aafbed1e3ccbbd89bd40183942c2084f.ppt
- Количество слайдов: 50

 GAMP/JETT AGENDA l l l Introduction and GAMP Organization GAMP Process Control Supplement Benefits of the GAMP/JETT Methodology Working Session Questions & Answers Dale Noteboom Jim John Chris Roerig JETT Team
GAMP/JETT AGENDA l l l Introduction and GAMP Organization GAMP Process Control Supplement Benefits of the GAMP/JETT Methodology Working Session Questions & Answers Dale Noteboom Jim John Chris Roerig JETT Team
 GAMP Forum Developments ~ 12 Special Interest Groups (SIG's) l GAMP Americas established Sept 2000 l – 8 New Special Interest Groups established l New groups incorporated into GAMP Forum – North American JETT Consortium joined (2000) – UK Suppliers Forum joining (2001)
GAMP Forum Developments ~ 12 Special Interest Groups (SIG's) l GAMP Americas established Sept 2000 l – 8 New Special Interest Groups established l New groups incorporated into GAMP Forum – North American JETT Consortium joined (2000) – UK Suppliers Forum joining (2001)
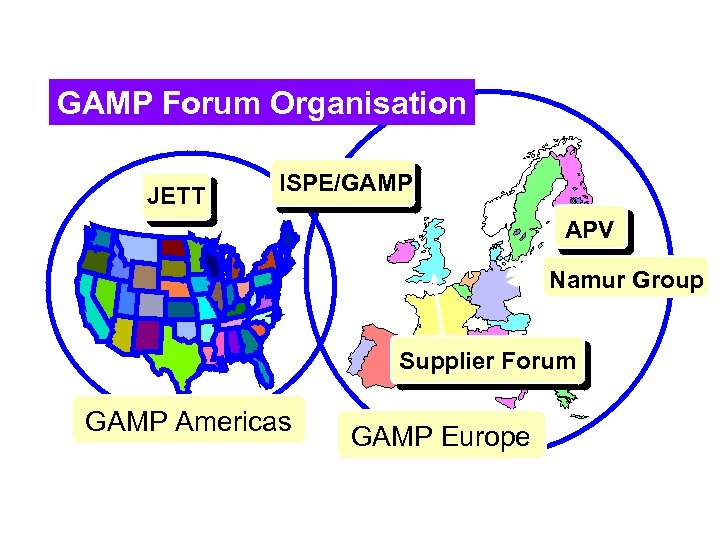 GAMP Forum Organisation JETT ISPE/GAMP APV Namur Group Supplier Forum GAMP Americas GAMP Europe
GAMP Forum Organisation JETT ISPE/GAMP APV Namur Group Supplier Forum GAMP Americas GAMP Europe
 Executive Summary of Good Automated Manufacturing Practice (GAMP) Guide
Executive Summary of Good Automated Manufacturing Practice (GAMP) Guide
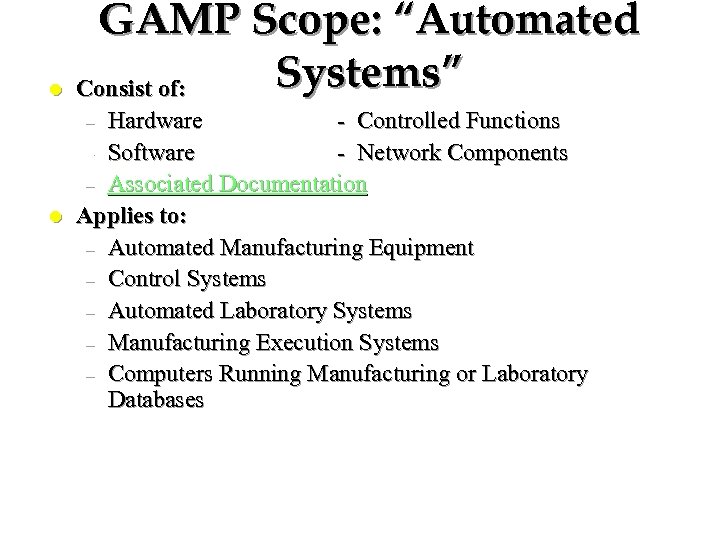 GAMP Scope: “Automated Systems” l Consist of: Hardware - Controlled Functions – Software - Network Components – Associated Documentation Applies to: – Automated Manufacturing Equipment – Control Systems – Automated Laboratory Systems – Manufacturing Execution Systems – Computers Running Manufacturing or Laboratory Databases – l
GAMP Scope: “Automated Systems” l Consist of: Hardware - Controlled Functions – Software - Network Components – Associated Documentation Applies to: – Automated Manufacturing Equipment – Control Systems – Automated Laboratory Systems – Manufacturing Execution Systems – Computers Running Manufacturing or Laboratory Databases – l
