88ff2d5a44aecf43b27f335a76c2e602.ppt
- Количество слайдов: 53
 Gastric lymphoma: changing role of surgery Joint Hospital Surgical Grand Round Dr Bonita HK Mark RHTSK
Gastric lymphoma: changing role of surgery Joint Hospital Surgical Grand Round Dr Bonita HK Mark RHTSK
 Gastric lymphoma What is gastric lymphoma? Why do we need to know about it? What is the evidence in literature? How to treat? When to operate / not to operate?
Gastric lymphoma What is gastric lymphoma? Why do we need to know about it? What is the evidence in literature? How to treat? When to operate / not to operate?
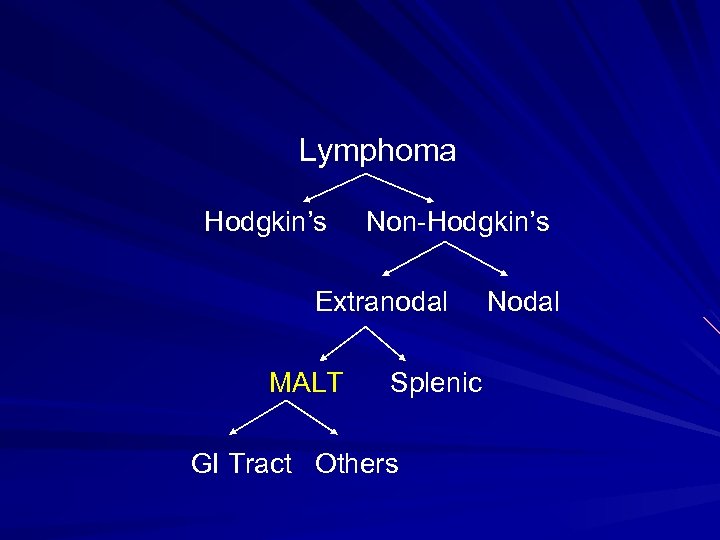 Lymphoma Hodgkin’s Non-Hodgkin’s Extranodal MALT Splenic GI Tract Others Nodal
Lymphoma Hodgkin’s Non-Hodgkin’s Extranodal MALT Splenic GI Tract Others Nodal
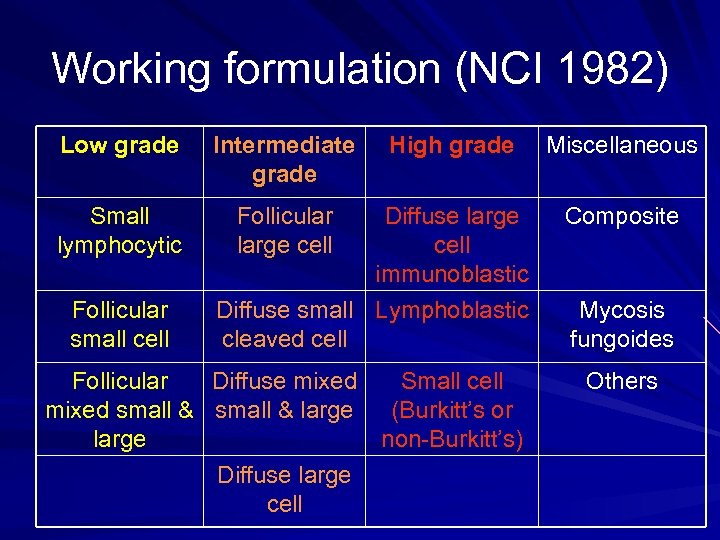 Working formulation (NCI 1982) Low grade Intermediate grade Small lymphocytic Follicular large cell Follicular small cell High grade Diffuse large cell immunoblastic Diffuse small Lymphoblastic cleaved cell Follicular Diffuse mixed Small cell mixed small & large (Burkitt’s or large non-Burkitt’s) Diffuse large cell Miscellaneous Composite Mycosis fungoides Others
Working formulation (NCI 1982) Low grade Intermediate grade Small lymphocytic Follicular large cell Follicular small cell High grade Diffuse large cell immunoblastic Diffuse small Lymphoblastic cleaved cell Follicular Diffuse mixed Small cell mixed small & large (Burkitt’s or large non-Burkitt’s) Diffuse large cell Miscellaneous Composite Mycosis fungoides Others
 Revised European-American Lymphoma (REAL) (WHO 1993) B-cell lymphoma – – – Lymphoblastic Small lymphocytic Lymphoplasmacytoid Mantle-cell Follicular center (follicular, diffuse, small) – Marginal-zone (nodal, extranodal, splenic) – Diffuse large B-cell Burkitt’s / Burkitt-like T cell lymphoma – – Lymphoblastic Mycosis fungoides/ sezary syndrome – Peripheral T-cell
