a66680495286be059b2388fd93878e96.ppt
- Количество слайдов: 76
 Funding Opportunities at Wellcome Fiona Mac. Laughlin Heather Chaffey
Funding Opportunities at Wellcome Fiona Mac. Laughlin Heather Chaffey
 The Wellcome Trust • independent research-funding charity • largest charity in UK • established 1936 • founder of company that developed AZT, FMD and Rubella • funded from private endowment valued at £ 15 billion (March 2008) • managed for long-term stability and growth • interests range from science to history of medicine
The Wellcome Trust • independent research-funding charity • largest charity in UK • established 1936 • founder of company that developed AZT, FMD and Rubella • funded from private endowment valued at £ 15 billion (March 2008) • managed for long-term stability and growth • interests range from science to history of medicine
 Mission and strategy “To foster and promote research with the aim of improving human and animal health”
Mission and strategy “To foster and promote research with the aim of improving human and animal health”
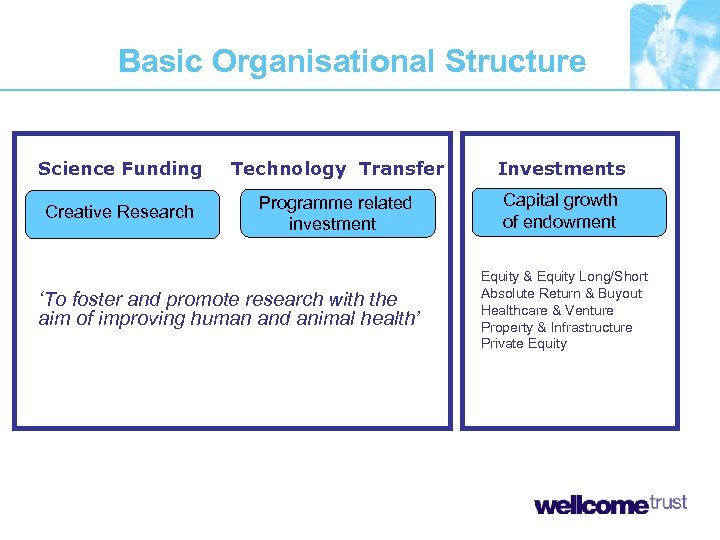 Basic Organisational Structure Science Funding Technology Transfer Investments Creative Research Programme related investment Capital growth of endowment ‘To foster and promote research with the aim of improving human and animal health’ Equity & Equity Long/Short Absolute Return & Buyout Healthcare & Venture Property & Infrastructure Private Equity
Basic Organisational Structure Science Funding Technology Transfer Investments Creative Research Programme related investment Capital growth of endowment ‘To foster and promote research with the aim of improving human and animal health’ Equity & Equity Long/Short Absolute Return & Buyout Healthcare & Venture Property & Infrastructure Private Equity
 Wellcome Trust Philosophy • spend up to £ 4 bn over next 5 years • how we work: • people & ideas - focus on individuals • programmes - big & brave grants • places - fund where we can make a difference • partnership - strategic partnerships • a committed funder of translational research • public engagement that aims to create an informed climate within which biomedical science can flourish
Wellcome Trust Philosophy • spend up to £ 4 bn over next 5 years • how we work: • people & ideas - focus on individuals • programmes - big & brave grants • places - fund where we can make a difference • partnership - strategic partnerships • a committed funder of translational research • public engagement that aims to create an informed climate within which biomedical science can flourish
 Improving innovation Translational medicine Key to an innovative healthcare and pharmaceutical industry we must: • develop bench to bedside – bedside to bench approaches • optimize academia & industry links • develop innovative public private partnerships • reinvigorate undergraduate medical curriculum • provide flexible training routes • streamline approval processes
Improving innovation Translational medicine Key to an innovative healthcare and pharmaceutical industry we must: • develop bench to bedside – bedside to bench approaches • optimize academia & industry links • develop innovative public private partnerships • reinvigorate undergraduate medical curriculum • provide flexible training routes • streamline approval processes
 Dr Heather Chaffey Grants Adviser, Neuroscience & Mental Health Grants Management Department (t) +44 (0)20 7611 8786 (e) h. chaffey@wellcome. ac. uk
Dr Heather Chaffey Grants Adviser, Neuroscience & Mental Health Grants Management Department (t) +44 (0)20 7611 8786 (e) h. chaffey@wellcome. ac. uk
 Overview • What we fund • Funding schemes • General information • The application process - background & tips