 GAMP Purpose l “Help suppliers of automated systems to the pharmaceutical industry ensure that systems are developed following good practice and to provide proper documentary evidence that their systems meet the agreed specifications. ”
GAMP Purpose l “Help suppliers of automated systems to the pharmaceutical industry ensure that systems are developed following good practice and to provide proper documentary evidence that their systems meet the agreed specifications. ”
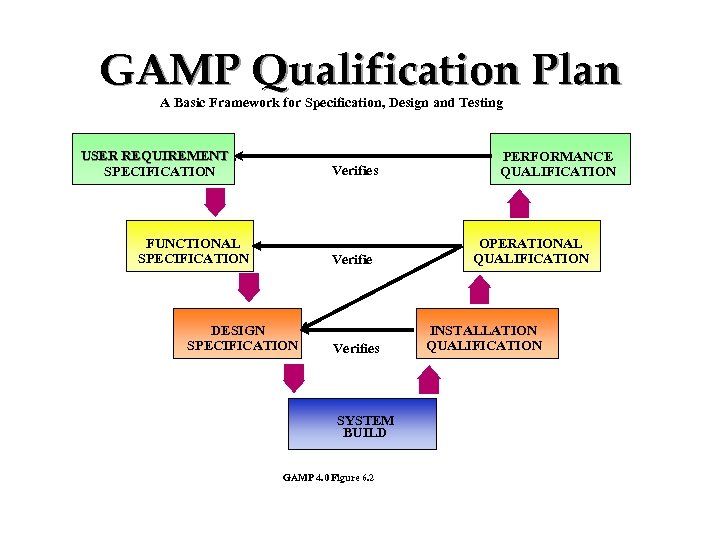 GAMP Qualification Plan A Basic Framework for Specification, Design and Testing USER REQUIREMENT SPECIFICATION Verifies FUNCTIONAL SPECIFICATION Verifies DESIGN SPECIFICATION Verifies SYSTEM BUILD GAMP 4. 0 Figure 6. 2 PERFORMANCE QUALIFICATION OPERATIONAL QUALIFICATION INSTALLATION QUALIFICATION
GAMP Qualification Plan A Basic Framework for Specification, Design and Testing USER REQUIREMENT SPECIFICATION Verifies FUNCTIONAL SPECIFICATION Verifies DESIGN SPECIFICATION Verifies SYSTEM BUILD GAMP 4. 0 Figure 6. 2 PERFORMANCE QUALIFICATION OPERATIONAL QUALIFICATION INSTALLATION QUALIFICATION
 Part 1: User Responsibilities l Validation (Master) Plan & System Specific Validation Plan(s) – l Supplier Audit – l Appendix 7 User Requirements Specification – l Appendix 6 Appendix 5 Supplier Education
Part 1: User Responsibilities l Validation (Master) Plan & System Specific Validation Plan(s) – l Supplier Audit – l Appendix 7 User Requirements Specification – l Appendix 6 Appendix 5 Supplier Education
 New Edition: GAMP 4 Strategic Framework Quality Management Procedures Practical Guidance (Good Practice) Training/Materials Workshop
New Edition: GAMP 4 Strategic Framework Quality Management Procedures Practical Guidance (Good Practice) Training/Materials Workshop
 GAMP 4 Goals l l l Software categories developed further Validation needs to be scaleable Global acceptance Examine the balance of work conducted by pharmaceutical manufacturers and their suppliers. Harmonization of terminology with other industry forums.
GAMP 4 Goals l l l Software categories developed further Validation needs to be scaleable Global acceptance Examine the balance of work conducted by pharmaceutical manufacturers and their suppliers. Harmonization of terminology with other industry forums.
