025765c2655225ec047072c80f3d0354.ppt
- Количество слайдов: 29
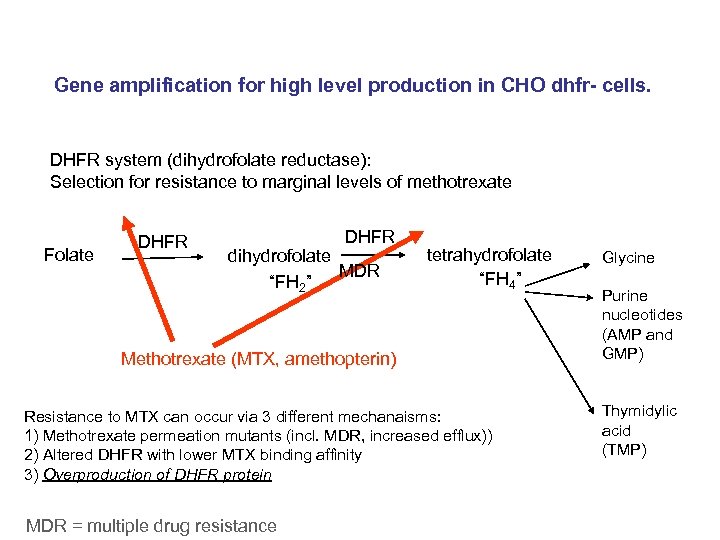 Gene amplification for high level production in CHO dhfr- cells. DHFR system (dihydrofolate reductase): Selection for resistance to marginal levels of methotrexate Folate DHFR dihydrofolate MDR “FH 2” tetrahydrofolate “FH 4” Methotrexate (MTX, amethopterin) Resistance to MTX can occur via 3 different mechanaisms: 1) Methotrexate permeation mutants (incl. MDR, increased efflux)) 2) Altered DHFR with lower MTX binding affinity 3) Overproduction of DHFR protein MDR = multiple drug resistance Glycine Purine nucleotides (AMP and GMP) Thymidylic acid (TMP)
Gene amplification for high level production in CHO dhfr- cells. DHFR system (dihydrofolate reductase): Selection for resistance to marginal levels of methotrexate Folate DHFR dihydrofolate MDR “FH 2” tetrahydrofolate “FH 4” Methotrexate (MTX, amethopterin) Resistance to MTX can occur via 3 different mechanaisms: 1) Methotrexate permeation mutants (incl. MDR, increased efflux)) 2) Altered DHFR with lower MTX binding affinity 3) Overproduction of DHFR protein MDR = multiple drug resistance Glycine Purine nucleotides (AMP and GMP) Thymidylic acid (TMP)
 Gene amplification: dhfr Historically: • Methotrexate resistance • MTX inhibits dihydrofolate reductase (DHFR) • MTX-resistant cells have (in order of discovery, 1970’s): • High DHFR enzyme activity • High DHFR protein • High protein synthetic rate • High translatable m. RNA • High m. RNA level (by hybridization) • High DNA level. Homogeneously staining, expanded chromosomal regions (HSRs) HSRs are the location of the high number of dhfr genes. Double minute chromosomes are an occasional alternative form. Amplicons (distance between repeated genes) are large (300 KB). (dhfr gene = ~ 25 kb) HSRs can shrink, migrate.
Gene amplification: dhfr Historically: • Methotrexate resistance • MTX inhibits dihydrofolate reductase (DHFR) • MTX-resistant cells have (in order of discovery, 1970’s): • High DHFR enzyme activity • High DHFR protein • High protein synthetic rate • High translatable m. RNA • High m. RNA level (by hybridization) • High DNA level. Homogeneously staining, expanded chromosomal regions (HSRs) HSRs are the location of the high number of dhfr genes. Double minute chromosomes are an occasional alternative form. Amplicons (distance between repeated genes) are large (300 KB). (dhfr gene = ~ 25 kb) HSRs can shrink, migrate.
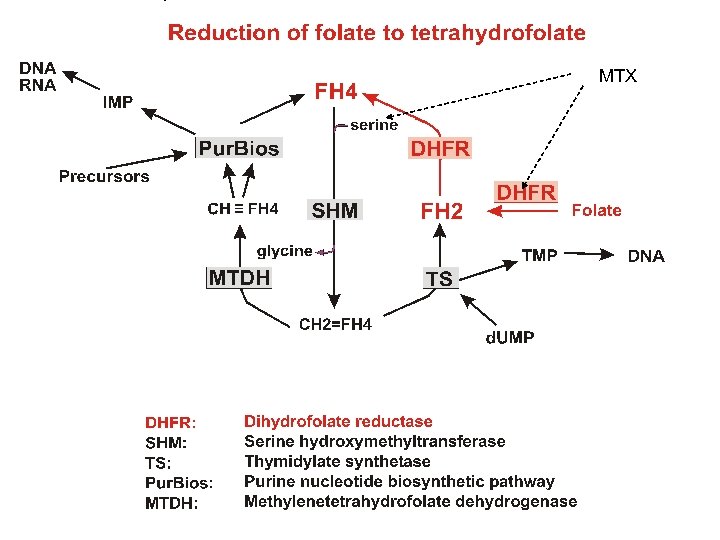 Reduction of folate to tetrahydrofolate MTX