Revised European-American Lymphoma (REAL) (WHO 1993) B-cell lymphoma – – – Lymphoblastic Small lymphocytic Lymphoplasmacytoid Mantle-cell Follicular center (follicular, diffuse, small) – Marginal-zone (nodal, extranodal, splenic) – Diffuse large B-cell Burkitt’s / Burkitt-like T cell lymphoma – – Lymphoblastic Mycosis fungoides/ sezary syndrome – Peripheral T-cell
 MALT lymphoma MALT (mucosa associated lymphoid tissue) lymphoma First described in 1983 Extra-nodal marginal zone B-cell lymphoma Indolent (low grade) Most common in GI tract (50%) Stomach mostly involved (50 -70% of GI MALT) 4% primary gastric tumours 40 -50% primary gastric lymphomas
MALT lymphoma MALT (mucosa associated lymphoid tissue) lymphoma First described in 1983 Extra-nodal marginal zone B-cell lymphoma Indolent (low grade) Most common in GI tract (50%) Stomach mostly involved (50 -70% of GI MALT) 4% primary gastric tumours 40 -50% primary gastric lymphomas
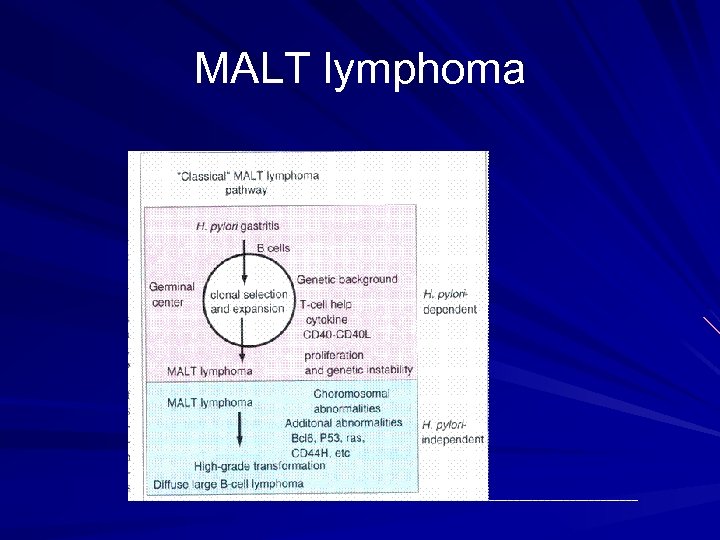 MALT lymphoma
MALT lymphoma
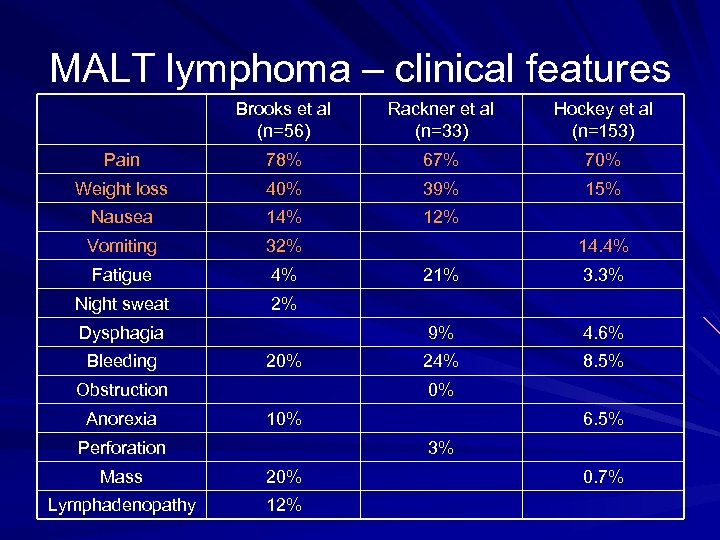 MALT lymphoma – clinical features Brooks et al (n=56) Rackner et al (n=33) Hockey et al (n=153) Pain 78% 67% 70% Weight loss 40% 39% 15% Nausea 14% 12% Vomiting 32% Fatigue 4% Night sweat 2% 20% Obstruction Anorexia 21% 3. 3% 9% Dysphagia Bleeding 14. 4% 4. 6% 24% 8. 5% 0% 10% Perforation 6. 5% 3% Mass 20% Lymphadenopathy 12% 0. 7%
MALT lymphoma – clinical features Brooks et al (n=56) Rackner et al (n=33) Hockey et al (n=153) Pain 78% 67% 70% Weight loss 40% 39% 15% Nausea 14% 12% Vomiting 32% Fatigue 4% Night sweat 2% 20% Obstruction Anorexia 21% 3. 3% 9% Dysphagia Bleeding 14. 4% 4. 6% 24% 8. 5% 0% 10% Perforation 6. 5% 3% Mass 20% Lymphadenopathy 12% 0. 7%
 MALT lymphoma - diagnosis Upper endoscopy Biopsy of suspicious area Ø Ulceration Ø Nodular mass Ø Diffuse infiltration Antral biopsy for H pylori Endoscopic ultrasound Ø Depth of tumour invasion Ø Perigastric LN enlargement CT chest, abdomen and pelvis/ PET scan Bone marrow biopsy