 Best Practice Guides l First Wave – Calibration – IT Infrastructure: Networks, Desktop, – Harmonized Terminology – Electronic Records and Signatures
Best Practice Guides l First Wave – Calibration – IT Infrastructure: Networks, Desktop, – Harmonized Terminology – Electronic Records and Signatures
 Additional Planned Guides l Second Wave – Control Systems: including Standalone PLC/SCADA/DCS and Packaged Systems”/”Skid Mount Equipment” – Supplier Management – Analytical Laboratory Equipment – Global Systems: ERP, MRPII, LIMS, EDMS
Additional Planned Guides l Second Wave – Control Systems: including Standalone PLC/SCADA/DCS and Packaged Systems”/”Skid Mount Equipment” – Supplier Management – Analytical Laboratory Equipment – Global Systems: ERP, MRPII, LIMS, EDMS
 Additional Planned Guides l Third Wave – Legacy Systems – Clinical Systems – Medical Devices – E-Applications: Web-based software – Manufacturing Execution Systems
Additional Planned Guides l Third Wave – Legacy Systems – Clinical Systems – Medical Devices – E-Applications: Web-based software – Manufacturing Execution Systems
 GAMP Summary International guideline. l Good starter system. l Continuing to evolve (GAMP 4) l Basis for Regulatory Agency Training and expectations l Good “common ground” for CSV International Alignment l
GAMP Summary International guideline. l Good starter system. l Continuing to evolve (GAMP 4) l Basis for Regulatory Agency Training and expectations l Good “common ground” for CSV International Alignment l
 Joint Equipment Transition Team (www. JETTconsortium. com)
Joint Equipment Transition Team (www. JETTconsortium. com)
 Mission Statement l Improve communications between Users and Suppliers to more effectively meet the “validation” requirements of the pharmaceutical industry.
Mission Statement l Improve communications between Users and Suppliers to more effectively meet the “validation” requirements of the pharmaceutical industry.
 JETT MEMBERS USER Representatives • • • Abbott Labs – Tim Schuetter Pharmacia - Dale Noteboom Eli Lilly - Bret Fisk Aventis Behring – John Dexter Aventis Pasteur – Jeff O’Donel Perrigo – Paul Coury SUPPLIER Representatives • • Fisher-Rosemount – Jon Lustri Bosch /TL Systems - Terry Petro Vector Corp - Don Rosendale Rockwell – Mc. Carthy, Jiang BOC Edwards – Mike Stella Millipore – Ramon Le. Doux Invensys – Russell Regan CONSULTANT Reps • • VAI Automation - Chris Roerig Jacobs Engrg – Brokamp, Buede PV - Filary, Rivera, Lauderman PAC – Bruce Lauderman Fluor-Daniel – Mike Humphries BE&K Engr – Vince Miller Brock Solutions – John, Casey ~ 14 Active Members + 45 Assoc. Members & Growing
JETT MEMBERS USER Representatives • • • Abbott Labs – Tim Schuetter Pharmacia - Dale Noteboom Eli Lilly - Bret Fisk Aventis Behring – John Dexter Aventis Pasteur – Jeff O’Donel Perrigo – Paul Coury SUPPLIER Representatives • • Fisher-Rosemount – Jon Lustri Bosch /TL Systems - Terry Petro Vector Corp - Don Rosendale Rockwell – Mc. Carthy, Jiang BOC Edwards – Mike Stella Millipore – Ramon Le. Doux Invensys – Russell Regan CONSULTANT Reps • • VAI Automation - Chris Roerig Jacobs Engrg – Brokamp, Buede PV - Filary, Rivera, Lauderman PAC – Bruce Lauderman Fluor-Daniel – Mike Humphries BE&K Engr – Vince Miller Brock Solutions – John, Casey ~ 14 Active Members + 45 Assoc. Members & Growing
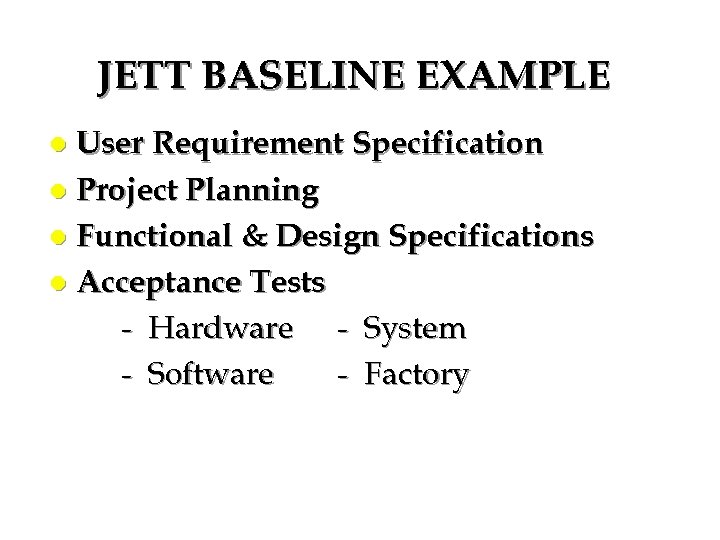 JETT BASELINE EXAMPLE User Requirement Specification l Project Planning l Functional & Design Specifications l Acceptance Tests - Hardware - System - Software - Factory l