Reduction of folate to tetrahydrofolate MTX
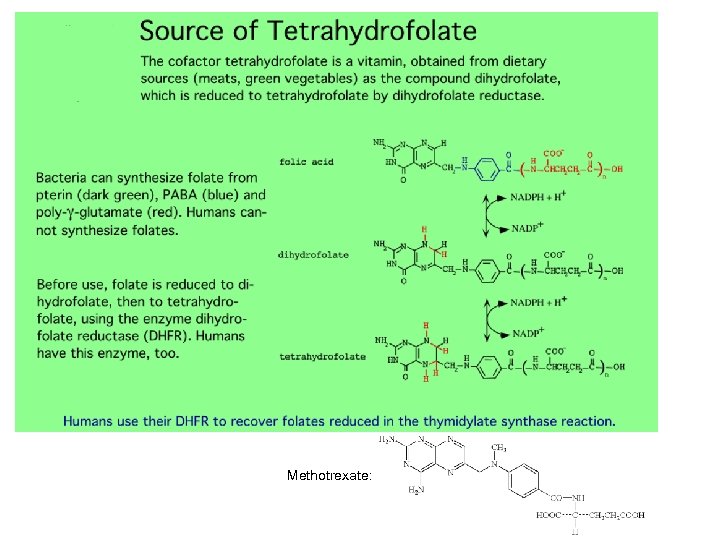 Methotrexate:
Methotrexate:
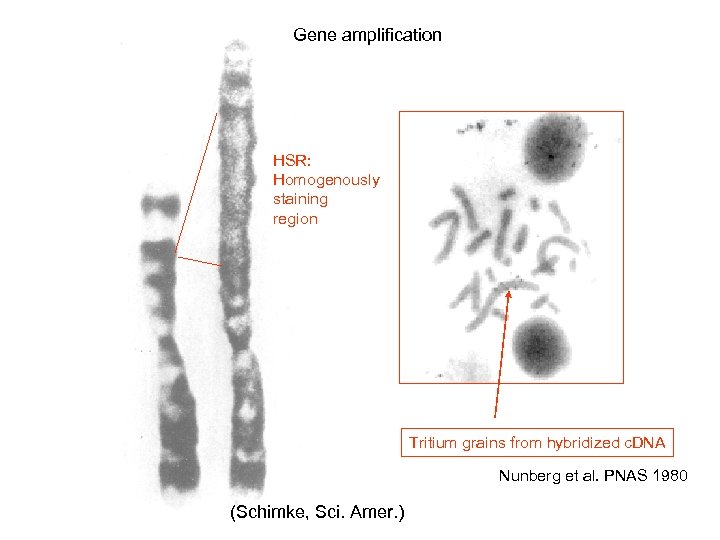 Gene amplification HSR: Homogenously staining region Tritium grains from hybridized c. DNA Nunberg et al. PNAS 1980 (Schimke, Sci. Amer. )
Gene amplification HSR: Homogenously staining region Tritium grains from hybridized c. DNA Nunberg et al. PNAS 1980 (Schimke, Sci. Amer. )
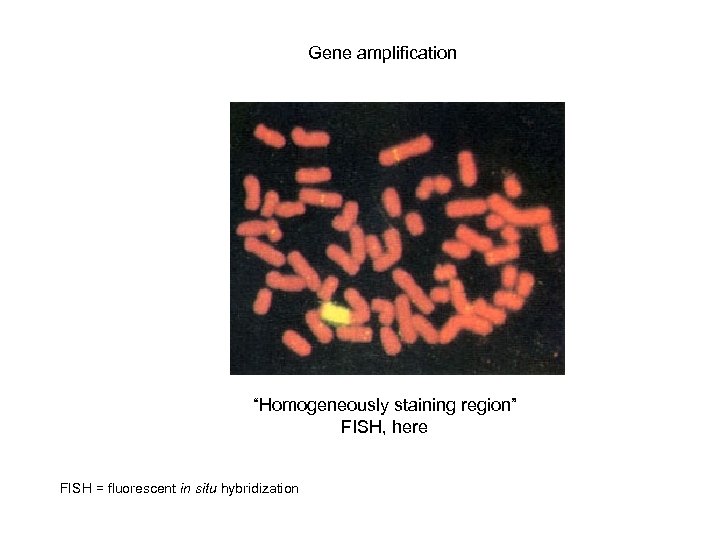 Gene amplification “Homogeneously staining region” FISH, here FISH = fluorescent in situ hybridization
Gene amplification “Homogeneously staining region” FISH, here FISH = fluorescent in situ hybridization
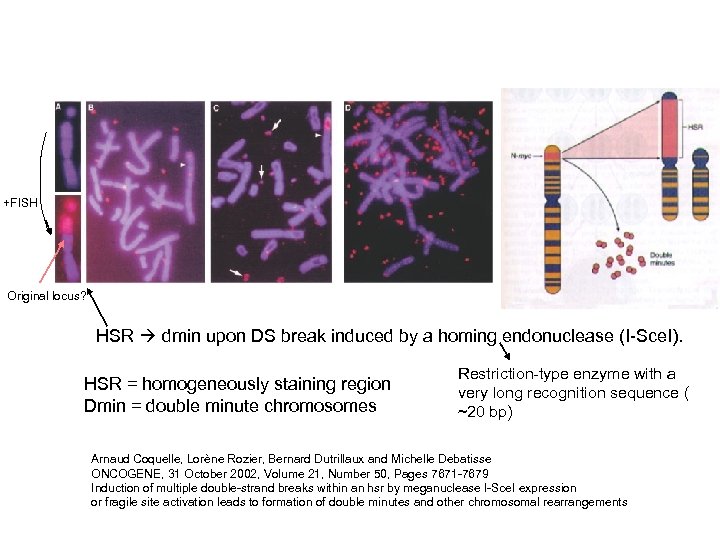 +FISH Original locus? HSR dmin upon DS break induced by a homing endonuclease (I-Sce. I). HSR = homogeneously staining region Dmin = double minute chromosomes Restriction-type enzyme with a very long recognition sequence ( ~20 bp) Arnaud Coquelle, Lorène Rozier, Bernard Dutrillaux and Michelle Debatisse ONCOGENE, 31 October 2002, Volume 21, Number 50, Pages 7671 -7679 Induction of multiple double-strand breaks within an hsr by meganuclease I-Sce. I expression or fragile site activation leads to formation of double minutes and other chromosomal rearrangements
+FISH Original locus? HSR dmin upon DS break induced by a homing endonuclease (I-Sce. I). HSR = homogeneously staining region Dmin = double minute chromosomes Restriction-type enzyme with a very long recognition sequence ( ~20 bp) Arnaud Coquelle, Lorène Rozier, Bernard Dutrillaux and Michelle Debatisse ONCOGENE, 31 October 2002, Volume 21, Number 50, Pages 7671 -7679 Induction of multiple double-strand breaks within an hsr by meganuclease I-Sce. I expression or fragile site activation leads to formation of double minutes and other chromosomal rearrangements
 Ampification models: over-replication, unequal sister chromatid exchange, breakage and fusion (Tanaka et al. ). Map dhfr amplicons (Schimke, Hamlin): ~ 300 kb , but wide range Gene amplification is rare in normal cells (Wahl, Tslty). p 53 - mutation allows it. In nature: r. DNA in oocytes; Drosophila chorion genes. In medicine: chemotherapy resistance (MDR, P-glycoprotein, efflux pump) cancer (myc, ras) In biotechnology: high level recombinant protein production in mammalian cells MDR = multiple drug resistance