Overview • What we fund • Funding schemes • General information • The application process - background & tips
 What do we fund? • Biomedical and Veterinary Research • Technology Transfer Activities • History of Medicine Research • Biomedical Ethics Research • Public Engagement Activity • Direct Activities (e. g. Sanger Institute)
What do we fund? • Biomedical and Veterinary Research • Technology Transfer Activities • History of Medicine Research • Biomedical Ethics Research • Public Engagement Activity • Direct Activities (e. g. Sanger Institute)
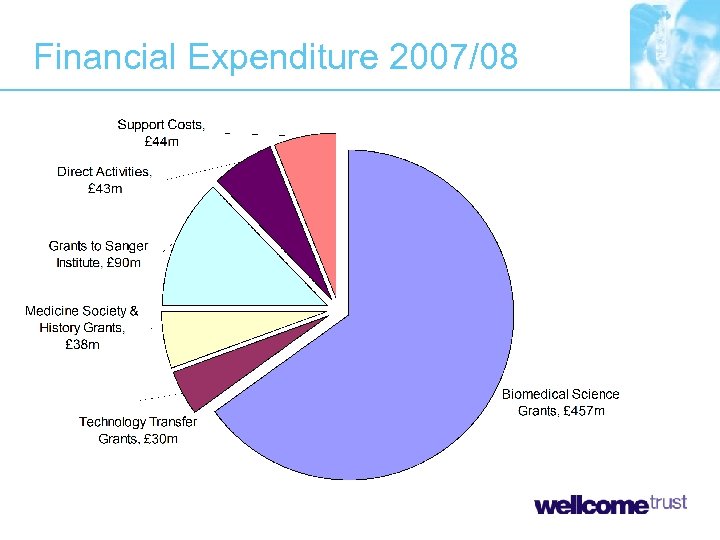 Financial Expenditure 2007/08
Financial Expenditure 2007/08
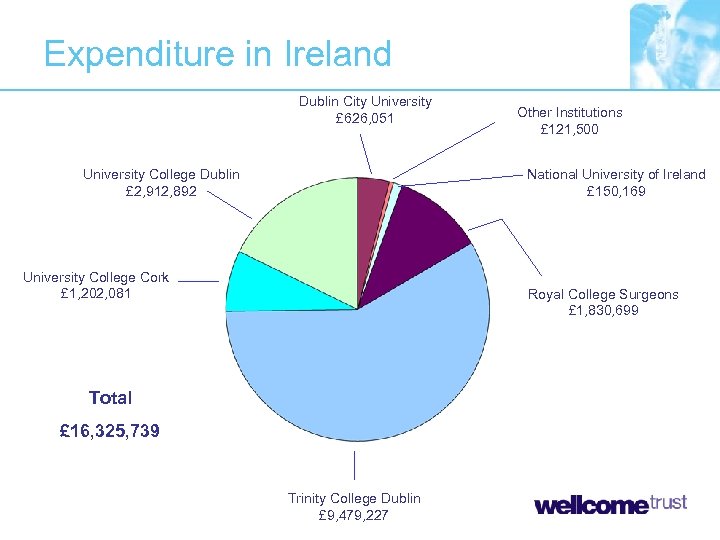 Expenditure in Ireland Dublin City University £ 626, 051 University College Dublin £ 2, 912, 892 Other Institutions £ 121, 500 National University of Ireland £ 150, 169 University College Cork £ 1, 202, 081 Royal College Surgeons £ 1, 830, 699 Total £ 16, 325, 739 Trinity College Dublin £ 9, 479, 227
Expenditure in Ireland Dublin City University £ 626, 051 University College Dublin £ 2, 912, 892 Other Institutions £ 121, 500 National University of Ireland £ 150, 169 University College Cork £ 1, 202, 081 Royal College Surgeons £ 1, 830, 699 Total £ 16, 325, 739 Trinity College Dublin £ 9, 479, 227
 Funding Schemes • Response - Mode Funding • Strategic Award Funding • Fellowships
Funding Schemes • Response - Mode Funding • Strategic Award Funding • Fellowships
 Response - Mode Funding
Response - Mode Funding
 Eligibility Requirements • Hold a research post in an eligible organisation • Have 5 years postdoctoral research experience • Must be able to sign up to the Trust’s grant conditions
Eligibility Requirements • Hold a research post in an eligible organisation • Have 5 years postdoctoral research experience • Must be able to sign up to the Trust’s grant conditions
 Project Grants • High-quality, hypothesis-driven research • Salaries for 1 -2 posts plus equipment, travel and materials for up to 3 years • Awards in the region of £ 150 -£ 400 k. • Support for pilot studies and international collaborations
Project Grants • High-quality, hypothesis-driven research • Salaries for 1 -2 posts plus equipment, travel and materials for up to 3 years • Awards in the region of £ 150 -£ 400 k. • Support for pilot studies and international collaborations
 Programme Grants • Internationally competitive research programmes • Proven track record of research and funding. • Salaries for 3 -4 posts for 5 years plus equipment, travel & materials • Awards typically in the region of £ 900 k-£ 1. 5 m I
Programme Grants • Internationally competitive research programmes • Proven track record of research and funding. • Salaries for 3 -4 posts for 5 years plus equipment, travel & materials • Awards typically in the region of £ 900 k-£ 1. 5 m I
 Other Schemes • Equipment, Biomedical Resources & Technology Development Grants • Flexible Travel Awards: Sabbaticals
Other Schemes • Equipment, Biomedical Resources & Technology Development Grants • Flexible Travel Awards: Sabbaticals
 Strategic Awards • Flexible forms of support to facilitate research &/or training. • To 'add value' to excellent research groups. • Must involve a partnership with the host institution. • Normally for 5 years. • Open rolling call for proposals but the Trust may highlight particular areas of research.
Strategic Awards • Flexible forms of support to facilitate research &/or training. • To 'add value' to excellent research groups. • Must involve a partnership with the host institution. • Normally for 5 years. • Open rolling call for proposals but the Trust may highlight particular areas of research.
