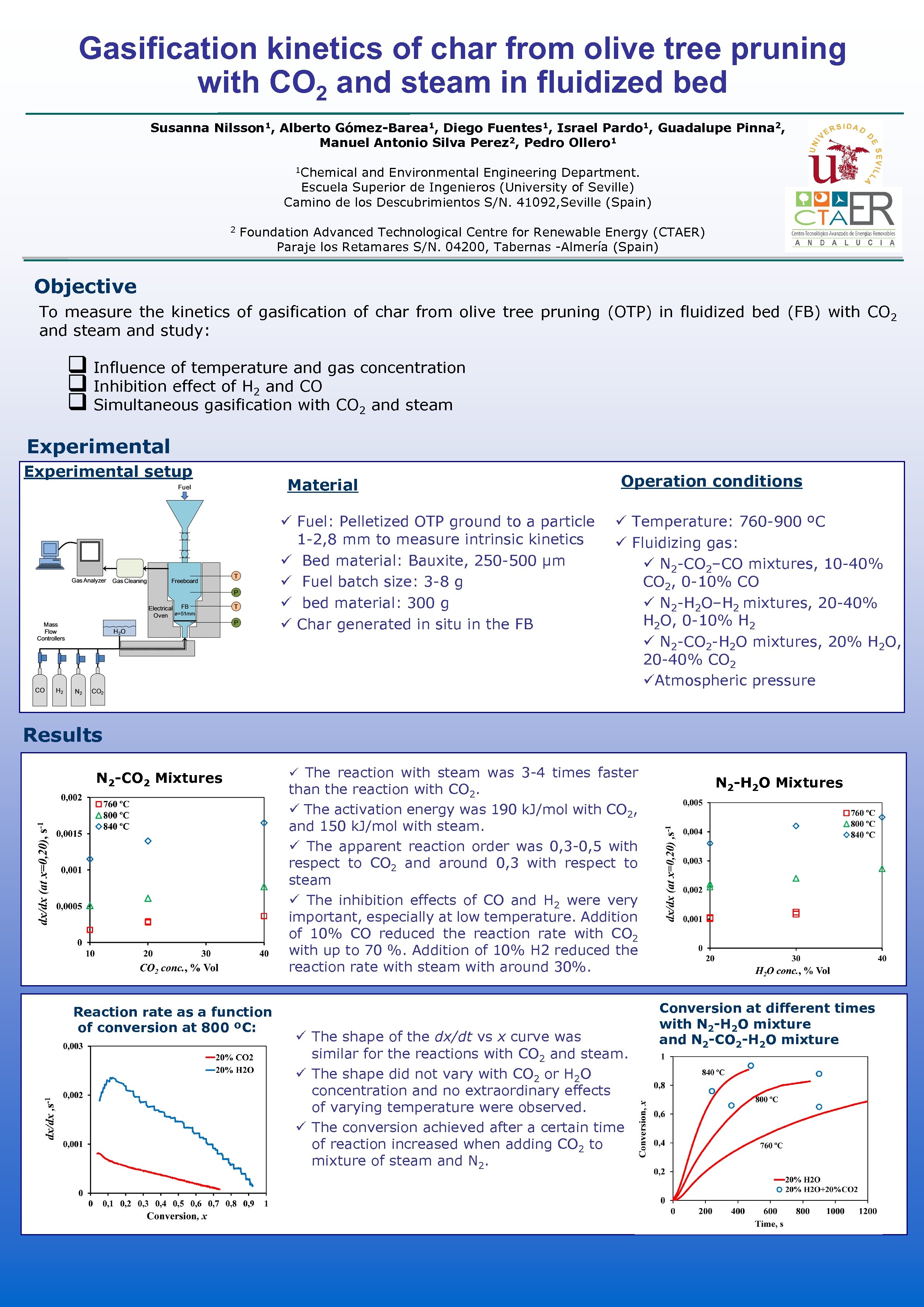
 Gasification kinetics of char from olive tree pruning with CO 2 and steam in fluidized bed Susanna Nilsson 1, Alberto Gómez-Barea 1, Diego Fuentes 1, Israel Pardo 1, Guadalupe Pinna 2, Manuel Antonio Silva Perez 2, Pedro Ollero 1 1 Chemical and Environmental Engineering Department. Escuela Superior de Ingenieros (University of Seville) Camino de los Descubrimientos S/N. 41092, Seville (Spain) 2 Foundation Advanced Technological Centre for Renewable Energy (CTAER) Paraje los Retamares S/N. 04200, Tabernas -Almería (Spain) Objective To measure the kinetics of gasification of char from olive tree pruning (OTP) in fluidized bed (FB) with CO 2 and steam and study: q Influence of temperature and gas concentration q Inhibition effect of H 2 and CO q Simultaneous gasification with CO 2 and steam Experimental setup Material ü Fuel: Pelletized OTP ground to a particle 1 -2, 8 mm to measure intrinsic kinetics ü Bed material: Bauxite, 250 -500 µm ü Fuel batch size: 3 -8 g ü bed material: 300 g ü Char generated in situ in the FB Operation conditions ü Temperature: 760 -900 ºC ü Fluidizing gas: ü N 2 -CO 2–CO mixtures, 10 -40% CO 2, 0 -10% CO ü N 2 -H 2 O–H 2 mixtures, 20 -40% H 2 O, 0 -10% H 2 ü N 2 -CO 2 -H 2 O mixtures, 20% H 2 O, 20 -40% CO 2 üAtmospheric pressure Results N 2 -CO 2 Mixtures Reaction rate as a function of conversion at 800 ºC: ü The reaction with steam was 3 -4 times faster than the reaction with CO 2. ü The activation energy was 190 k. J/mol with CO 2, and 150 k. J/mol with steam. ü The apparent reaction order was 0, 3 -0, 5 with respect to CO 2 and around 0, 3 with respect to steam ü The inhibition effects of CO and H 2 were very important, especially at low temperature. Addition of 10% CO reduced the reaction rate with CO 2 with up to 70 %. Addition of 10% H 2 reduced the reaction rate with steam with around 30%. ü The shape of the dx/dt vs x curve was similar for the reactions with CO 2 and steam. ü The shape did not vary with CO 2 or H 2 O concentration and no extraordinary effects of varying temperature were observed. ü The conversion achieved after a certain time of reaction increased when adding CO 2 to mixture of steam and N 2 -H 2 O Mixtures Conversion at different times with N 2 -H 2 O mixture and N 2 -CO 2 -H 2 O mixture