JETT BASELINE EXAMPLE User Requirement Specification l Project Planning l Functional & Design Specifications l Acceptance Tests - Hardware - System - Software - Factory l
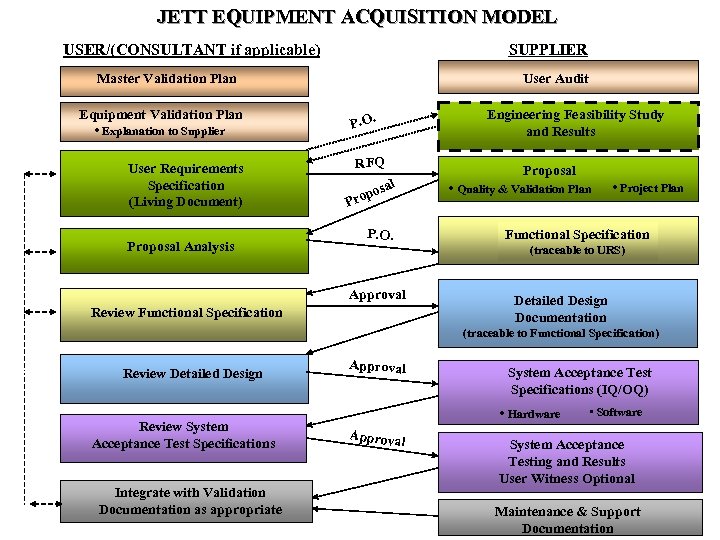 JETT EQUIPMENT ACQUISITION MODEL SUPPLIER USER/(CONSULTANT if applicable) User Audit Master Validation Plan Equipment Validation Plan • Explanation to Supplier User Requirements Specification (Living Document) Proposal Analysis Engineering Feasibility Study and Results P. O. RFQ Proposal al pos o • Quality & Validation Plan Pr P. O. • Project Plan Functional Specification (traceable to URS) Approval Review Functional Specification Detailed Design Documentation (traceable to Functional Specification) Review Detailed Design Review System Acceptance Test Specifications Integrate with Validation Documentation as appropriate Approval System Acceptance Test Specifications (IQ/OQ) • Hardware Approva l • Software System Acceptance Testing and Results User Witness Optional Maintenance & Support Documentation
JETT EQUIPMENT ACQUISITION MODEL SUPPLIER USER/(CONSULTANT if applicable) User Audit Master Validation Plan Equipment Validation Plan • Explanation to Supplier User Requirements Specification (Living Document) Proposal Analysis Engineering Feasibility Study and Results P. O. RFQ Proposal al pos o • Quality & Validation Plan Pr P. O. • Project Plan Functional Specification (traceable to URS) Approval Review Functional Specification Detailed Design Documentation (traceable to Functional Specification) Review Detailed Design Review System Acceptance Test Specifications Integrate with Validation Documentation as appropriate Approval System Acceptance Test Specifications (IQ/OQ) • Hardware Approva l • Software System Acceptance Testing and Results User Witness Optional Maintenance & Support Documentation
 JETT Efforts Applying GAMP to Automated Equipment l Communicating GAMP Approach l – – – Interphex 1997, 2000, & 2001 ISPE - Regional & National Meetings Pharmaceutical Online Articles Pharmaceutical Engineering Articles Institute of Validation Technology – Conferences and Articles – Published in VPCS Supplement to GAMP 4
JETT Efforts Applying GAMP to Automated Equipment l Communicating GAMP Approach l – – – Interphex 1997, 2000, & 2001 ISPE - Regional & National Meetings Pharmaceutical Online Articles Pharmaceutical Engineering Articles Institute of Validation Technology – Conferences and Articles – Published in VPCS Supplement to GAMP 4
 JETT Efforts Providing Input to GAMP Forum l Developing Guidance for Equipment URS’s, Equipment Validation Plans, Functional and Design Specifications and IQ/OQ’s l Working directly with Industry OEM’s l
JETT Efforts Providing Input to GAMP Forum l Developing Guidance for Equipment URS’s, Equipment Validation Plans, Functional and Design Specifications and IQ/OQ’s l Working directly with Industry OEM’s l
 JETT Efforts Equipment Validation Plan Template l URS Template l Common equipment URS examples and templates l Baseline example for Pure Steam Generator l – URS, FRS, HDS, SDS, FAT
JETT Efforts Equipment Validation Plan Template l URS Template l Common equipment URS examples and templates l Baseline example for Pure Steam Generator l – URS, FRS, HDS, SDS, FAT
 JETT Efforts l Released URS Documents Glassware Washers Vial Washer Saturated Steam Autoclave Barrier Isolator & HVAC System Label Rewinder Chromatography Labeler Pure Steam Generator Multiple-Effect Still
