39014e0dbb2c21c33c7e03a73246fcbd.ppt
- Количество слайдов: 14
 Gas Chromatography n Overview n Determining Identity n Quantitative Analysis
Gas Chromatography n Overview n Determining Identity n Quantitative Analysis
 Gas Chromatography n Gas Chromatography (GC) n A type of partition chromatography used to analyze very small quantities of volatile materials n Qualitative technique: § Can be used to identify compounds if standards are used n Quantitative technique: § Can be used to determine relative amounts of compounds present in a mixture
Gas Chromatography n Gas Chromatography (GC) n A type of partition chromatography used to analyze very small quantities of volatile materials n Qualitative technique: § Can be used to identify compounds if standards are used n Quantitative technique: § Can be used to determine relative amounts of compounds present in a mixture
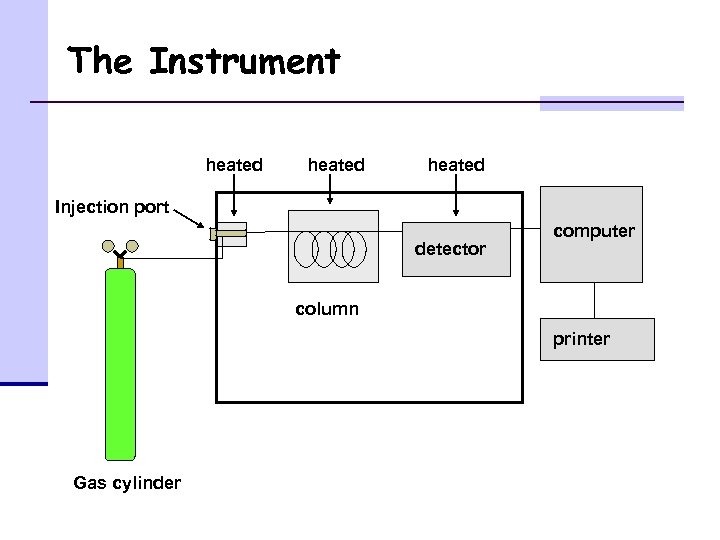 The Instrument heated Injection port detector computer column printer Gas cylinder
The Instrument heated Injection port detector computer column printer Gas cylinder
 Overview n During gas chromatography: n The injected sample vaporizes and is carried onto the column by an inert carrier gas (mobile phase) n Nitrogen n Helium n The sample adsorbs on the stationary phase, a nonvolatile liquid. n Usually liquid polymers with a high BP: § Polydimethylsiloxane § Carbowax (polyethylene glycol)
Overview n During gas chromatography: n The injected sample vaporizes and is carried onto the column by an inert carrier gas (mobile phase) n Nitrogen n Helium n The sample adsorbs on the stationary phase, a nonvolatile liquid. n Usually liquid polymers with a high BP: § Polydimethylsiloxane § Carbowax (polyethylene glycol)
 Overview n As the mobile phase passes across the stationary phase, the compounds in the mixture partition between the stationary phase and the mobile phase. n Partitioning depends on: n Column temperature n Gas flow rate n Affinity of the components for the stationary phase
Overview n As the mobile phase passes across the stationary phase, the compounds in the mixture partition between the stationary phase and the mobile phase. n Partitioning depends on: n Column temperature n Gas flow rate n Affinity of the components for the stationary phase
 Overview n Partitioning between the stationary and mobile phases depends on both a compound’s: n affinity for the stationary phase and n its vapor pressure or boiling point. n In general, compounds with low boiling points (and high vapor pressures) spend more time in the mobile phase and elute from the column in a shorter amount of time than compounds with high boiling points.
Overview n Partitioning between the stationary and mobile phases depends on both a compound’s: n affinity for the stationary phase and n its vapor pressure or boiling point. n In general, compounds with low boiling points (and high vapor pressures) spend more time in the mobile phase and elute from the column in a shorter amount of time than compounds with high boiling points.