Ampification models: over-replication, unequal sister chromatid exchange, breakage and fusion (Tanaka et al. ). Map dhfr amplicons (Schimke, Hamlin): ~ 300 kb , but wide range Gene amplification is rare in normal cells (Wahl, Tslty). p 53 - mutation allows it. In nature: r. DNA in oocytes; Drosophila chorion genes. In medicine: chemotherapy resistance (MDR, P-glycoprotein, efflux pump) cancer (myc, ras) In biotechnology: high level recombinant protein production in mammalian cells MDR = multiple drug resistance
 Some notable gene amplification players John Littlefield Geoff Wahl Bob Schimke Fred Alt Joyce Hamlin George Stark
Some notable gene amplification players John Littlefield Geoff Wahl Bob Schimke Fred Alt Joyce Hamlin George Stark
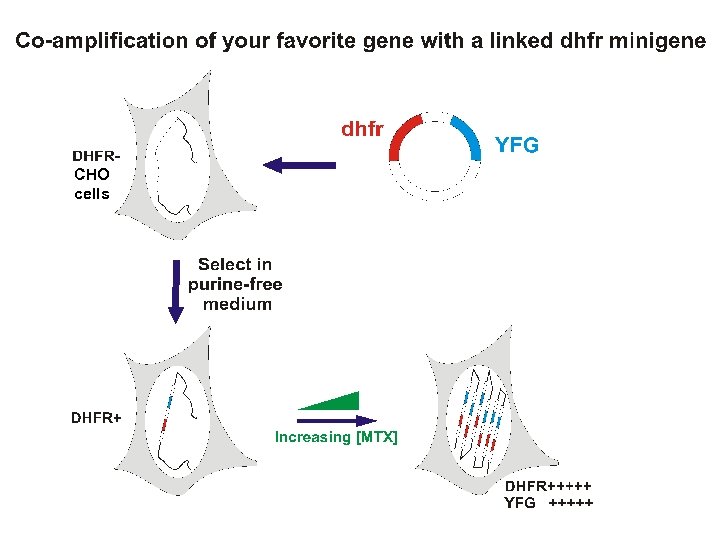
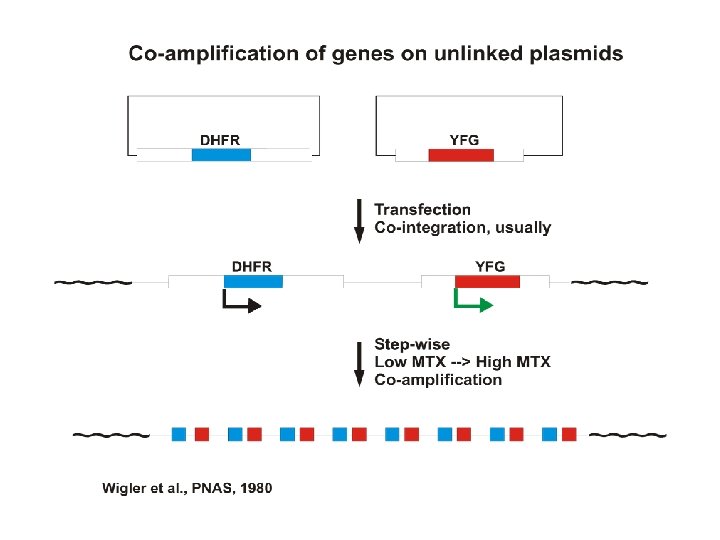
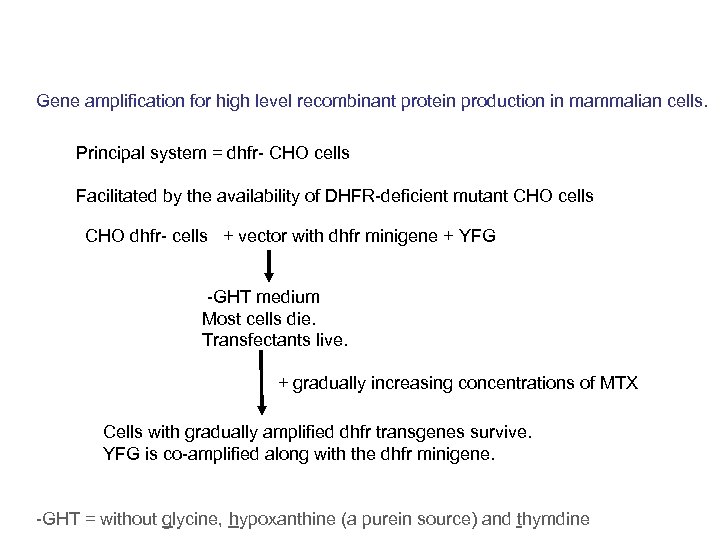 Gene amplification for high level recombinant protein production in mammalian cells. Principal system = dhfr- CHO cells Facilitated by the availability of DHFR-deficient mutant CHO cells CHO dhfr- cells + vector with dhfr minigene + YFG -GHT medium Most cells die. Transfectants live. + gradually increasing concentrations of MTX Cells with gradually amplified dhfr transgenes survive. YFG is co-amplified along with the dhfr minigene. -GHT = without glycine, hypoxanthine (a purein source) and thymdine
Gene amplification for high level recombinant protein production in mammalian cells. Principal system = dhfr- CHO cells Facilitated by the availability of DHFR-deficient mutant CHO cells CHO dhfr- cells + vector with dhfr minigene + YFG -GHT medium Most cells die. Transfectants live. + gradually increasing concentrations of MTX Cells with gradually amplified dhfr transgenes survive. YFG is co-amplified along with the dhfr minigene. -GHT = without glycine, hypoxanthine (a purein source) and thymdine
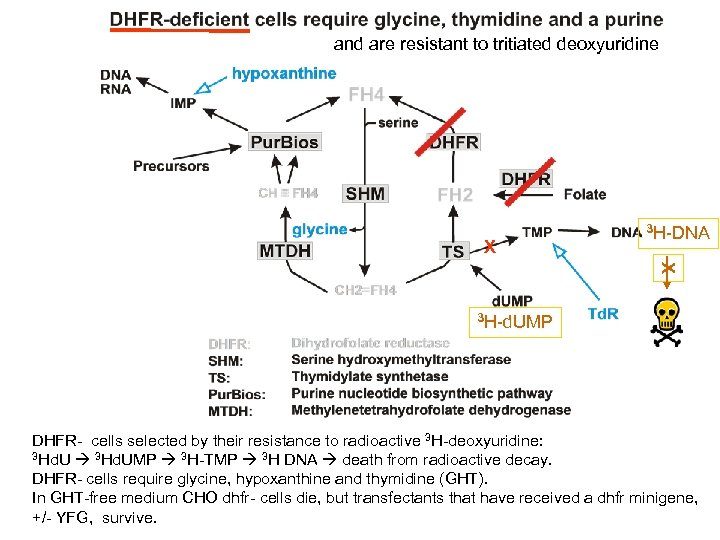 and are resistant to tritiated deoxyuridine X 3 H-DNA X 3 H-d. UMP DHFR- cells selected by their resistance to radioactive 3 H-deoxyuridine: 3 Hd. UMP 3 H-TMP 3 H DNA death from radioactive decay. DHFR- cells require glycine, hypoxanthine and thymidine (GHT). In GHT-free medium CHO dhfr- cells die, but transfectants that have received a dhfr minigene, +/- YFG, survive.
and are resistant to tritiated deoxyuridine X 3 H-DNA X 3 H-d. UMP DHFR- cells selected by their resistance to radioactive 3 H-deoxyuridine: 3 Hd. UMP 3 H-TMP 3 H DNA death from radioactive decay. DHFR- cells require glycine, hypoxanthine and thymidine (GHT). In GHT-free medium CHO dhfr- cells die, but transfectants that have received a dhfr minigene, +/- YFG, survive.