 Strategic & Themed Initiatives • African Institutions Initiative • Medical Engineering • Genome-wide Association Studies in Disease • Research in Neurodegenerative Diseases • Capital Awards in Biomedical Science
Strategic & Themed Initiatives • African Institutions Initiative • Medical Engineering • Genome-wide Association Studies in Disease • Research in Neurodegenerative Diseases • Capital Awards in Biomedical Science
 Fellowships
Fellowships
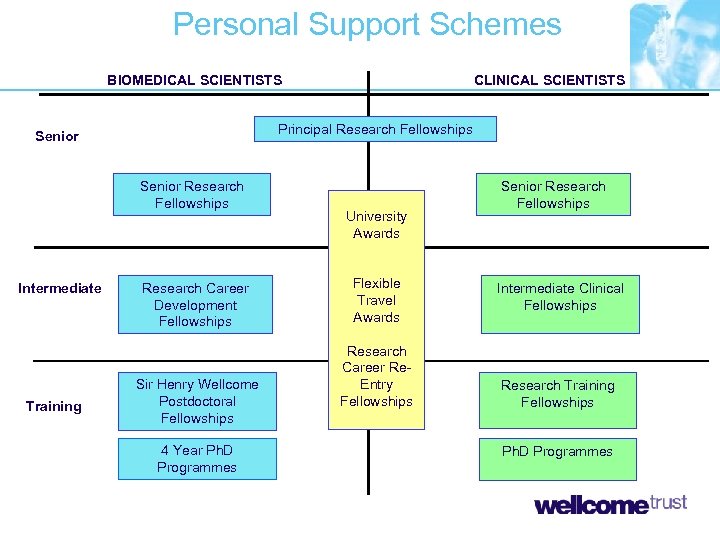 Personal Support Schemes BIOMEDICAL SCIENTISTS Principal Research Fellowships Senior Research Fellowships Intermediate Training CLINICAL SCIENTISTS Research Career Development Fellowships Sir Henry Wellcome Postdoctoral Fellowships 4 Year Ph. D Programmes University Awards Flexible Travel Awards Research Career Re. Entry Fellowships Senior Research Fellowships Intermediate Clinical Fellowships Research Training Fellowships Ph. D Programmes
Personal Support Schemes BIOMEDICAL SCIENTISTS Principal Research Fellowships Senior Research Fellowships Intermediate Training CLINICAL SCIENTISTS Research Career Development Fellowships Sir Henry Wellcome Postdoctoral Fellowships 4 Year Ph. D Programmes University Awards Flexible Travel Awards Research Career Re. Entry Fellowships Senior Research Fellowships Intermediate Clinical Fellowships Research Training Fellowships Ph. D Programmes
 Schemes for nonclinical scientists
Schemes for nonclinical scientists
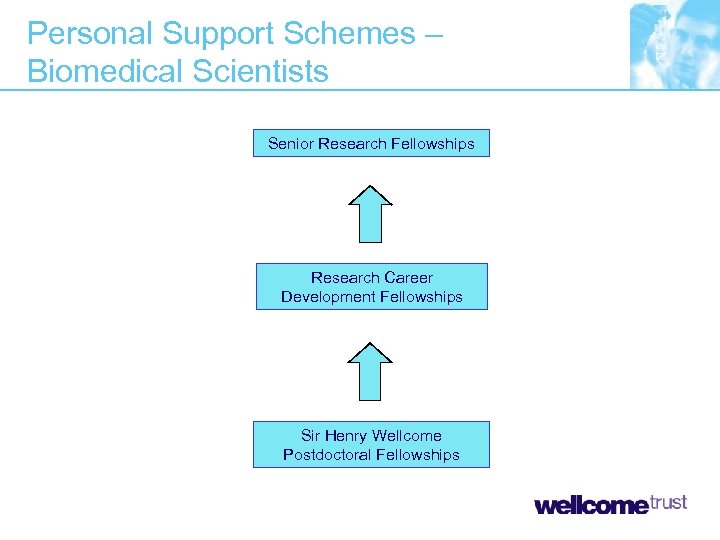 Personal Support Schemes – Biomedical Scientists Senior Research Fellowships Research Career Development Fellowships Sir Henry Wellcome Postdoctoral Fellowships
Personal Support Schemes – Biomedical Scientists Senior Research Fellowships Research Career Development Fellowships Sir Henry Wellcome Postdoctoral Fellowships
 Schemes for clinically and veterinary qualified scientists
Schemes for clinically and veterinary qualified scientists
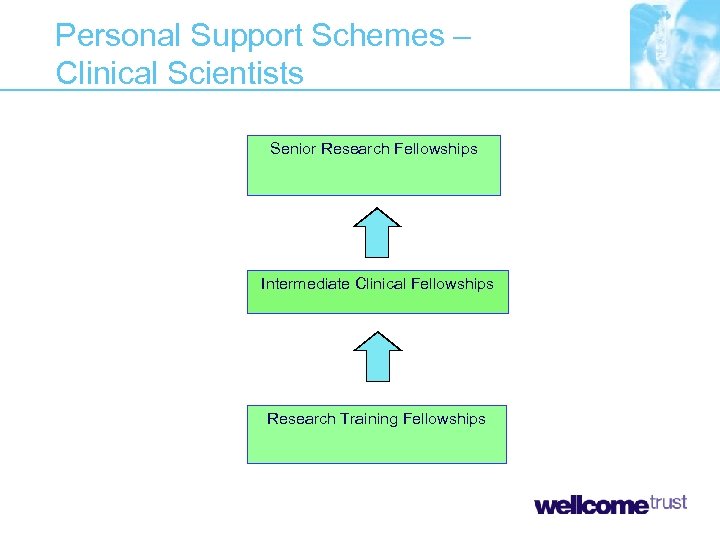 Personal Support Schemes – Clinical Scientists Senior Research Fellowships Intermediate Clinical Fellowships Research Training Fellowships
Personal Support Schemes – Clinical Scientists Senior Research Fellowships Intermediate Clinical Fellowships Research Training Fellowships
 Other Personal Support Schemes • Flexible Travel Awards: Fellowships • Research Career Re-entry Fellowships • University Awards
Other Personal Support Schemes • Flexible Travel Awards: Fellowships • Research Career Re-entry Fellowships • University Awards
 Alternatives • Request personal salary support on a project grant
Alternatives • Request personal salary support on a project grant
 General Information
General Information
 What funding do we provide? • Directly incurred costs of research. We do not fund on a proportion of f. EC • Travel allowances • Flexible funding allowance • We also fund some additional costs, including: – – animal house facility f. EC charge out rates open access publishing – national and international resources
What funding do we provide? • Directly incurred costs of research. We do not fund on a proportion of f. EC • Travel allowances • Flexible funding allowance • We also fund some additional costs, including: – – animal house facility f. EC charge out rates open access publishing – national and international resources
 The Application Process – Background & Tips
The Application Process – Background & Tips
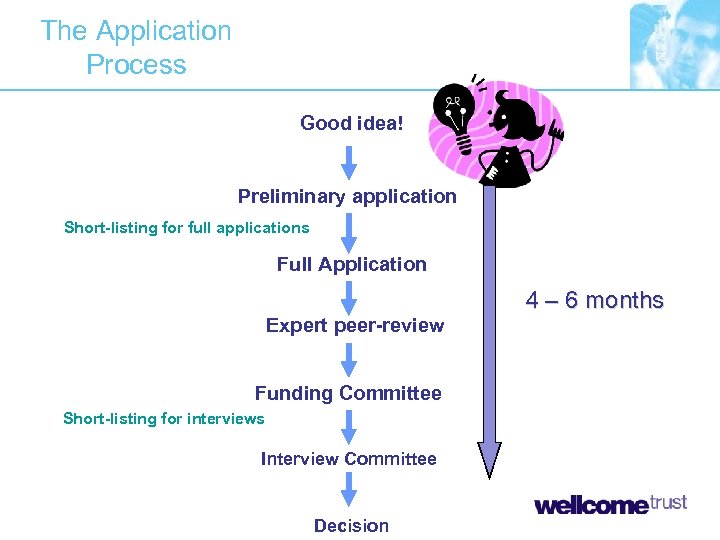 The Application Process Good idea! Preliminary application Short-listing for full applications Full Application 4 – 6 months Expert peer-review Funding Committee Short-listing for interviews Interview Committee Decision
The Application Process Good idea! Preliminary application Short-listing for full applications Full Application 4 – 6 months Expert peer-review Funding Committee Short-listing for interviews Interview Committee Decision
 Writing the Application
Writing the Application
 A ‘Good’ Application • A strong and original central hypothesis • Evident knowledge of the area and appropriate expertise • Clear research plan/easy to understand • Convincing preliminary data • Not over- or under- ambitious • All staff, equipment & animals carefully justified • Seek advice!