Gasification kinetics of char from olive tree pruning with CO 2 and steam in fluidized bed Susanna Nilsson 1, Alberto Gómez-Barea 1, Diego Fuentes 1, Israel Pardo 1, Guadalupe Pinna 2, Manuel Antonio Silva Perez 2, Pedro Ollero 1 1 Chemical and Environmental Engineering Department. Escuela Superior de Ingenieros (University of Seville) Camino de los Descubrimientos S/N. 41092, Seville (Spain) 2 Foundation Advanced Technological Centre for Renewable Energy (CTAER) Paraje los Retamares S/N. 04200, Tabernas -Almería (Spain) Objective To measure the kinetics of gasification of char from olive tree pruning (OTP) in fluidized bed (FB) with CO 2 and steam and study: q Influence of temperature and gas concentration q Inhibition effect of H 2 and CO q Simultaneous gasification with CO 2 and steam Experimental setup Material ü Fuel: Pelletized OTP ground to a particle 1 -2, 8 mm to measure intrinsic kinetics ü Bed material: Bauxite, 250 -500 µm ü Fuel batch size: 3 -8 g ü bed material: 300 g ü Char generated in situ in the FB Operation conditions ü Temperature: 760 -900 ºC ü Fluidizing gas: ü N 2 -CO 2–CO mixtures, 10 -40% CO 2, 0 -10% CO ü N 2 -H 2 O–H 2 mixtures, 20 -40% H 2 O, 0 -10% H 2 ü N 2 -CO 2 -H 2 O mixtures, 20% H 2 O, 20 -40% CO 2 üAtmospheric pressure Results N 2 -CO 2 Mixtures Reaction rate as a function of conversion at 800 ºC: ü The reaction with steam was 3 -4 times faster than the reaction with CO 2. ü The activation energy was 190 k. J/mol with CO 2, and 150 k. J/mol with steam. ü The apparent reaction order was 0, 3 -0, 5 with respect to CO 2 and around 0, 3 with respect to steam ü The inhibition effects of CO and H 2 were very important, especially at low temperature. Addition of 10% CO reduced the reaction rate with CO 2 with up to 70 %. Addition of 10% H 2 reduced the reaction rate with steam with around 30%. ü The shape of the dx/dt vs x curve was similar for the reactions with CO 2 and steam. ü The shape did not vary with CO 2 or H 2 O concentration and no extraordinary effects of varying temperature were observed. ü The conversion achieved after a certain time of reaction increased when adding CO 2 to mixture of steam and N 2 -H 2 O Mixtures Conversion at different times with N 2 -H 2 O mixture and N 2 -CO 2 -H 2 O mixture