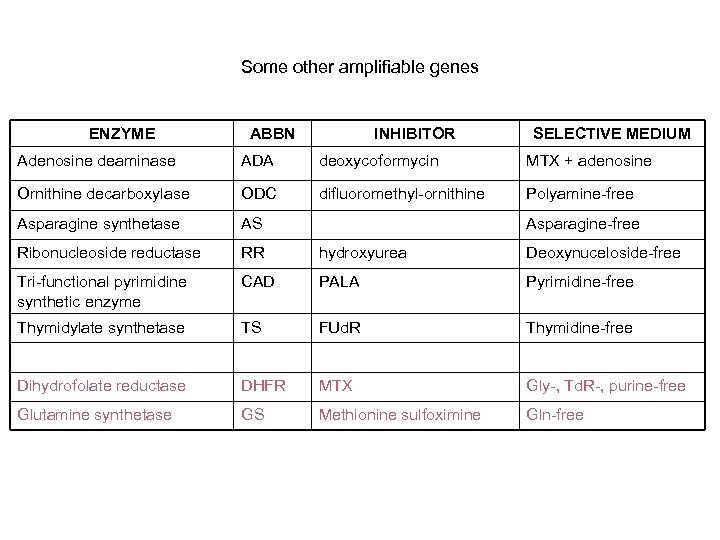 Some other amplifiable genes ENZYME ABBN INHIBITOR SELECTIVE MEDIUM Adenosine deaminase ADA deoxycoformycin MTX + adenosine Ornithine decarboxylase ODC difluoromethyl-ornithine Polyamine-free Asparagine synthetase AS Ribonucleoside reductase RR hydroxyurea Deoxynuceloside-free Tri-functional pyrimidine synthetic enzyme CAD PALA Pyrimidine-free Thymidylate synthetase TS FUd. R Thymidine-free Dihydrofolate reductase DHFR MTX Gly-, Td. R-, purine-free Glutamine synthetase GS Methionine sulfoximine Gln-free Asparagine-free
Some other amplifiable genes ENZYME ABBN INHIBITOR SELECTIVE MEDIUM Adenosine deaminase ADA deoxycoformycin MTX + adenosine Ornithine decarboxylase ODC difluoromethyl-ornithine Polyamine-free Asparagine synthetase AS Ribonucleoside reductase RR hydroxyurea Deoxynuceloside-free Tri-functional pyrimidine synthetic enzyme CAD PALA Pyrimidine-free Thymidylate synthetase TS FUd. R Thymidine-free Dihydrofolate reductase DHFR MTX Gly-, Td. R-, purine-free Glutamine synthetase GS Methionine sulfoximine Gln-free Asparagine-free
 A different major system for high level Mab production NS 0 cells: Mouse myeloma cells, high Ig. G producers Ig. G- variants = NS 0 No endogenous Ig. G, but cell is a natural Ig. G secretor. Lack glutamine synthetase (GS): glutamate + NH 3 + ATP glutamine + ADP + Pi Vector = MAb genes driven by strong promoters (H-chain, L-chain) + GS c. DNA gene (Bebbington) Select on glutamine-free medium Inhibit GS with methionine sulfoximine (gln analog) Select for GS overproducers --->--> (gene amplification does not seem to be operating in this system of the GS c. DNA gene and linked Mab genes) Proprietary (Lonza Biologics)
A different major system for high level Mab production NS 0 cells: Mouse myeloma cells, high Ig. G producers Ig. G- variants = NS 0 No endogenous Ig. G, but cell is a natural Ig. G secretor. Lack glutamine synthetase (GS): glutamate + NH 3 + ATP glutamine + ADP + Pi Vector = MAb genes driven by strong promoters (H-chain, L-chain) + GS c. DNA gene (Bebbington) Select on glutamine-free medium Inhibit GS with methionine sulfoximine (gln analog) Select for GS overproducers --->--> (gene amplification does not seem to be operating in this system of the GS c. DNA gene and linked Mab genes) Proprietary (Lonza Biologics)
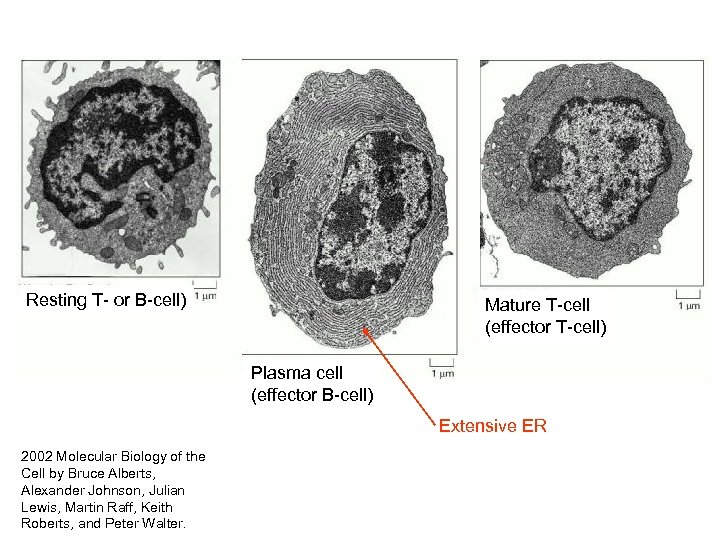 Resting T- or B-cell) Mature T-cell (effector T-cell) Plasma cell (effector B-cell) Extensive ER 2002 Molecular Biology of the Cell by Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter.