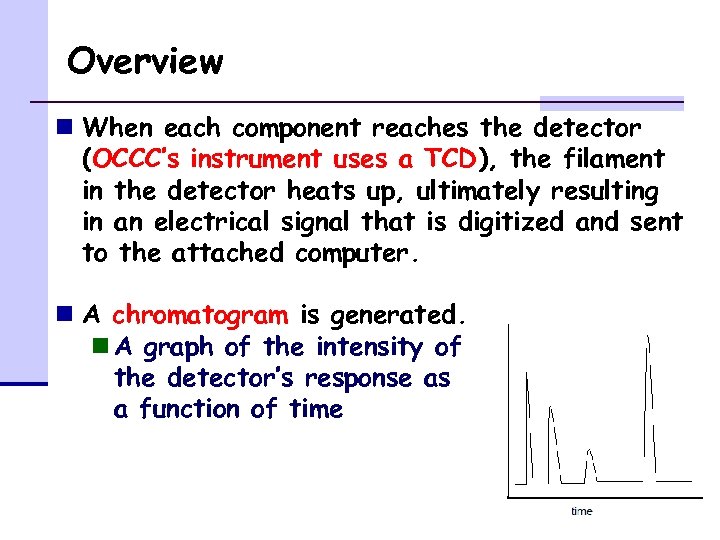 Overview n When each component reaches the detector (OCCC’s instrument uses a TCD), the filament in the detector heats up, ultimately resulting in an electrical signal that is digitized and sent to the attached computer. n A chromatogram is generated. n A graph of the intensity of the detector’s response as a function of time
Overview n When each component reaches the detector (OCCC’s instrument uses a TCD), the filament in the detector heats up, ultimately resulting in an electrical signal that is digitized and sent to the attached computer. n A chromatogram is generated. n A graph of the intensity of the detector’s response as a function of time
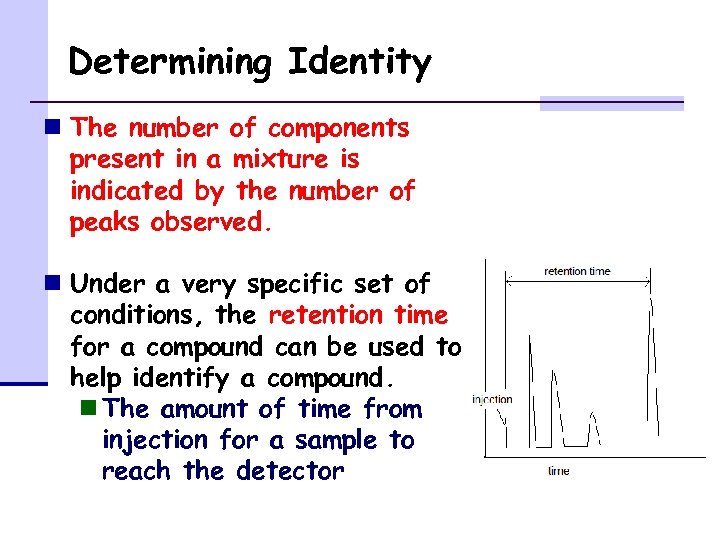 Determining Identity n The number of components present in a mixture is indicated by the number of peaks observed. n Under a very specific set of conditions, the retention time for a compound can be used to help identify a compound. n The amount of time from injection for a sample to reach the detector
Determining Identity n The number of components present in a mixture is indicated by the number of peaks observed. n Under a very specific set of conditions, the retention time for a compound can be used to help identify a compound. n The amount of time from injection for a sample to reach the detector
 Determining Identity n Retention time varies with changes in the identity of a compound and with experimental conditions including: n type and amount of stationary phase n length of column, n gas flow rate, n temperature profile n If the same experimental conditions are used, the retention time of standards can be used to identify the components of a mixture. n You must also have reason to expect that particular compound is present.
Determining Identity n Retention time varies with changes in the identity of a compound and with experimental conditions including: n type and amount of stationary phase n length of column, n gas flow rate, n temperature profile n If the same experimental conditions are used, the retention time of standards can be used to identify the components of a mixture. n You must also have reason to expect that particular compound is present.