MALT lymphoma - diagnosis Upper endoscopy Biopsy of suspicious area Ø Ulceration Ø Nodular mass Ø Diffuse infiltration Antral biopsy for H pylori Endoscopic ultrasound Ø Depth of tumour invasion Ø Perigastric LN enlargement CT chest, abdomen and pelvis/ PET scan Bone marrow biopsy
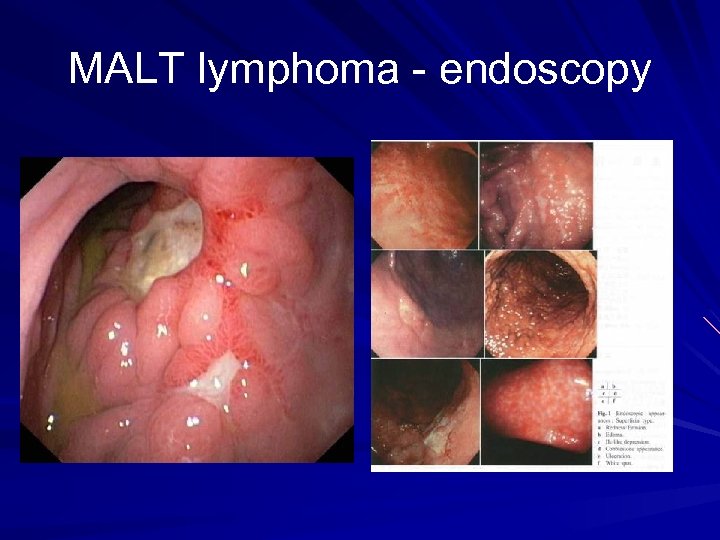 MALT lymphoma - endoscopy
MALT lymphoma - endoscopy
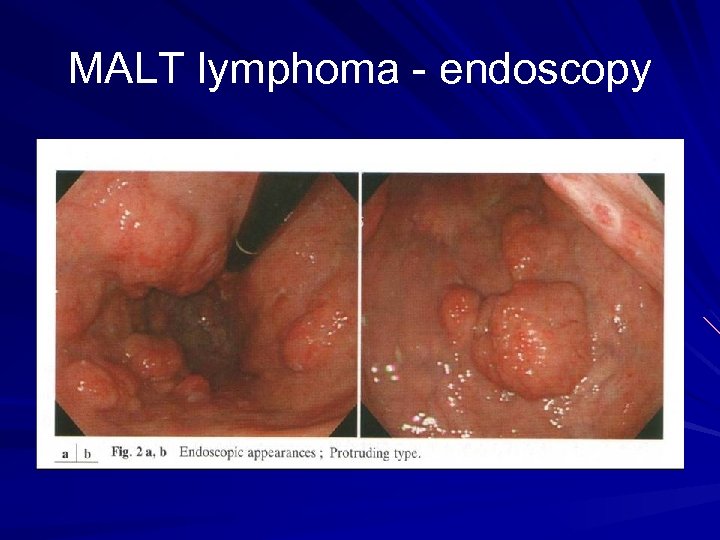 MALT lymphoma - endoscopy
MALT lymphoma - endoscopy
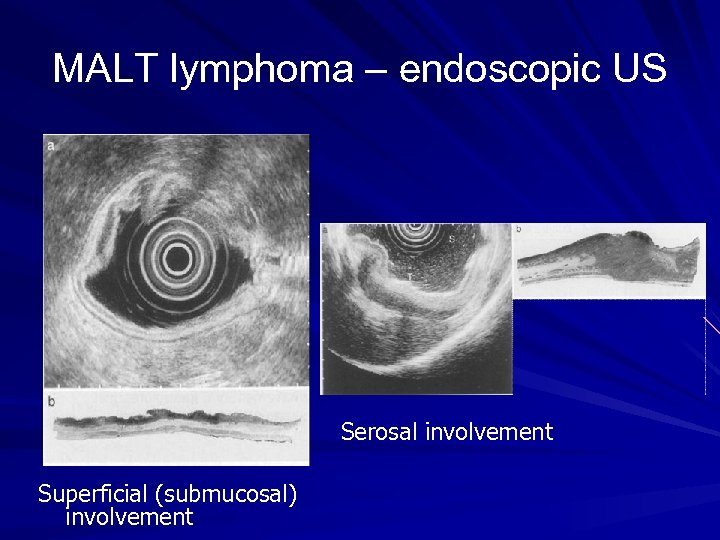 MALT lymphoma – endoscopic US Serosal involvement Superficial (submucosal) involvement
MALT lymphoma – endoscopic US Serosal involvement Superficial (submucosal) involvement
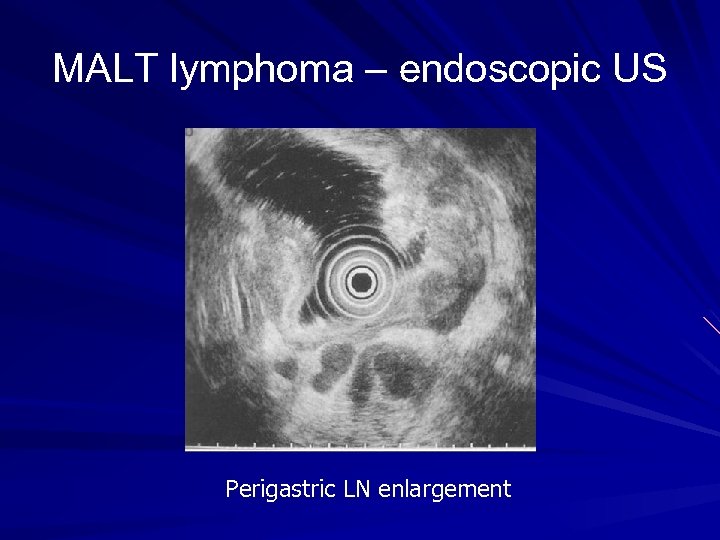 MALT lymphoma – endoscopic US Perigastric LN enlargement
MALT lymphoma – endoscopic US Perigastric LN enlargement
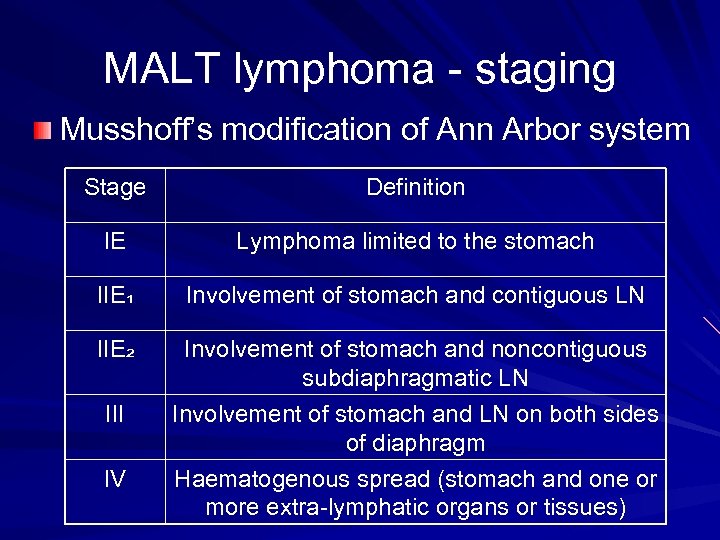 MALT lymphoma - staging Musshoff’s modification of Ann Arbor system Stage Definition IE Lymphoma limited to the stomach IIE₁ Involvement of stomach and contiguous LN IIE₂ Involvement of stomach and noncontiguous subdiaphragmatic LN Involvement of stomach and LN on both sides of diaphragm Haematogenous spread (stomach and one or more extra-lymphatic organs or tissues) III IV
MALT lymphoma - staging Musshoff’s modification of Ann Arbor system Stage Definition IE Lymphoma limited to the stomach IIE₁ Involvement of stomach and contiguous LN IIE₂ Involvement of stomach and noncontiguous subdiaphragmatic LN Involvement of stomach and LN on both sides of diaphragm Haematogenous spread (stomach and one or more extra-lymphatic organs or tissues) III IV
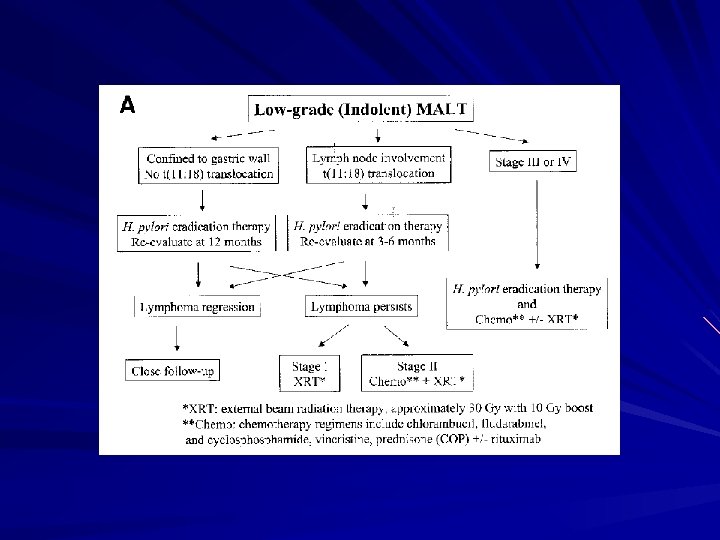
 H pylori eradication therapy Low grade MALT lymphoma: stage I or II disease with slow progression H pylori in 90% gastric MALT lymphoma 2/3 lymphoma regresses after eradication Prognosis good: 10 -year survival 80 -90%