Resting T- or B-cell) Mature T-cell (effector T-cell) Plasma cell (effector B-cell) Extensive ER 2002 Molecular Biology of the Cell by Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter.
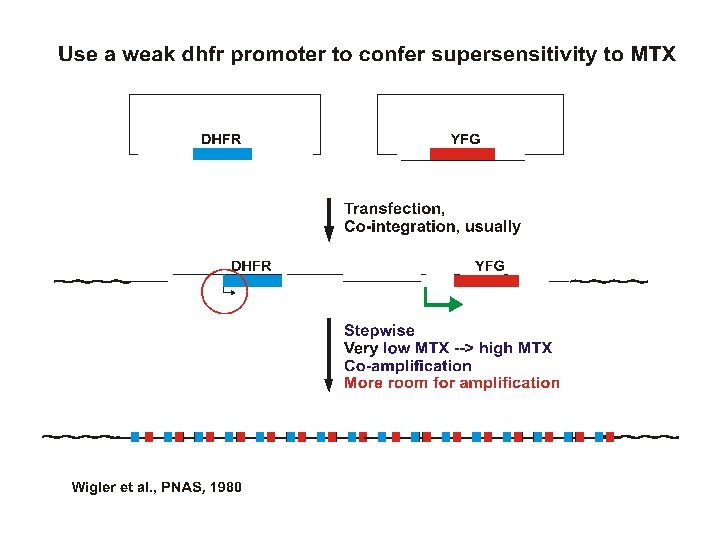
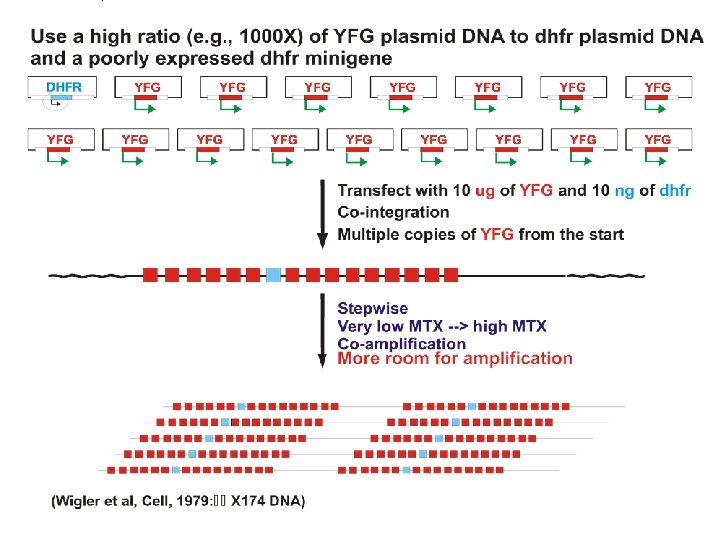
 Transfection strategies for gene amplification 1. YFG (Your Favorite Gene) linked to a dhfr minigene on a single plasmid A. ~Insures co-integration B. ~Insures co-amplification 2. YFG and dhfr on separate plasmids A. Allows a high ratio of YFG to dhfr to start B. Co-amplification not assured but commonly occurs.
Transfection strategies for gene amplification 1. YFG (Your Favorite Gene) linked to a dhfr minigene on a single plasmid A. ~Insures co-integration B. ~Insures co-amplification 2. YFG and dhfr on separate plasmids A. Allows a high ratio of YFG to dhfr to start B. Co-amplification not assured but commonly occurs.