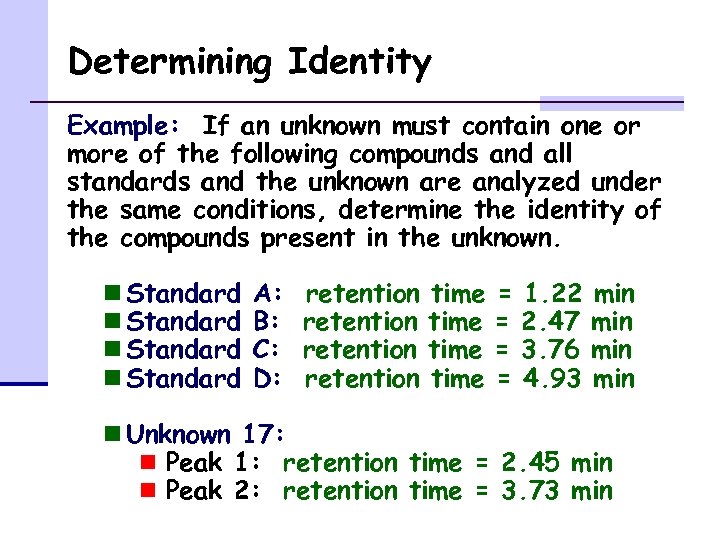 Determining Identity Example: If an unknown must contain one or more of the following compounds and all standards and the unknown are analyzed under the same conditions, determine the identity of the compounds present in the unknown. n Standard A: B: C: D: retention time = = 1. 22 2. 47 3. 76 4. 93 min min n Unknown 17: n Peak 1: retention time = 2. 45 min n Peak 2: retention time = 3. 73 min
Determining Identity Example: If an unknown must contain one or more of the following compounds and all standards and the unknown are analyzed under the same conditions, determine the identity of the compounds present in the unknown. n Standard A: B: C: D: retention time = = 1. 22 2. 47 3. 76 4. 93 min min n Unknown 17: n Peak 1: retention time = 2. 45 min n Peak 2: retention time = 3. 73 min
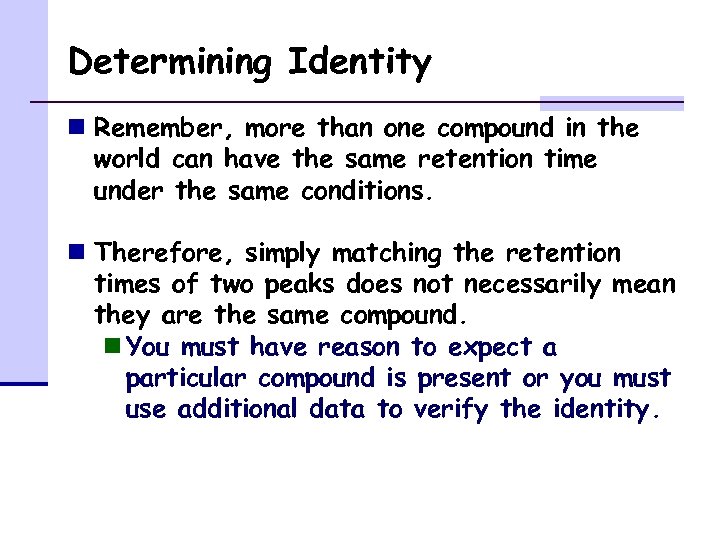 Determining Identity n Remember, more than one compound in the world can have the same retention time under the same conditions. n Therefore, simply matching the retention times of two peaks does not necessarily mean they are the same compound. n You must have reason to expect a particular compound is present or you must use additional data to verify the identity.
Determining Identity n Remember, more than one compound in the world can have the same retention time under the same conditions. n Therefore, simply matching the retention times of two peaks does not necessarily mean they are the same compound. n You must have reason to expect a particular compound is present or you must use additional data to verify the identity.