H pylori eradication therapy Low grade MALT lymphoma: stage I or II disease with slow progression H pylori in 90% gastric MALT lymphoma 2/3 lymphoma regresses after eradication Prognosis good: 10 -year survival 80 -90%
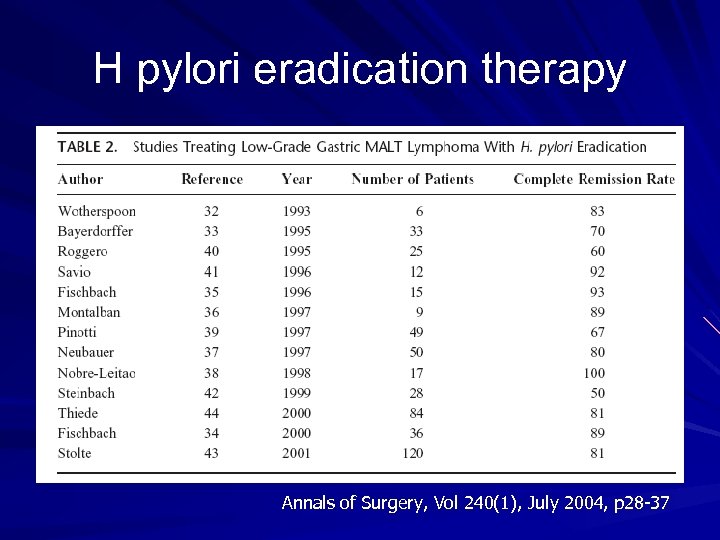 H pylori eradication therapy Annals of Surgery, Vol 240(1), July 2004, p 28 -37
H pylori eradication therapy Annals of Surgery, Vol 240(1), July 2004, p 28 -37
 Predictive factors for poor response to H pylori eradication therapy Perigastric LN involvement (stage II₁) – 0% with stage II vs. 79% with stage I (Multicentre French study, Gut 2001; 48: 297 -303) – 33% LN +ve vs. 76% LN –ve (Am J Gastroenterology 2002; 97: 292 -297) A t (11: 18) chromosomal translocation – review of 111 patients by Liu et al: 73% vs. 4% (Gastroenterology 2002; 122: 1286 -1294) H pylori -ve
Predictive factors for poor response to H pylori eradication therapy Perigastric LN involvement (stage II₁) – 0% with stage II vs. 79% with stage I (Multicentre French study, Gut 2001; 48: 297 -303) – 33% LN +ve vs. 76% LN –ve (Am J Gastroenterology 2002; 97: 292 -297) A t (11: 18) chromosomal translocation – review of 111 patients by Liu et al: 73% vs. 4% (Gastroenterology 2002; 122: 1286 -1294) H pylori -ve
 What is the best Rx modality?
What is the best Rx modality?
 Implications to surgeons
Implications to surgeons

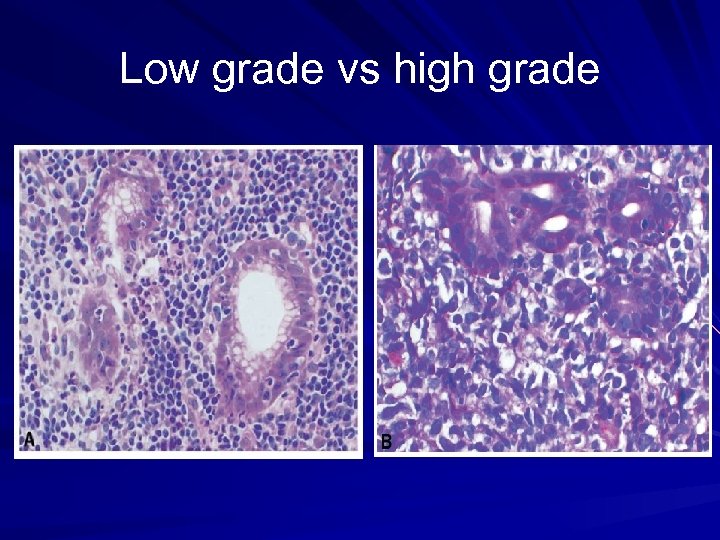 Low grade vs high grade
Low grade vs high grade
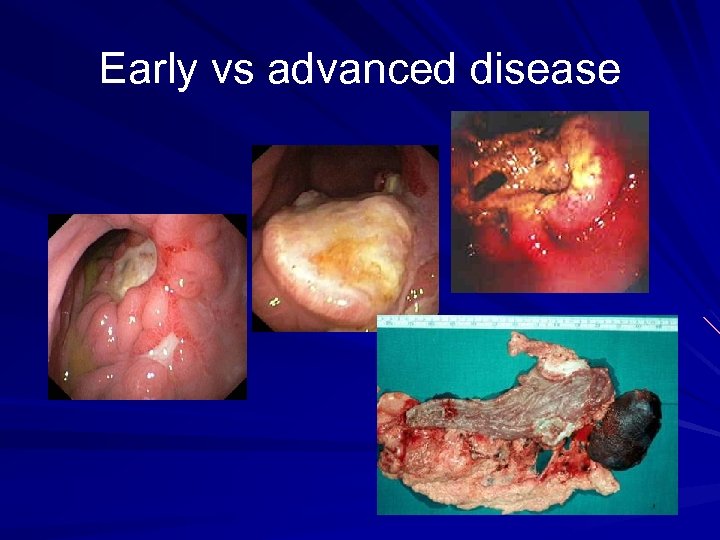 Early vs advanced disease
Early vs advanced disease

 Surgery for gastric lymphoma Brands et al reviewed 100 papers analyzing 3157 patients with all stages of gastric lymphoma Treated from 1974 to 1995 The overall survival during that time period ↑from 37% to 87%.
Surgery for gastric lymphoma Brands et al reviewed 100 papers analyzing 3157 patients with all stages of gastric lymphoma Treated from 1974 to 1995 The overall survival during that time period ↑from 37% to 87%.