 CHO cells
CHO cells
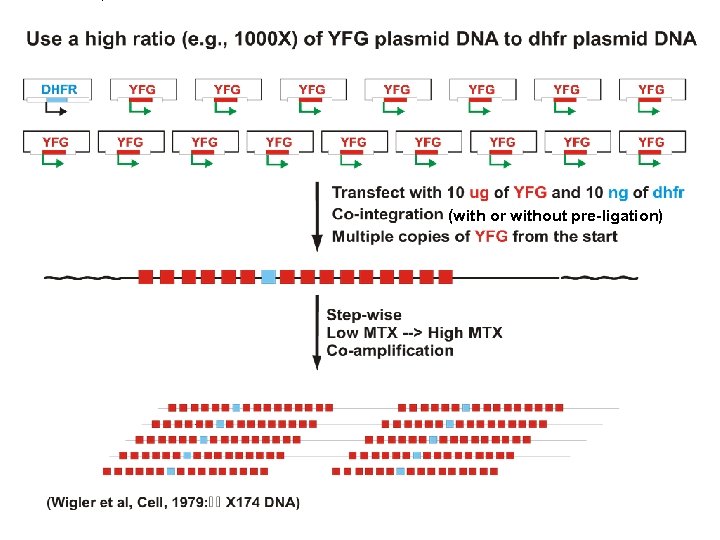 (with or without pre-ligation)
(with or without pre-ligation)
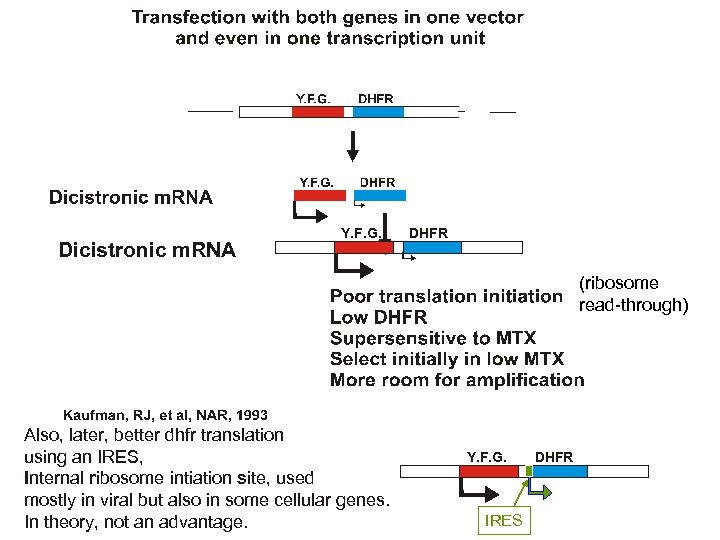 Dicistronic m. RNA Y. F. G. DHFR (ribosome read-through) Also, later, better dhfr translation using an IRES, Internal ribosome intiation site, used mostly in viral but also in some cellular genes. In theory, not an advantage. Y. F. G. IRES DHFR
Dicistronic m. RNA Y. F. G. DHFR (ribosome read-through) Also, later, better dhfr translation using an IRES, Internal ribosome intiation site, used mostly in viral but also in some cellular genes. In theory, not an advantage. Y. F. G. IRES DHFR
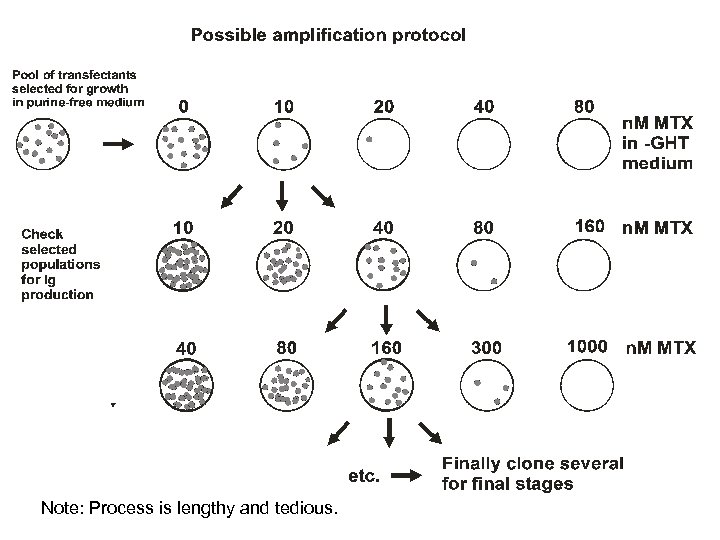 Note: Process is lengthy and tedious.
Note: Process is lengthy and tedious.
 Some marketed recombinant proteins Erythropoietin (Epogen, Procrit) J&J, Amgen Tissue plasminogen activator (TPA) Genentech Growth Hormone (Genentech) Insulin (Genentech) Beta-interferon (Avonex) Biogen-IDEC Alpha-interferon (Intron. A) Schering-Plough Neupogen (Amgen) Etanercept – TNF receptor + Ig. G (Enbrel) Amgen Monoclonal antibodies (m. Abs): see next
Some marketed recombinant proteins Erythropoietin (Epogen, Procrit) J&J, Amgen Tissue plasminogen activator (TPA) Genentech Growth Hormone (Genentech) Insulin (Genentech) Beta-interferon (Avonex) Biogen-IDEC Alpha-interferon (Intron. A) Schering-Plough Neupogen (Amgen) Etanercept – TNF receptor + Ig. G (Enbrel) Amgen Monoclonal antibodies (m. Abs): see next
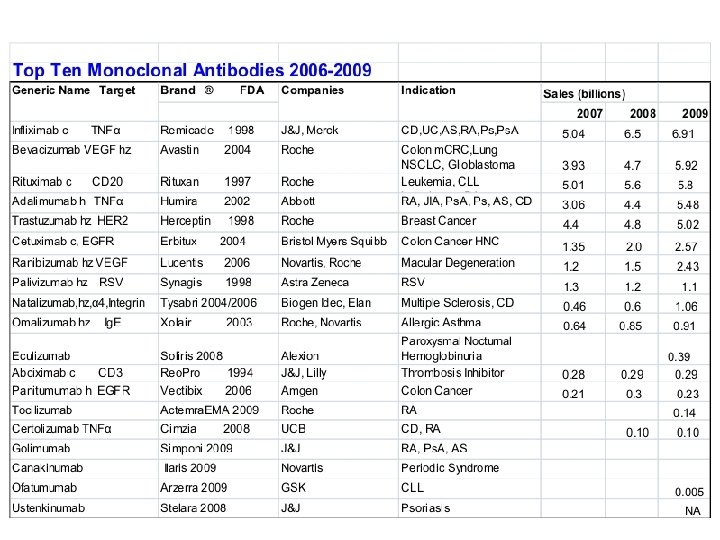
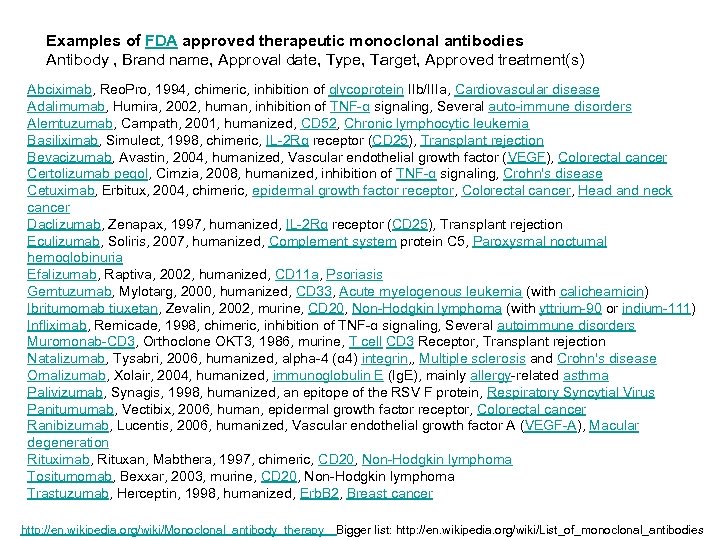 Examples of FDA approved therapeutic monoclonal antibodies Antibody , Brand name, Approval date, Type, Target, Approved treatment(s) Abciximab, Reo. Pro, 1994, chimeric, inhibition of glycoprotein IIb/IIIa, Cardiovascular disease Adalimumab, Humira, 2002, human, inhibition of TNF-α signaling, Several auto-immune disorders Alemtuzumab, Campath, 2001, humanized, CD 52, Chronic lymphocytic leukemia Basiliximab, Simulect, 1998, chimeric, IL-2 Rα receptor (CD 25), Transplant rejection Bevacizumab, Avastin, 2004, humanized, Vascular endothelial growth factor (VEGF), Colorectal cancer Certolizumab pegol, Cimzia, 2008, humanized, inhibition of TNF-α signaling, Crohn's disease Cetuximab, Erbitux, 2004, chimeric, epidermal growth factor receptor, Colorectal cancer, Head and neck cancer Daclizumab, Zenapax, 1997, humanized, IL-2 Rα receptor (CD 25), Transplant rejection Eculizumab, Soliris, 2007, humanized, Complement system protein C 5, Paroxysmal nocturnal hemoglobinuria Efalizumab, Raptiva, 2002, humanized, CD 11 a, Psoriasis Gemtuzumab, Mylotarg, 2000, humanized, CD 33, Acute myelogenous leukemia (with calicheamicin) Ibritumomab tiuxetan, Zevalin, 2002, murine, CD 20, Non-Hodgkin lymphoma (with yttrium-90 or indium-111) Infliximab, Remicade, 1998, chimeric, inhibition of TNF-α signaling, Several autoimmune disorders Muromonab-CD 3, Orthoclone OKT 3, 1986, murine, T cell CD 3 Receptor, Transplant rejection Natalizumab, Tysabri, 2006, humanized, alpha-4 (α 4) integrin, , Multiple sclerosis and Crohn's disease Omalizumab, Xolair, 2004, humanized, immunoglobulin E (Ig. E), mainly allergy-related asthma Palivizumab, Synagis, 1998, humanized, an epitope of the RSV F protein, Respiratory Syncytial Virus Panitumumab, Vectibix, 2006, human, epidermal growth factor receptor, Colorectal cancer Ranibizumab, Lucentis, 2006, humanized, Vascular endothelial growth factor A (VEGF-A), Macular degeneration Rituximab, Rituxan, Mabthera, 1997, chimeric, CD 20, Non-Hodgkin lymphoma Tositumomab, Bexxar, 2003, murine, CD 20, Non-Hodgkin lymphoma Trastuzumab, Herceptin, 1998, humanized, Erb. B 2, Breast cancer http: //en. wikipedia. org/wiki/Monoclonal_antibody_therapy Bigger list: http: //en. wikipedia. org/wiki/List_of_monoclonal_antibodies