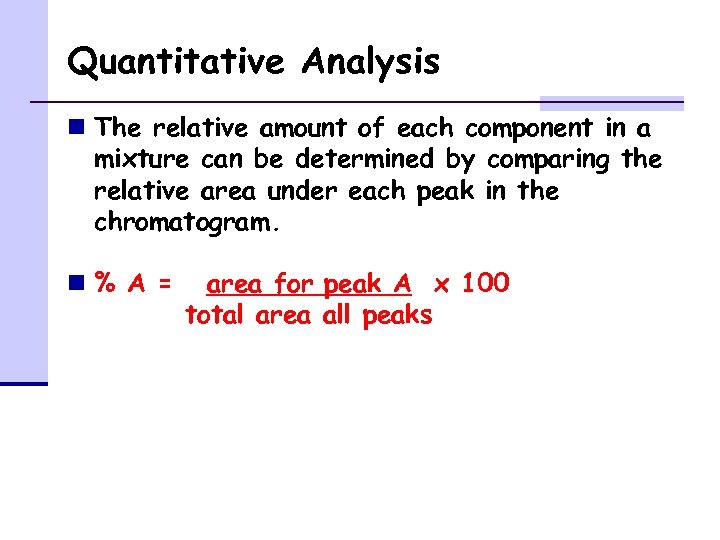 Quantitative Analysis n The relative amount of each component in a mixture can be determined by comparing the relative area under each peak in the chromatogram. n% A = area for peak A x 100 total area all peaks
Quantitative Analysis n The relative amount of each component in a mixture can be determined by comparing the relative area under each peak in the chromatogram. n% A = area for peak A x 100 total area all peaks
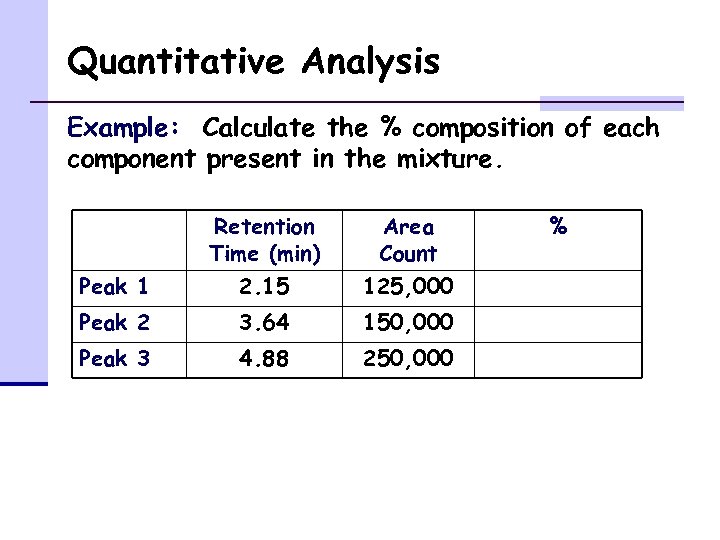 Quantitative Analysis Example: Calculate the % composition of each component present in the mixture. Retention Time (min) Area Count Peak 1 2. 15 125, 000 Peak 2 3. 64 150, 000 Peak 3 4. 88 250, 000 %
Quantitative Analysis Example: Calculate the % composition of each component present in the mixture. Retention Time (min) Area Count Peak 1 2. 15 125, 000 Peak 2 3. 64 150, 000 Peak 3 4. 88 250, 000 %
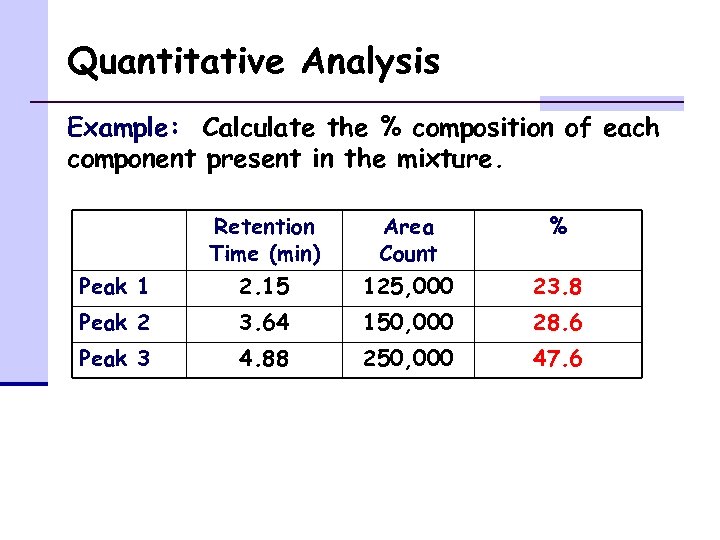 Quantitative Analysis Example: Calculate the % composition of each component present in the mixture. Retention Time (min) Area Count % Peak 1 2. 15 125, 000 23. 8 Peak 2 3. 64 150, 000 28. 6 Peak 3 4. 88 250, 000 47. 6
Quantitative Analysis Example: Calculate the % composition of each component present in the mixture. Retention Time (min) Area Count % Peak 1 2. 15 125, 000 23. 8 Peak 2 3. 64 150, 000 28. 6 Peak 3 4. 88 250, 000 47. 6