JETT Efforts l Released URS Documents Glassware Washers Vial Washer Saturated Steam Autoclave Barrier Isolator & HVAC System Label Rewinder Chromatography Labeler Pure Steam Generator Multiple-Effect Still
 JETT Efforts l URS Documents in development Granulators Centrifuge Wide Range Filler Tablet Press CIP Systems Variable Data Inspection Fluid Bed Dryer Tangential Flow Filtration System Bio. Reactors Building Management Systems Freeze Dryer Tablet Coater Purified Water System SCADA System Cappers Blender Depyrogenation Tunnel
JETT Efforts l URS Documents in development Granulators Centrifuge Wide Range Filler Tablet Press CIP Systems Variable Data Inspection Fluid Bed Dryer Tangential Flow Filtration System Bio. Reactors Building Management Systems Freeze Dryer Tablet Coater Purified Water System SCADA System Cappers Blender Depyrogenation Tunnel
 GAMP Process Control Supplement
GAMP Process Control Supplement
 GAMP Process Control Supplement - Purpose “This Guide is intended is a supplement to the GAMP Guide, and provides a harmonized overview of the key elements involved in the lifecycle of process control systems, from inception to retirement. As such, the Guide complements the current Baseline Guide on Commissioning and Qualification from ISPE. ”
GAMP Process Control Supplement - Purpose “This Guide is intended is a supplement to the GAMP Guide, and provides a harmonized overview of the key elements involved in the lifecycle of process control systems, from inception to retirement. As such, the Guide complements the current Baseline Guide on Commissioning and Qualification from ISPE. ”
 GAMP Process Control Supplement - Scope · Systems that control the manufacturing process, and have direct impact on product quality attributes at any stage in the life cycle. Product quality attributes include the identity, efficacy, strength, dosage, quality, disposition, safety, and purity of the product · Systems that process, transfer, or store process information in electronic format
GAMP Process Control Supplement - Scope · Systems that control the manufacturing process, and have direct impact on product quality attributes at any stage in the life cycle. Product quality attributes include the identity, efficacy, strength, dosage, quality, disposition, safety, and purity of the product · Systems that process, transfer, or store process information in electronic format
 GAMP Process Control Supplement - Benefits · Application and adaptation of the general principles of GAMP 4 to process control systems · A comprehensive overview of current best practice techniques for process control systems · Reduction of the cost and time required to achieve compliant process control systems · Application of good practice to the development and management of projects involving process control systems to meet regulatory expectations · Harmonized approaches for embedded as well as standalone systems · Detailed definition of engineering steps
GAMP Process Control Supplement - Benefits · Application and adaptation of the general principles of GAMP 4 to process control systems · A comprehensive overview of current best practice techniques for process control systems · Reduction of the cost and time required to achieve compliant process control systems · Application of good practice to the development and management of projects involving process control systems to meet regulatory expectations · Harmonized approaches for embedded as well as standalone systems · Detailed definition of engineering steps