Examples of FDA approved therapeutic monoclonal antibodies Antibody , Brand name, Approval date, Type, Target, Approved treatment(s) Abciximab, Reo. Pro, 1994, chimeric, inhibition of glycoprotein IIb/IIIa, Cardiovascular disease Adalimumab, Humira, 2002, human, inhibition of TNF-α signaling, Several auto-immune disorders Alemtuzumab, Campath, 2001, humanized, CD 52, Chronic lymphocytic leukemia Basiliximab, Simulect, 1998, chimeric, IL-2 Rα receptor (CD 25), Transplant rejection Bevacizumab, Avastin, 2004, humanized, Vascular endothelial growth factor (VEGF), Colorectal cancer Certolizumab pegol, Cimzia, 2008, humanized, inhibition of TNF-α signaling, Crohn's disease Cetuximab, Erbitux, 2004, chimeric, epidermal growth factor receptor, Colorectal cancer, Head and neck cancer Daclizumab, Zenapax, 1997, humanized, IL-2 Rα receptor (CD 25), Transplant rejection Eculizumab, Soliris, 2007, humanized, Complement system protein C 5, Paroxysmal nocturnal hemoglobinuria Efalizumab, Raptiva, 2002, humanized, CD 11 a, Psoriasis Gemtuzumab, Mylotarg, 2000, humanized, CD 33, Acute myelogenous leukemia (with calicheamicin) Ibritumomab tiuxetan, Zevalin, 2002, murine, CD 20, Non-Hodgkin lymphoma (with yttrium-90 or indium-111) Infliximab, Remicade, 1998, chimeric, inhibition of TNF-α signaling, Several autoimmune disorders Muromonab-CD 3, Orthoclone OKT 3, 1986, murine, T cell CD 3 Receptor, Transplant rejection Natalizumab, Tysabri, 2006, humanized, alpha-4 (α 4) integrin, , Multiple sclerosis and Crohn's disease Omalizumab, Xolair, 2004, humanized, immunoglobulin E (Ig. E), mainly allergy-related asthma Palivizumab, Synagis, 1998, humanized, an epitope of the RSV F protein, Respiratory Syncytial Virus Panitumumab, Vectibix, 2006, human, epidermal growth factor receptor, Colorectal cancer Ranibizumab, Lucentis, 2006, humanized, Vascular endothelial growth factor A (VEGF-A), Macular degeneration Rituximab, Rituxan, Mabthera, 1997, chimeric, CD 20, Non-Hodgkin lymphoma Tositumomab, Bexxar, 2003, murine, CD 20, Non-Hodgkin lymphoma Trastuzumab, Herceptin, 1998, humanized, Erb. B 2, Breast cancer http: //en. wikipedia. org/wiki/Monoclonal_antibody_therapy Bigger list: http: //en. wikipedia. org/wiki/List_of_monoclonal_antibodies
 Ways to increase production and/or lessen development time: Mitchell Reff (IDEC patent): Screen for a high production genomic position. Integrate YFG into it by homologous recombination, selecting for reconstitution of a split dhfr minigene, then amplify. Mitsubishi (T. Shibou, Mitsubishi Pharma Corporation. European Patent Application. Vol. EP 001293564 A 1, PCT/JP 01/04801): Same, but use a lox site and site-specific recombination to integrate YFG. Add chromatin remodeling sequences to vector to open chromatin. Add “insulator” sequences to vector to block postion specific repression. Search for even better promoters (current: CMV, EIFalpha, actin) Or even synthetic promoters (E. coli: Stephanopolos, MIT) Engineer cells with advantageous glycosylation patterns Engineer cell to eliminate or defer apoptosis (for longer productinon runs) Etc. (including Chasin lab project)