A ‘Good’ Application • A strong and original central hypothesis • Evident knowledge of the area and appropriate expertise • Clear research plan/easy to understand • Convincing preliminary data • Not over- or under- ambitious • All staff, equipment & animals carefully justified • Seek advice!
 “. . . it involves techniques with which the applicant appears to have no prior experience and for which no preliminary data are proposed. ” “. . . the work described in this application is over-ambitious, it could not be achieved in the life time of the investigator. ” “The poor writing, referencing and proof reading of this application significantly detract from its overall quality. ” “I had only one problem with this application, I had no idea what they were trying to do. . . ”
“. . . it involves techniques with which the applicant appears to have no prior experience and for which no preliminary data are proposed. ” “. . . the work described in this application is over-ambitious, it could not be achieved in the life time of the investigator. ” “The poor writing, referencing and proof reading of this application significantly detract from its overall quality. ” “I had only one problem with this application, I had no idea what they were trying to do. . . ”
 Peer Review Assessment • Importance of the problem • Strengths and weaknesses of application - quality of science, feasibility • Standing of applicants – track record • Resources requested – are they appropriate? • Fellowships: person, project, place
Peer Review Assessment • Importance of the problem • Strengths and weaknesses of application - quality of science, feasibility • Standing of applicants – track record • Resources requested – are they appropriate? • Fellowships: person, project, place
 Thank you! Dr Heather Chaffey Grants Management Department H. Chaffey@wellcome. ac. uk
Thank you! Dr Heather Chaffey Grants Management Department H. Chaffey@wellcome. ac. uk
 techtransfer@wellcome Purpose To maximise the impact of research innovations on health by facilitating their route to the ‘market’ • early-stage R&D funder • focused on funding gaps • motivated by public good
techtransfer@wellcome Purpose To maximise the impact of research innovations on health by facilitating their route to the ‘market’ • early-stage R&D funder • focused on funding gaps • motivated by public good
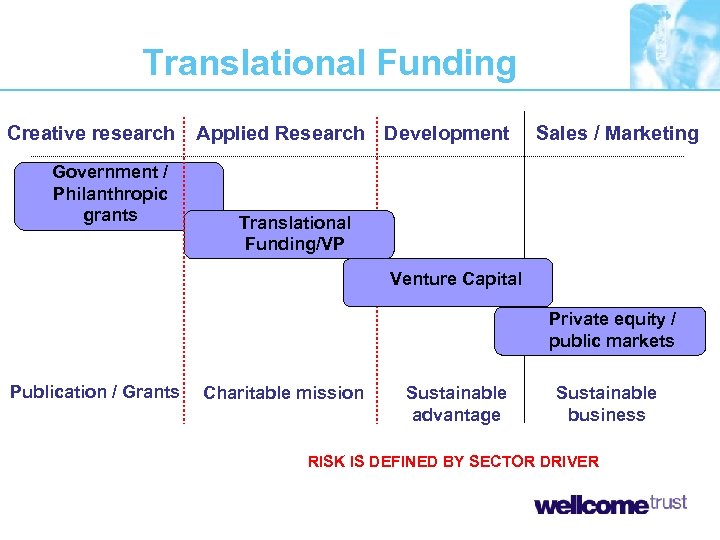 Translational Funding Creative research Government / Philanthropic grants Applied Research Development Sales / Marketing Translational Funding/VP Venture Capital Private equity / public markets Publication / Grants Charitable mission Sustainable advantage Sustainable business RISK IS DEFINED BY SECTOR DRIVER
Translational Funding Creative research Government / Philanthropic grants Applied Research Development Sales / Marketing Translational Funding/VP Venture Capital Private equity / public markets Publication / Grants Charitable mission Sustainable advantage Sustainable business RISK IS DEFINED BY SECTOR DRIVER
 Technology Transfer History • 1995 - Intellectual Property and Industrial Relations worked with Cancer Research Campaign Technology • 1997 – Catalyst Bio. Medica Limited, wholly owned trading subsidiary with own £ 20 Million fund • 2003 – Technology Transfer Division with Translation Awards Fund