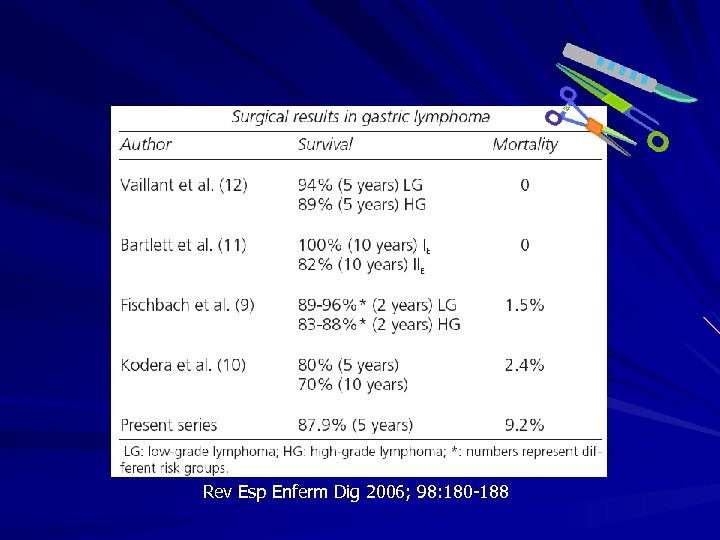 Rev Esp Enferm Dig 2006; 98: 180 -188
Rev Esp Enferm Dig 2006; 98: 180 -188
 Review article Ann Surg 2004; 240: 28 -37
Review article Ann Surg 2004; 240: 28 -37
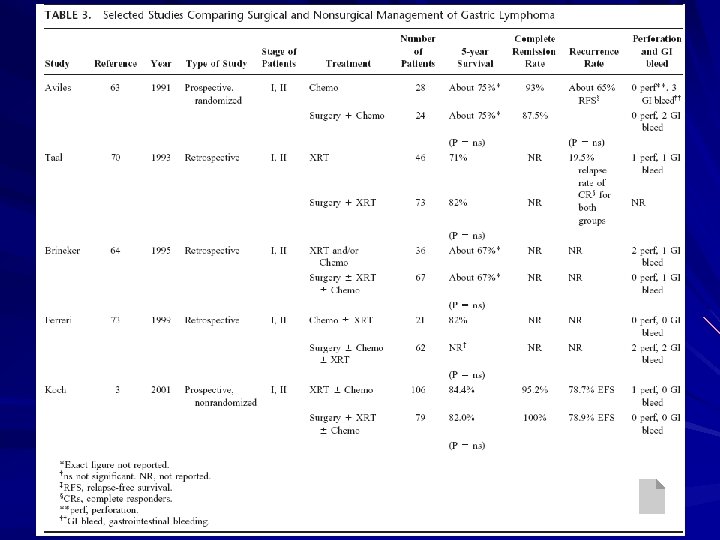

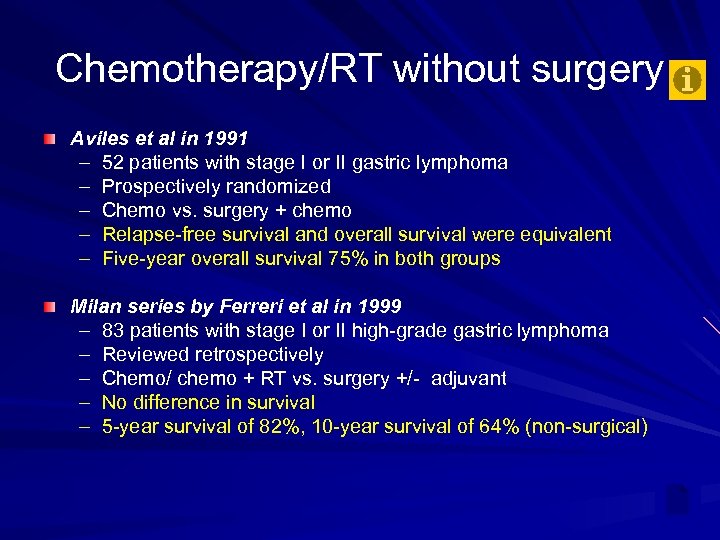 Chemotherapy/RT without surgery Aviles et al in 1991 – 52 patients with stage I or II gastric lymphoma – Prospectively randomized – Chemo vs. surgery + chemo – Relapse-free survival and overall survival were equivalent – Five-year overall survival 75% in both groups Milan series by Ferreri et al in 1999 – 83 patients with stage I or II high-grade gastric lymphoma – Reviewed retrospectively – Chemo/ chemo + RT vs. surgery +/- adjuvant – No difference in survival – 5 -year survival of 82%, 10 -year survival of 64% (non-surgical)
Chemotherapy/RT without surgery Aviles et al in 1991 – 52 patients with stage I or II gastric lymphoma – Prospectively randomized – Chemo vs. surgery + chemo – Relapse-free survival and overall survival were equivalent – Five-year overall survival 75% in both groups Milan series by Ferreri et al in 1999 – 83 patients with stage I or II high-grade gastric lymphoma – Reviewed retrospectively – Chemo/ chemo + RT vs. surgery +/- adjuvant – No difference in survival – 5 -year survival of 82%, 10 -year survival of 64% (non-surgical)
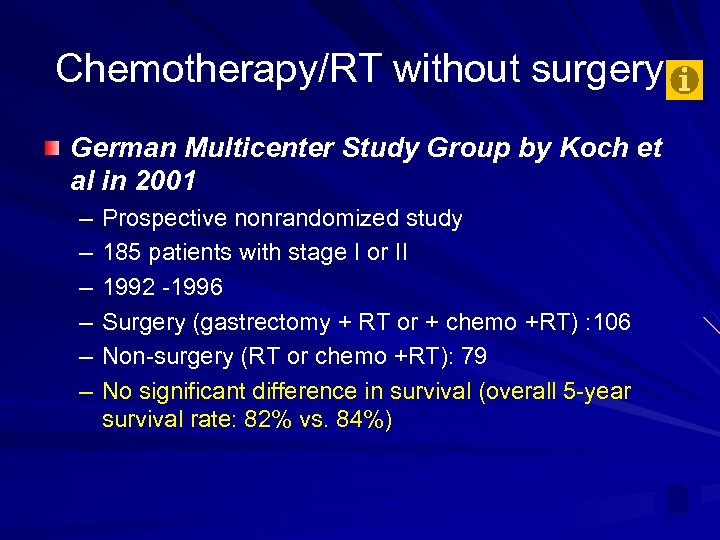 Chemotherapy/RT without surgery German Multicenter Study Group by Koch et al in 2001 – – – Prospective nonrandomized study 185 patients with stage I or II 1992 -1996 Surgery (gastrectomy + RT or + chemo +RT) : 106 Non-surgery (RT or chemo +RT): 79 No significant difference in survival (overall 5 -year survival rate: 82% vs. 84%)
Chemotherapy/RT without surgery German Multicenter Study Group by Koch et al in 2001 – – – Prospective nonrandomized study 185 patients with stage I or II 1992 -1996 Surgery (gastrectomy + RT or + chemo +RT) : 106 Non-surgery (RT or chemo +RT): 79 No significant difference in survival (overall 5 -year survival rate: 82% vs. 84%)
 Chemotherapy/RT without surgery Aviles et al – No perforation – Bleeding: 3 (non-surgical) vs. 2 (surgical) German Multicenter Study Group by Koch et al – Perforation: 1 (non-surgical) vs. none (surgical) – No bleeding
Chemotherapy/RT without surgery Aviles et al – No perforation – Bleeding: 3 (non-surgical) vs. 2 (surgical) German Multicenter Study Group by Koch et al – Perforation: 1 (non-surgical) vs. none (surgical) – No bleeding
