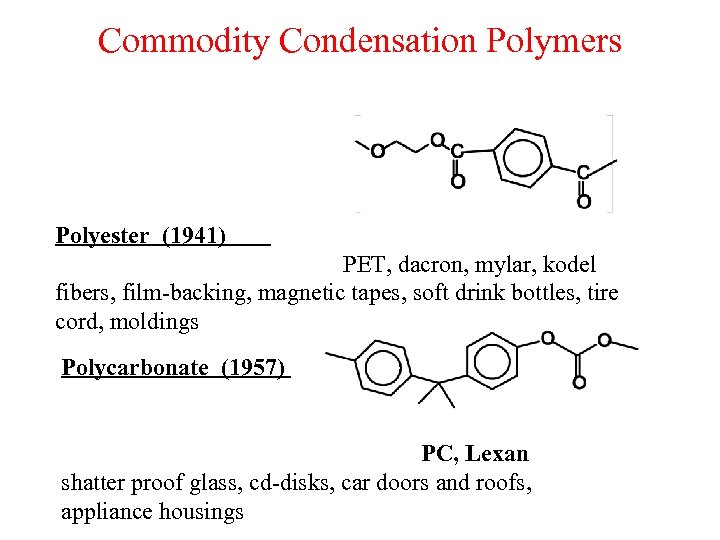 Commodity Condensation Polymers Polyester (1941) PET, dacron, mylar, kodel fibers, film-backing, magnetic tapes, soft drink bottles, tire cord, moldings Polycarbonate (1957) PC, Lexan shatter proof glass, cd-disks, car doors and roofs, appliance housings
Commodity Condensation Polymers Polyester (1941) PET, dacron, mylar, kodel fibers, film-backing, magnetic tapes, soft drink bottles, tire cord, moldings Polycarbonate (1957) PC, Lexan shatter proof glass, cd-disks, car doors and roofs, appliance housings