 GAMP Process Control Supplement - Benefits Detailed guidance on the generation of user requirements specifications · Guidance on functionality and structures of process control systems as well as supplier services required · Guidance on the supplier services required for regulated environments · Avoids extensive and time-consuming retrospective validation of legacy process control systems, but provides for the application of a risk based approach, if required · Clarifies the collaboration between user and supplier · Guidance on incorporation of supplier documentation into the user validation documentation Modified and extended supplier audit to ensure compliance of the supplier’s development processes and documentation l
GAMP Process Control Supplement - Benefits Detailed guidance on the generation of user requirements specifications · Guidance on functionality and structures of process control systems as well as supplier services required · Guidance on the supplier services required for regulated environments · Avoids extensive and time-consuming retrospective validation of legacy process control systems, but provides for the application of a risk based approach, if required · Clarifies the collaboration between user and supplier · Guidance on incorporation of supplier documentation into the user validation documentation Modified and extended supplier audit to ensure compliance of the supplier’s development processes and documentation l
 GAMP Process Control Supplement - Release Global Introduction of the VPCS Guide ISPE Washington Continuing Advancement Conference – June 4 Arlington, VA
GAMP Process Control Supplement - Release Global Introduction of the VPCS Guide ISPE Washington Continuing Advancement Conference – June 4 Arlington, VA
 JETT Benefits Analysis Chris Roerig
JETT Benefits Analysis Chris Roerig
 Benefits of JETT Approach Provides Standards/Guidelines for ® Project Lifecycle ® Deliverables ® Documentation ® Approvals Industry Consistency
Benefits of JETT Approach Provides Standards/Guidelines for ® Project Lifecycle ® Deliverables ® Documentation ® Approvals Industry Consistency
 Benefits of JETT Approach "Speed to Market" ® Smoother Procurement Process ® Smoother Validation Process ® Shorter Project Schedule Reduced Project Costs ® Integration Services ® Validation ® Re-work
Benefits of JETT Approach "Speed to Market" ® Smoother Procurement Process ® Smoother Validation Process ® Shorter Project Schedule Reduced Project Costs ® Integration Services ® Validation ® Re-work
 Savings Analysis % of Purchase Price l User 5 -6 % savings – Gains: l l Qualification Protocol development & execution Life Cycle support (maintenance, upgrades) – Losses: l l Additional Auditing Validation Plan URS development Time Savings 3 - 14 weeks
Savings Analysis % of Purchase Price l User 5 -6 % savings – Gains: l l Qualification Protocol development & execution Life Cycle support (maintenance, upgrades) – Losses: l l Additional Auditing Validation Plan URS development Time Savings 3 - 14 weeks
 Savings Analysis % of Purchase Price l Supplier 3 - 6% savings – Gains: l l l Functional, Design, & Test spec development System production costs FAT – Losses l l Supplier Audits Time Savings 6 - 8 weeks
Savings Analysis % of Purchase Price l Supplier 3 - 6% savings – Gains: l l l Functional, Design, & Test spec development System production costs FAT – Losses l l Supplier Audits Time Savings 6 - 8 weeks
 Savings Analysis % of Purchase Price l Consultant 3 - 4 % savings – Gains: l l Functional, Design, & Test Spec Development FAT – Losses l l Integrator Audits Time savings 3 -10 weeks