Technology Transfer History • 1995 - Intellectual Property and Industrial Relations worked with Cancer Research Campaign Technology • 1997 – Catalyst Bio. Medica Limited, wholly owned trading subsidiary with own £ 20 Million fund • 2003 – Technology Transfer Division with Translation Awards Fund
 Early stage funding : Bridging the R&D gap
Early stage funding : Bridging the R&D gap
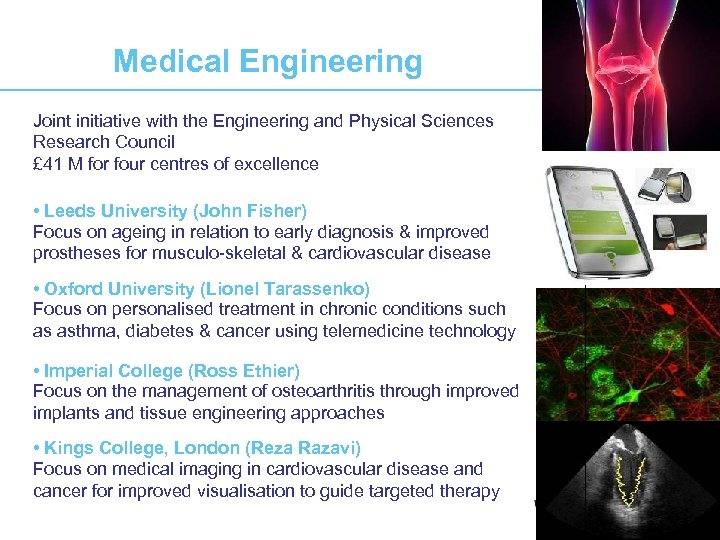 Medical Engineering Joint initiative with the Engineering and Physical Sciences Research Council £ 41 M for four centres of excellence • Leeds University (John Fisher) Focus on ageing in relation to early diagnosis & improved prostheses for musculo-skeletal & cardiovascular disease • Oxford University (Lionel Tarassenko) Focus on personalised treatment in chronic conditions such as asthma, diabetes & cancer using telemedicine technology • Imperial College (Ross Ethier) Focus on the management of osteoarthritis through improved implants and tissue engineering approaches • Kings College, London (Reza Razavi) Focus on medical imaging in cardiovascular disease and cancer for improved visualisation to guide targeted therapy
Medical Engineering Joint initiative with the Engineering and Physical Sciences Research Council £ 41 M for four centres of excellence • Leeds University (John Fisher) Focus on ageing in relation to early diagnosis & improved prostheses for musculo-skeletal & cardiovascular disease • Oxford University (Lionel Tarassenko) Focus on personalised treatment in chronic conditions such as asthma, diabetes & cancer using telemedicine technology • Imperial College (Ross Ethier) Focus on the management of osteoarthritis through improved implants and tissue engineering approaches • Kings College, London (Reza Razavi) Focus on medical imaging in cardiovascular disease and cancer for improved visualisation to guide targeted therapy

 What types of opportunity are charities looking for ? Projects that relate to their mission § Usually disease-focused § Sometimes problem-oriented § Always fulfil an unmet need § Offer solutions that are ‘fit for purpose’ Projects at the appropriate stage § Different charities engage at various stages § Fund-raising charities are more patient-oriented § Many charities look for leverage of their funds
What types of opportunity are charities looking for ? Projects that relate to their mission § Usually disease-focused § Sometimes problem-oriented § Always fulfil an unmet need § Offer solutions that are ‘fit for purpose’ Projects at the appropriate stage § Different charities engage at various stages § Fund-raising charities are more patient-oriented § Many charities look for leverage of their funds
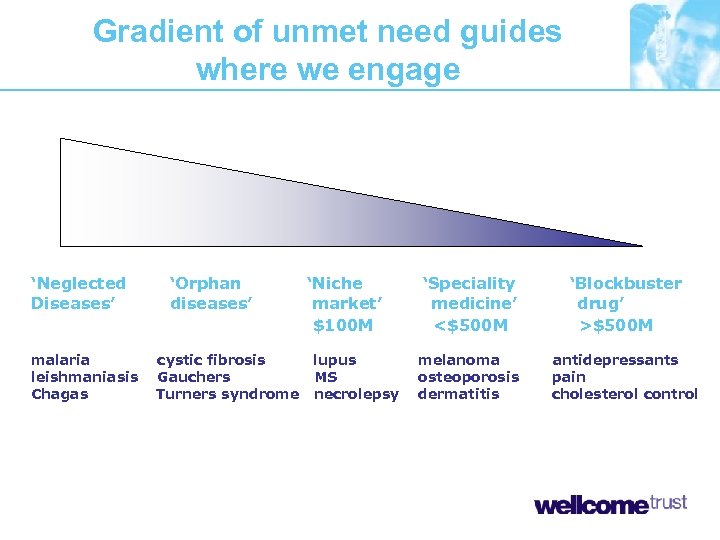 Gradient of unmet need guides where we engage ‘Neglected Diseases’ malaria leishmaniasis Chagas ‘Orphan diseases’ cystic fibrosis Gauchers Turners syndrome ‘Niche market’ $100 M lupus MS necrolepsy ‘Speciality medicine’ <$500 M ‘Blockbuster drug’ >$500 M melanoma osteoporosis dermatitis antidepressants pain cholesterol control
Gradient of unmet need guides where we engage ‘Neglected Diseases’ malaria leishmaniasis Chagas ‘Orphan diseases’ cystic fibrosis Gauchers Turners syndrome ‘Niche market’ $100 M lupus MS necrolepsy ‘Speciality medicine’ <$500 M ‘Blockbuster drug’ >$500 M melanoma osteoporosis dermatitis antidepressants pain cholesterol control
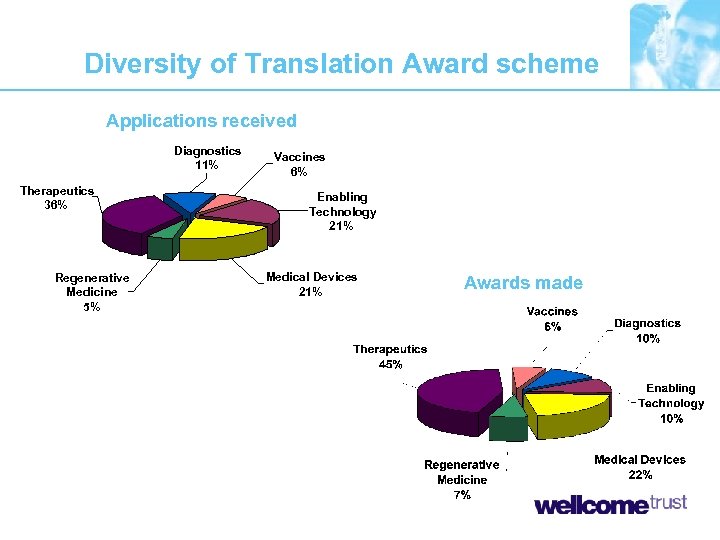 Diversity of Translation Award scheme Applications received Diagnostics 11% Therapeutics 36% Regenerative Medicine 5% Vaccines 6% Enabling Technology 21% Medical Devices 21% Awards made