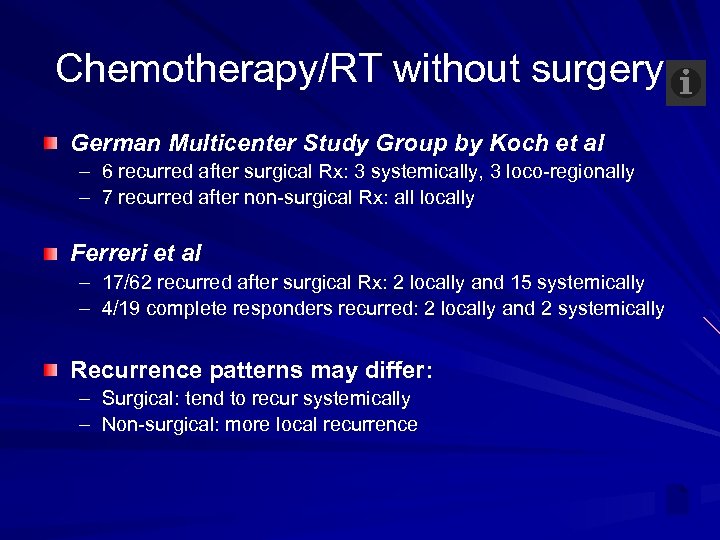 Chemotherapy/RT without surgery German Multicenter Study Group by Koch et al – 6 recurred after surgical Rx: 3 systemically, 3 loco-regionally – 7 recurred after non-surgical Rx: all locally Ferreri et al – 17/62 recurred after surgical Rx: 2 locally and 15 systemically – 4/19 complete responders recurred: 2 locally and 2 systemically Recurrence patterns may differ: – Surgical: tend to recur systemically – Non-surgical: more local recurrence
Chemotherapy/RT without surgery German Multicenter Study Group by Koch et al – 6 recurred after surgical Rx: 3 systemically, 3 loco-regionally – 7 recurred after non-surgical Rx: all locally Ferreri et al – 17/62 recurred after surgical Rx: 2 locally and 15 systemically – 4/19 complete responders recurred: 2 locally and 2 systemically Recurrence patterns may differ: – Surgical: tend to recur systemically – Non-surgical: more local recurrence
 Retrospective review J Formos Med Assoc 2006; 105(3): 194 -202
Retrospective review J Formos Med Assoc 2006; 105(3): 194 -202
 Retrospective review Objective: – To evaluate the outcome of PGL (except MALT lymphoma) treated with chemo alone or surgery followed by chemo Methods: – – – 1986 -2003 55 PGL patients (MALT lymphoma excluded) Localized 32 (IE 15 + IIE 17) Advanced 23 Chemo alone vs. Combination (surgery + chemo)
Retrospective review Objective: – To evaluate the outcome of PGL (except MALT lymphoma) treated with chemo alone or surgery followed by chemo Methods: – – – 1986 -2003 55 PGL patients (MALT lymphoma excluded) Localized 32 (IE 15 + IIE 17) Advanced 23 Chemo alone vs. Combination (surgery + chemo)
 Retrospective review Results: – Complete remission no sig. difference: Chemo: 84. 2% Combination: 92. 3% – 5 -year overall survival no sig. difference: Chemo: 73. 4% Combination: 87. 5% – 5 -year disease-free survival no sig. difference: Chemo: 68. 4% Combination: 84. 6%
Retrospective review Results: – Complete remission no sig. difference: Chemo: 84. 2% Combination: 92. 3% – 5 -year overall survival no sig. difference: Chemo: 73. 4% Combination: 87. 5% – 5 -year disease-free survival no sig. difference: Chemo: 68. 4% Combination: 84. 6%
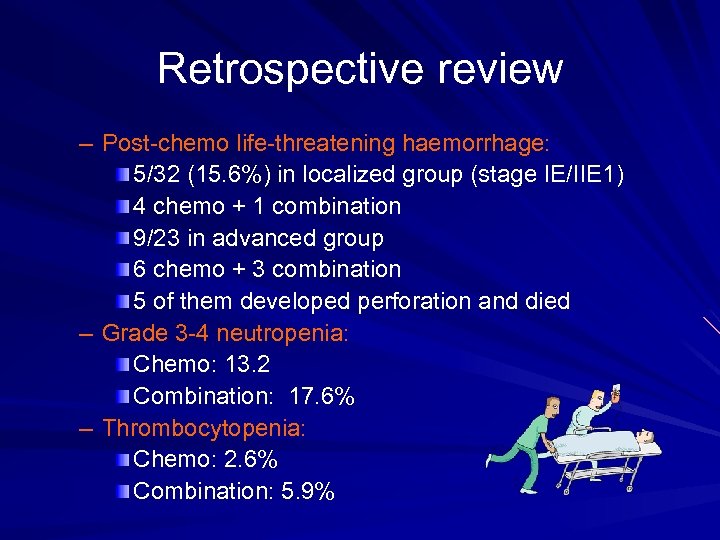 Retrospective review – Post-chemo life-threatening haemorrhage: 5/32 (15. 6%) in localized group (stage IE/IIE 1) 4 chemo + 1 combination 9/23 in advanced group 6 chemo + 3 combination 5 of them developed perforation and died – Grade 3 -4 neutropenia: Chemo: 13. 2 Combination: 17. 6% – Thrombocytopenia: Chemo: 2. 6% Combination: 5. 9%
Retrospective review – Post-chemo life-threatening haemorrhage: 5/32 (15. 6%) in localized group (stage IE/IIE 1) 4 chemo + 1 combination 9/23 in advanced group 6 chemo + 3 combination 5 of them developed perforation and died – Grade 3 -4 neutropenia: Chemo: 13. 2 Combination: 17. 6% – Thrombocytopenia: Chemo: 2. 6% Combination: 5. 9%