Savings Analysis % of Purchase Price l Consultant 3 - 4 % savings – Gains: l l Functional, Design, & Test Spec Development FAT – Losses l l Integrator Audits Time savings 3 -10 weeks
 Real World Example Courtesy of: Dr. David Selby, David Begg Associates, Kirkbymoorside, N. Yorks, UK YO 6 6 AX
Real World Example Courtesy of: Dr. David Selby, David Begg Associates, Kirkbymoorside, N. Yorks, UK YO 6 6 AX
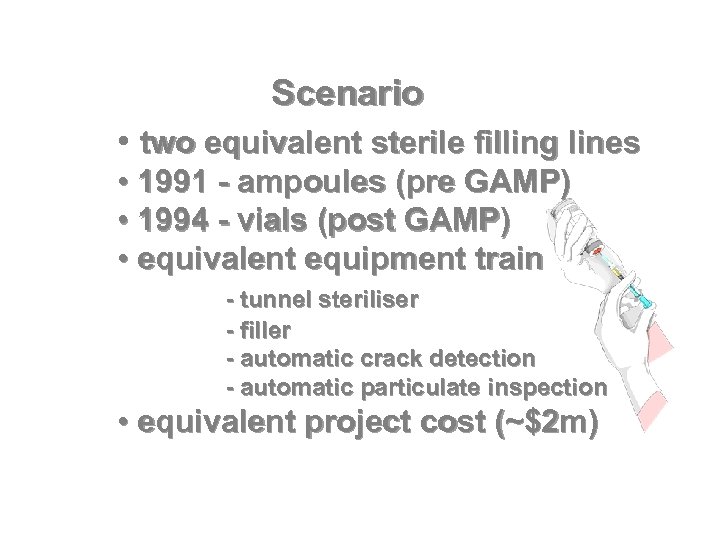 Scenario • two equivalent sterile filling lines • 1991 - ampoules (pre GAMP) • 1994 - vials (post GAMP) • equivalent equipment train - tunnel steriliser - filler - automatic crack detection - automatic particulate inspection • equivalent project cost (~$2 m)
Scenario • two equivalent sterile filling lines • 1991 - ampoules (pre GAMP) • 1994 - vials (post GAMP) • equivalent equipment train - tunnel steriliser - filler - automatic crack detection - automatic particulate inspection • equivalent project cost (~$2 m)
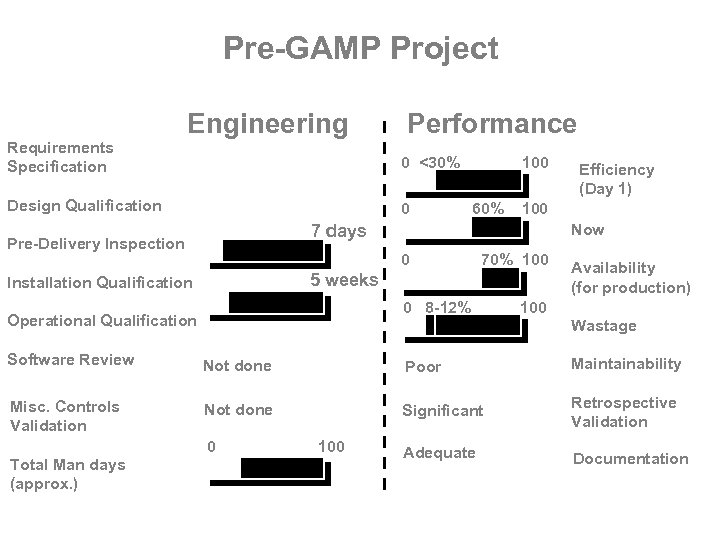 Pre-GAMP Project Requirements Specification Engineering Design Qualification Pre-Delivery Inspection Performance Minimal 0 <30% Not done 0 0 100 60% 100 7 days Now 0 70% 100 5 weeks Installation Qualification 0 0 8 -12% Operational Qualification Efficiency (Day 1) Availability (for production) 100 Wastage Software Review Not done Poor Maintainability Misc. Controls Validation Not done Significant Retrospective Validation Adequate Documentation Total Man days (approx. ) 0 100 30 days
Pre-GAMP Project Requirements Specification Engineering Design Qualification Pre-Delivery Inspection Performance Minimal 0 <30% Not done 0 0 100 60% 100 7 days Now 0 70% 100 5 weeks Installation Qualification 0 0 8 -12% Operational Qualification Efficiency (Day 1) Availability (for production) 100 Wastage Software Review Not done Poor Maintainability Misc. Controls Validation Not done Significant Retrospective Validation Adequate Documentation Total Man days (approx. ) 0 100 30 days
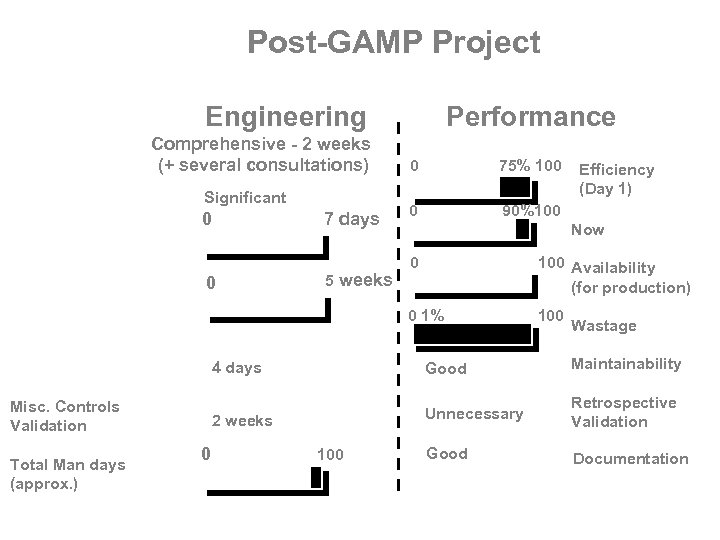 Post-GAMP Project Engineering Requirements Specification Comprehensive - 2 weeks (+ several consultations) Design Qualification Significant 0 7 days Performance 0 75% 100 0 90%100 Now Pre-Delivery Inspection Operational Qualification Software Review 0 100 Availability (for production) 0 1% 5 weeks Installation Qualification 0 100 Wastage 4 days Good Maintainability 2 weeks Misc. Controls Validation Total Man days (approx. ) Efficiency (Day 1) Unnecessary Retrospective Validation Good Documentation 0 100 90 days
Post-GAMP Project Engineering Requirements Specification Comprehensive - 2 weeks (+ several consultations) Design Qualification Significant 0 7 days Performance 0 75% 100 0 90%100 Now Pre-Delivery Inspection Operational Qualification Software Review 0 100 Availability (for production) 0 1% 5 weeks Installation Qualification 0 100 Wastage 4 days Good Maintainability 2 weeks Misc. Controls Validation Total Man days (approx. ) Efficiency (Day 1) Unnecessary Retrospective Validation Good Documentation 0 100 90 days
 Summary To derive benefit when validating automated systems : - post GAMP 0 • adopt “good engineering practices” • adopt the common approach (GAMP) • manage the benefits through • measure them 75% 0 90% 0 100% 0 1%
Summary To derive benefit when validating automated systems : - post GAMP 0 • adopt “good engineering practices” • adopt the common approach (GAMP) • manage the benefits through • measure them 75% 0 90% 0 100% 0 1%