Diversity of Translation Award scheme Applications received Diagnostics 11% Therapeutics 36% Regenerative Medicine 5% Vaccines 6% Enabling Technology 21% Medical Devices 21% Awards made
 Venture Philanthropy versus Private Financing? Advantages § Charity aims don’t keep changing § Financial return is not the priority § Charities are well informed & well connected § Charity backing gives a company good PR Disadvantages § Cultural differences may lead to tensions § Conflicts of interest can be tricky to manage § Shareholders need to understand the implications
Venture Philanthropy versus Private Financing? Advantages § Charity aims don’t keep changing § Financial return is not the priority § Charities are well informed & well connected § Charity backing gives a company good PR Disadvantages § Cultural differences may lead to tensions § Conflicts of interest can be tricky to manage § Shareholders need to understand the implications
 Value of Philanthropic funding? “Increase number of scientists who can translate drug discoveries into effective new medicines” “One proven route to [drug discovery] is innovation forged through synergistic industrial-academic collaborations” “Enhance drug R & D efforts in non-profit institutes”
Value of Philanthropic funding? “Increase number of scientists who can translate drug discoveries into effective new medicines” “One proven route to [drug discovery] is innovation forged through synergistic industrial-academic collaborations” “Enhance drug R & D efforts in non-profit institutes”
 Funding in Europe (inc. R. of Ireland) • Strategic mode • Institutions/registered company • SDDI – follow usual route • Translation award – invite only
Funding in Europe (inc. R. of Ireland) • Strategic mode • Institutions/registered company • SDDI – follow usual route • Translation award – invite only
 Strategic Translational funding Ireland • Core to the Trust’s own initiatives and objectives • Exceptional science • Trusts investment is of the order necessary to achieve programme goals thus no financial ceiling (within reason!) • Co-funding is welcome • Invite only (except SDDI) • Funding meetings approximately every 12 weeks • No small-molecule therapeutic programmes • Work in partnership with the Trust to achieve the commercial translation of targeted technologies
Strategic Translational funding Ireland • Core to the Trust’s own initiatives and objectives • Exceptional science • Trusts investment is of the order necessary to achieve programme goals thus no financial ceiling (within reason!) • Co-funding is welcome • Invite only (except SDDI) • Funding meetings approximately every 12 weeks • No small-molecule therapeutic programmes • Work in partnership with the Trust to achieve the commercial translation of targeted technologies
 Key Criteria • • • Ultimate healthcare benefit and impact Scientific rationale and evidence Competitive advantage / differentiation Downstream feasibility, risks identified Background assets and new assets for exit Competencies and skills for implementation
Key Criteria • • • Ultimate healthcare benefit and impact Scientific rationale and evidence Competitive advantage / differentiation Downstream feasibility, risks identified Background assets and new assets for exit Competencies and skills for implementation