Ways to increase production and/or lessen development time: Mitchell Reff (IDEC patent): Screen for a high production genomic position. Integrate YFG into it by homologous recombination, selecting for reconstitution of a split dhfr minigene, then amplify. Mitsubishi (T. Shibou, Mitsubishi Pharma Corporation. European Patent Application. Vol. EP 001293564 A 1, PCT/JP 01/04801): Same, but use a lox site and site-specific recombination to integrate YFG. Add chromatin remodeling sequences to vector to open chromatin. Add “insulator” sequences to vector to block postion specific repression. Search for even better promoters (current: CMV, EIFalpha, actin) Or even synthetic promoters (E. coli: Stephanopolos, MIT) Engineer cells with advantageous glycosylation patterns Engineer cell to eliminate or defer apoptosis (for longer productinon runs) Etc. (including Chasin lab project)
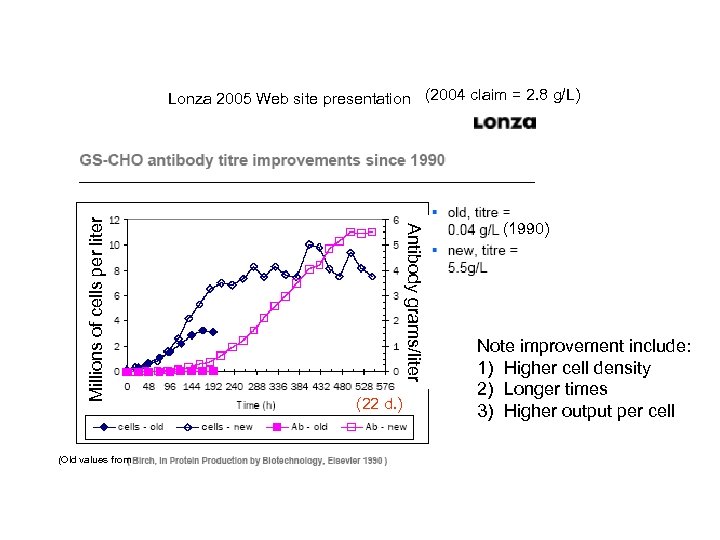 (Old values from Antibody grams/liter Millions of cells per liter Lonza 2005 Web site presentation (2004 claim = 2. 8 g/L) (22 d. ) (1990) Note improvement include: 1) Higher cell density 2) Longer times 3) Higher output per cell
(Old values from Antibody grams/liter Millions of cells per liter Lonza 2005 Web site presentation (2004 claim = 2. 8 g/L) (22 d. ) (1990) Note improvement include: 1) Higher cell density 2) Longer times 3) Higher output per cell
 20, 000 liter fermentor 20, 000 liter mammalian cell fermentor - Lonza Biologics - Portsmouth, NH
20, 000 liter fermentor 20, 000 liter mammalian cell fermentor - Lonza Biologics - Portsmouth, NH
 High level production in mammalian cells. Do the math (back of the envelope): Reff patent (IDEC): 55 pg/cell/day Max cell density = 107/ml ? So 1010 cells/L Therefore 55 x 10 -12 g/cell/day x 1010 cells/L = 55 x 10 -2 g/L/day = 0. 55 g/L/day = 11 g/L/20 days, calculated Lonza (contract manufacturer) claims (2005) = 5 g/L yield Same ballpark. 30, 000 L reactor (largest): 30, 000 L. X 5 g/L. = 150 kg in 20 days, or say one month x 12 months = 1800 kg/year = 1, 800, 000 g/year One MAb dose = ~500 mg = 0. 5 g 1, 800, 000/0. 5 = 3. 6 million doses per reactor per year. 6 doses per patient per year? 3, 600, 000/6 = 600, 000 patients per year per reactor. At $15, 000 per patient per year $9 B in sales /per 30 k. L reactor
High level production in mammalian cells. Do the math (back of the envelope): Reff patent (IDEC): 55 pg/cell/day Max cell density = 107/ml ? So 1010 cells/L Therefore 55 x 10 -12 g/cell/day x 1010 cells/L = 55 x 10 -2 g/L/day = 0. 55 g/L/day = 11 g/L/20 days, calculated Lonza (contract manufacturer) claims (2005) = 5 g/L yield Same ballpark. 30, 000 L reactor (largest): 30, 000 L. X 5 g/L. = 150 kg in 20 days, or say one month x 12 months = 1800 kg/year = 1, 800, 000 g/year One MAb dose = ~500 mg = 0. 5 g 1, 800, 000/0. 5 = 3. 6 million doses per reactor per year. 6 doses per patient per year? 3, 600, 000/6 = 600, 000 patients per year per reactor. At $15, 000 per patient per year $9 B in sales /per 30 k. L reactor