 Retrospective review - conclusion Clinical outcome of localized PGL treated by chemo alone is comparable to that treated by combination therapy In terms of : tumour response, disease-free survival and overall survival Bulky tumours: tumour bleeding/perforation Debulking surgery followed by chemo can offer better tumour control / ↓complication
Retrospective review - conclusion Clinical outcome of localized PGL treated by chemo alone is comparable to that treated by combination therapy In terms of : tumour response, disease-free survival and overall survival Bulky tumours: tumour bleeding/perforation Debulking surgery followed by chemo can offer better tumour control / ↓complication

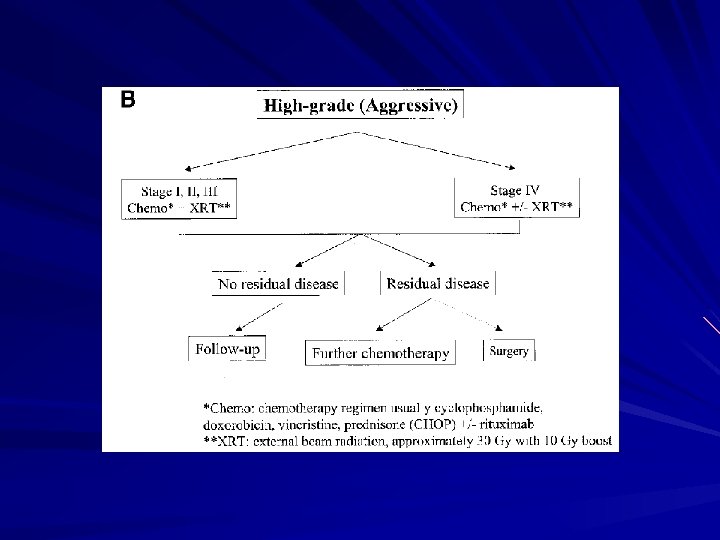
 Gastric lymphoma Rx MALT lymphoma – H pylori eradication therapy High-grade (non-MALT) – Chemo +/-RT – Surgery For bulky tumour to prevent bleeding/perforation For local residual disease post chemo/RT For palliation of symptoms like obstruction
Gastric lymphoma Rx MALT lymphoma – H pylori eradication therapy High-grade (non-MALT) – Chemo +/-RT – Surgery For bulky tumour to prevent bleeding/perforation For local residual disease post chemo/RT For palliation of symptoms like obstruction

 Some additional information For discussion
Some additional information For discussion
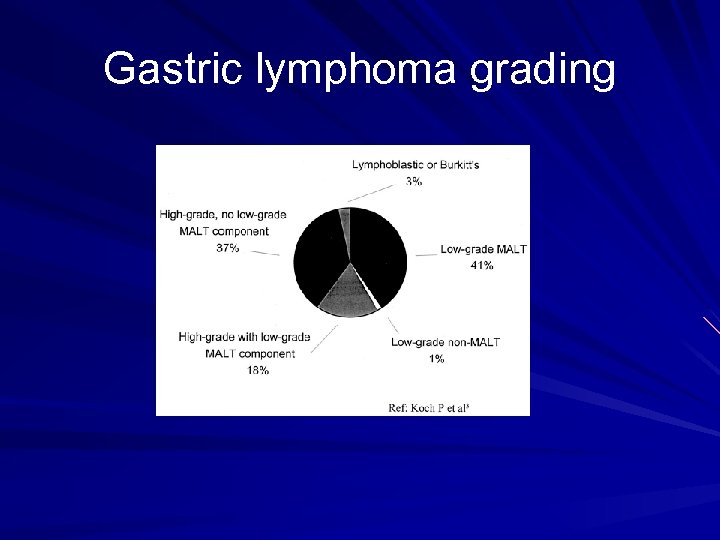 Gastric lymphoma grading
Gastric lymphoma grading
 International prognostic index 1) Age: <60 years vs. >60 years 2) Serum LDH: normal vs. elevated 3) Performance status: 0 or 1 vs. 2 -4 4) Stage: stage I / II vs. stage III / IV 5) Extranodal site involvement: 0 or 1 vs. 2 -4
International prognostic index 1) Age: <60 years vs. >60 years 2) Serum LDH: normal vs. elevated 3) Performance status: 0 or 1 vs. 2 -4 4) Stage: stage I / II vs. stage III / IV 5) Extranodal site involvement: 0 or 1 vs. 2 -4
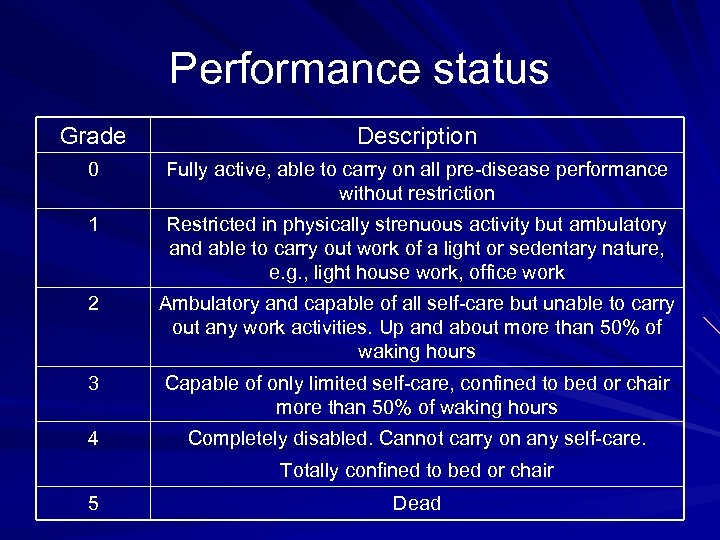 Performance status Grade Description 0 Fully active, able to carry on all pre-disease performance without restriction 1 Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e. g. , light house work, office work 2 Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours 3 Capable of only limited self-care, confined to bed or chair more than 50% of waking hours 4 Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair 5 Dead