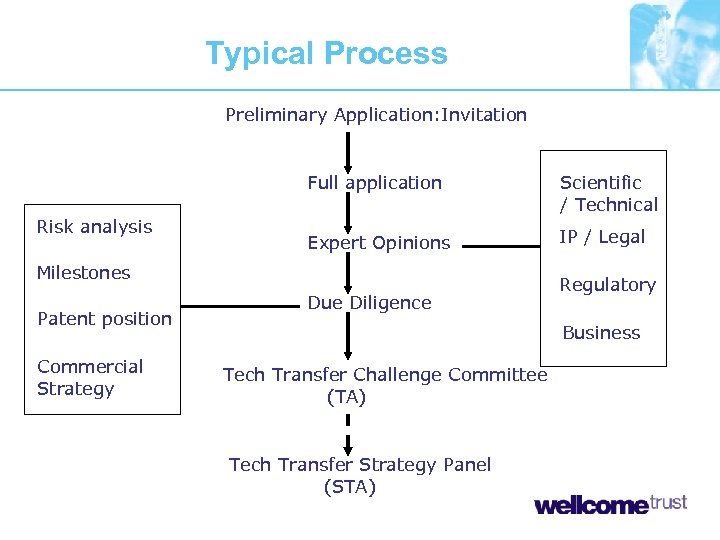 Typical Process Preliminary Application: Invitation Full application Risk analysis Scientific / Technical Expert Opinions IP / Legal Milestones Patent position Commercial Strategy Due Diligence Regulatory Business Tech Transfer Challenge Committee (TA) Tech Transfer Strategy Panel (STA)
Typical Process Preliminary Application: Invitation Full application Risk analysis Scientific / Technical Expert Opinions IP / Legal Milestones Patent position Commercial Strategy Due Diligence Regulatory Business Tech Transfer Challenge Committee (TA) Tech Transfer Strategy Panel (STA)
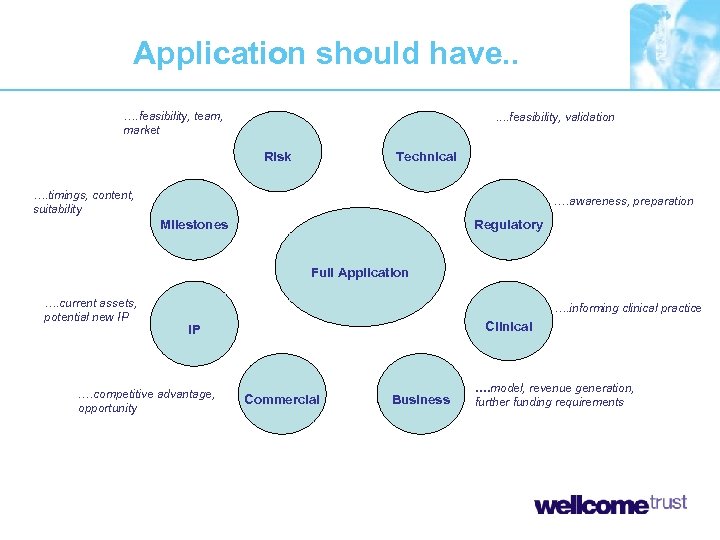 Application should have. . …. feasibility, team, market . . feasibility, validation Risk Technical …. timings, content, suitability …. awareness, preparation Milestones Regulatory Full Application …. current assets, potential new IP …. informing clinical practice Clinical IP …. competitive advantage, opportunity Commercial Business …. model, revenue generation, further funding requirements
Application should have. . …. feasibility, team, market . . feasibility, validation Risk Technical …. timings, content, suitability …. awareness, preparation Milestones Regulatory Full Application …. current assets, potential new IP …. informing clinical practice Clinical IP …. competitive advantage, opportunity Commercial Business …. model, revenue generation, further funding requirements
 The Seven Deadly Sins • Poor project plan • Lack of key expertise/experience • Unclear competitive advantage • Unrealistic commercial exit • • • Under-resourced project Major product development hurdles Weak scientific rationale
The Seven Deadly Sins • Poor project plan • Lack of key expertise/experience • Unclear competitive advantage • Unrealistic commercial exit • • • Under-resourced project Major product development hurdles Weak scientific rationale
 “A few misconceptions” “……you won’t fund companies” Wrong - >£ 35 M committed since 2003 with approximately 50: 50 academia: company settings “……of course the Trust will want to have some ownership of IP arising from the project” No. The IP is owned by the university or company. “…. you only fund projects arising from Trust funded research” No. We look to fund the best projects from any background “. . only interested in Biology” Wrong. Technology Transfer has a history of funding projects ranging from computation through to devices
“A few misconceptions” “……you won’t fund companies” Wrong - >£ 35 M committed since 2003 with approximately 50: 50 academia: company settings “……of course the Trust will want to have some ownership of IP arising from the project” No. The IP is owned by the university or company. “…. you only fund projects arising from Trust funded research” No. We look to fund the best projects from any background “. . only interested in Biology” Wrong. Technology Transfer has a history of funding projects ranging from computation through to devices
 Irish projects funded to date Jim Mc. Laughlin John Anderson Louise Kenny Phil Baker Neil Frankish Liam Marnane
Irish projects funded to date Jim Mc. Laughlin John Anderson Louise Kenny Phil Baker Neil Frankish Liam Marnane
 Devices Vital Signs Monitor Objective Vital. Sens Vital Signs Monitor for hospital use Team Jim Mc. Laughlin, John Anderson, Michael Caulfield, ST&D Features Non-invasive patient worn monitor Five Vital Signs : ECG, respiration rate, skin temperature, gait, Sp. O 2 Data transmission via hospital wireless networks