 How Do I Get Started? l JETT Web Site – www. jettconsortium. com GAMP Web Site l Contact JETT Members for Help l Start with Baseline Piece of Equip. l Promote with Users, QA, & Suppliers l Integrate in your Stds and Methods l
How Do I Get Started? l JETT Web Site – www. jettconsortium. com GAMP Web Site l Contact JETT Members for Help l Start with Baseline Piece of Equip. l Promote with Users, QA, & Suppliers l Integrate in your Stds and Methods l
 Working Session l Break into functional groups – – l l l QA/Validation Manufacturing/Operations Environmental/Safety/Utilities/Maintenance Engineering Select a piece of OEM equipment (simple) Develop portions of a URS Develop portions of project validation plan
Working Session l Break into functional groups – – l l l QA/Validation Manufacturing/Operations Environmental/Safety/Utilities/Maintenance Engineering Select a piece of OEM equipment (simple) Develop portions of a URS Develop portions of project validation plan
 URS Guidelines l Each statement: – Uniquely referenced – Less than 250 words l l l Express requirements, not design solution Each requirement should be testable URS should be understandable by user and supplier – – l No ambiguity No contradictions Distinguish between mandatory and desirable items/requirements
URS Guidelines l Each statement: – Uniquely referenced – Less than 250 words l l l Express requirements, not design solution Each requirement should be testable URS should be understandable by user and supplier – – l No ambiguity No contradictions Distinguish between mandatory and desirable items/requirements
 URS Content Checklist ü Functions required Ø Product requirement Ø Functional requirement Ø Design requirement ü ü ü Modes of operation Performance and timing Failure actions Ø Hardware Ø Software
URS Content Checklist ü Functions required Ø Product requirement Ø Functional requirement Ø Design requirement ü ü ü Modes of operation Performance and timing Failure actions Ø Hardware Ø Software
 URS Content Checklist ü Safety and security ü Data ØArchive ØCapacity ØSpeed ØDefinition of data and valid ranges ü Interfaces ü Environment
URS Content Checklist ü Safety and security ü Data ØArchive ØCapacity ØSpeed ØDefinition of data and valid ranges ü Interfaces ü Environment
 URS Constraints l l l Schedule Compatibility with existing networks, hardware, etc. Reliability requirements Legal issues, working methods, user skill levels, etc. Maintenance – – Ease of maintenance Expansion capability Expected lifetime Long-term support
URS Constraints l l l Schedule Compatibility with existing networks, hardware, etc. Reliability requirements Legal issues, working methods, user skill levels, etc. Maintenance – – Ease of maintenance Expansion capability Expected lifetime Long-term support
 URS Lifecycle l l Development – e. g. project management/QA/mandatory design methods Testing – – – l Special testing under load conditions Test data Simulations Delivery – Shipment directions – Documents – what supplier is expected to deliver
URS Lifecycle l l Development – e. g. project management/QA/mandatory design methods Testing – – – l Special testing under load conditions Test data Simulations Delivery – Shipment directions – Documents – what supplier is expected to deliver
 URS Lifecycle Tools l Training l – Engineering – Operations – Maintenance l Support from vendor after: – FAT – Validation complete
URS Lifecycle Tools l Training l – Engineering – Operations – Maintenance l Support from vendor after: – FAT – Validation complete