Performance status Grade Description 0 Fully active, able to carry on all pre-disease performance without restriction 1 Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e. g. , light house work, office work 2 Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours 3 Capable of only limited self-care, confined to bed or chair more than 50% of waking hours 4 Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair 5 Dead
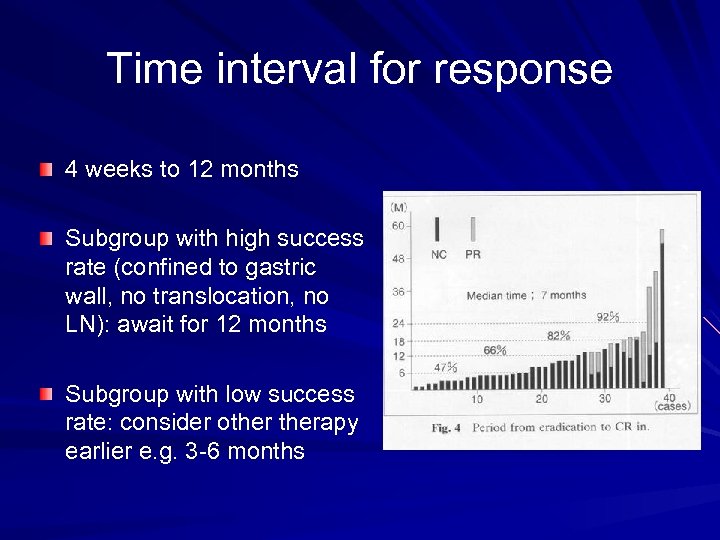 Time interval for response 4 weeks to 12 months Subgroup with high success rate (confined to gastric wall, no translocation, no LN): await for 12 months Subgroup with low success rate: consider otherapy earlier e. g. 3 -6 months
Time interval for response 4 weeks to 12 months Subgroup with high success rate (confined to gastric wall, no translocation, no LN): await for 12 months Subgroup with low success rate: consider otherapy earlier e. g. 3 -6 months
 Retrospective study
Retrospective study
 Retrospective study Objective: – To assess whether surgical excision is still a valid therapeutic option Patients and method: – A retrospective study – 1974 - 1999 – 69 consecutive patients stage IE-IIE – 65 (94. 2%) gastrectomy – Mean age: 62. 6 years (28 -85)
Retrospective study Objective: – To assess whether surgical excision is still a valid therapeutic option Patients and method: – A retrospective study – 1974 - 1999 – 69 consecutive patients stage IE-IIE – 65 (94. 2%) gastrectomy – Mean age: 62. 6 years (28 -85)
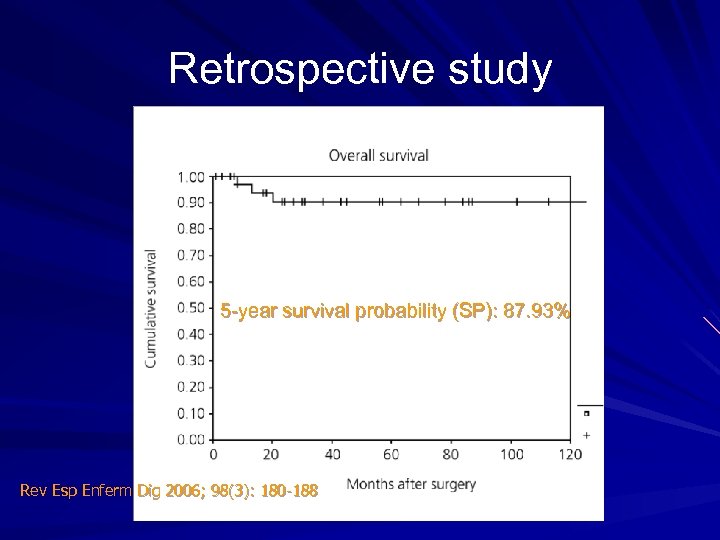 Retrospective study 5 -year survival probability (SP): 87. 93% Rev Esp Enferm Dig 2006; 98(3): 180 -188
Retrospective study 5 -year survival probability (SP): 87. 93% Rev Esp Enferm Dig 2006; 98(3): 180 -188
 Retrospective study Statistical analysis: – Ann Arbor stage: – Gastric wall invasion, H. pylori , margin: – Histological type: borderline significance (p = 0. 056)
Retrospective study Statistical analysis: – Ann Arbor stage: – Gastric wall invasion, H. pylori , margin: – Histological type: borderline significance (p = 0. 056)
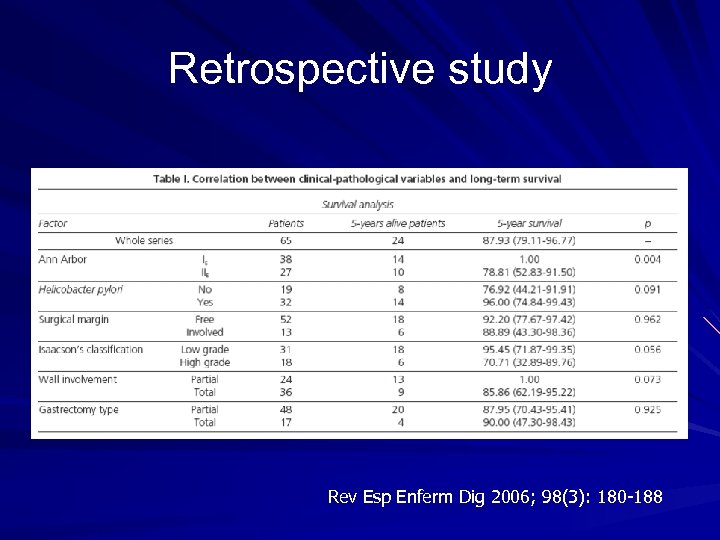 Retrospective study Rev Esp Enferm Dig 2006; 98(3): 180 -188
Retrospective study Rev Esp Enferm Dig 2006; 98(3): 180 -188
 Retrospective study - conclusion Good long-term survival (> 87% after 5 years) No prognostic value in surgical margin involvement. Radical excision (R 0), according to the criteria used in carcinomas, was not associated with a significantly longer survival than excisions leaving microscopic residual tumor (R 1).
Retrospective study - conclusion Good long-term survival (> 87% after 5 years) No prognostic value in surgical margin involvement. Radical excision (R 0), according to the criteria used in carcinomas, was not associated with a significantly longer survival than excisions leaving microscopic residual tumor (R 1).