Devices Vital Signs Monitor Objective Vital. Sens Vital Signs Monitor for hospital use Team Jim Mc. Laughlin, John Anderson, Michael Caulfield, ST&D Features Non-invasive patient worn monitor Five Vital Signs : ECG, respiration rate, skin temperature, gait, Sp. O 2 Data transmission via hospital wireless networks
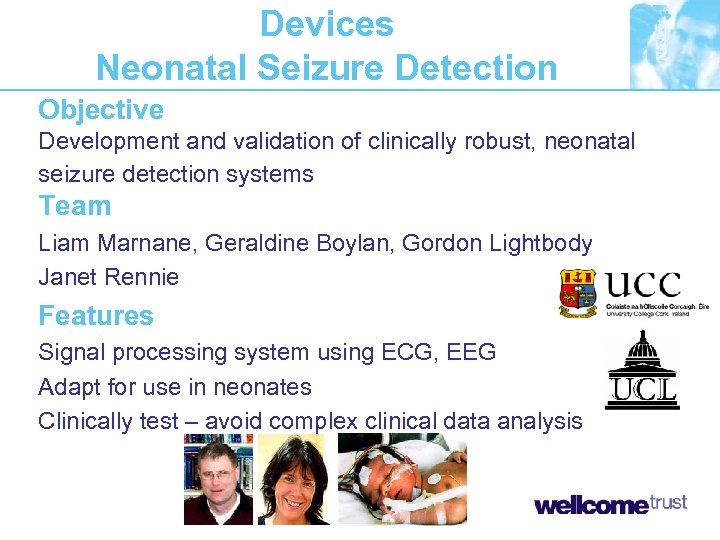 Devices Neonatal Seizure Detection Objective Development and validation of clinically robust, neonatal seizure detection systems Team Liam Marnane, Geraldine Boylan, Gordon Lightbody Janet Rennie Features Signal processing system using ECG, EEG Adapt for use in neonates Clinically test – avoid complex clinical data analysis
Devices Neonatal Seizure Detection Objective Development and validation of clinically robust, neonatal seizure detection systems Team Liam Marnane, Geraldine Boylan, Gordon Lightbody Janet Rennie Features Signal processing system using ECG, EEG Adapt for use in neonates Clinically test – avoid complex clinical data analysis
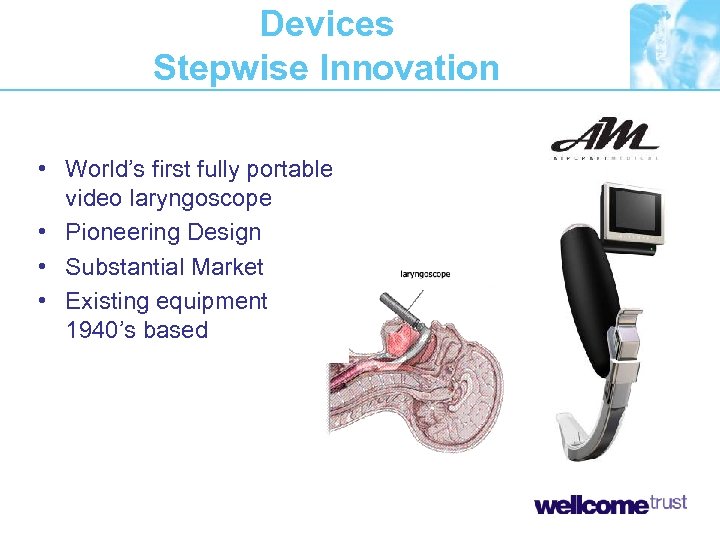 Devices Stepwise Innovation • World’s first fully portable video laryngoscope • Pioneering Design • Substantial Market • Existing equipment 1940’s based
Devices Stepwise Innovation • World’s first fully portable video laryngoscope • Pioneering Design • Substantial Market • Existing equipment 1940’s based
 Diagnostics Re-profiling platform technologies Objective The rapid and selective detection of Mycobacterium tuberculosis by field deployable thermochemolysis gas chromatograph mass spectrometry Strategic Features Space research meets tropical medicine WT had funded Beagle mission
Diagnostics Re-profiling platform technologies Objective The rapid and selective detection of Mycobacterium tuberculosis by field deployable thermochemolysis gas chromatograph mass spectrometry Strategic Features Space research meets tropical medicine WT had funded Beagle mission
 Device Pheromone baited traps for Sand-fly vectors Objective Field feasibility trial of synthetic sex pheromone analogue of Sand-flies Team Gordon Hamilton Features • High efficiency trap • Minimise spread of leishmaniasis
Device Pheromone baited traps for Sand-fly vectors Objective Field feasibility trial of synthetic sex pheromone analogue of Sand-flies Team Gordon Hamilton Features • High efficiency trap • Minimise spread of leishmaniasis
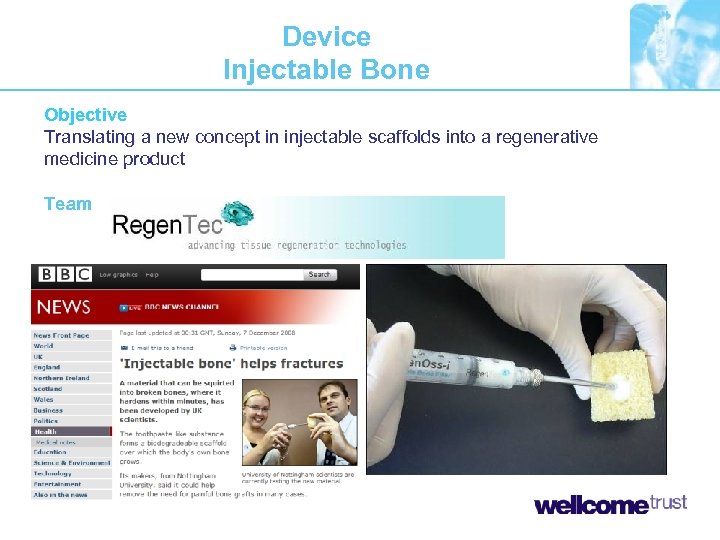 Device Injectable Bone Objective Translating a new concept in injectable scaffolds into a regenerative medicine product Team
Device Injectable Bone Objective Translating a new concept in injectable scaffolds into a regenerative medicine product Team
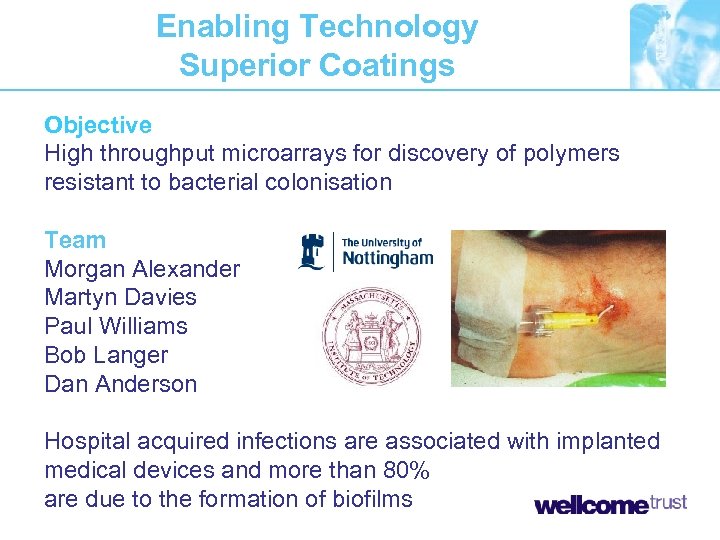 Enabling Technology Superior Coatings Objective High throughput microarrays for discovery of polymers resistant to bacterial colonisation Team Morgan Alexander Martyn Davies Paul Williams Bob Langer Dan Anderson Hospital acquired infections are associated with implanted medical devices and more than 80% are due to the formation of biofilms
Enabling Technology Superior Coatings Objective High throughput microarrays for discovery of polymers resistant to bacterial colonisation Team Morgan Alexander Martyn Davies Paul Williams Bob Langer Dan Anderson Hospital acquired infections are associated with implanted medical devices and more than 80% are due to the formation of biofilms
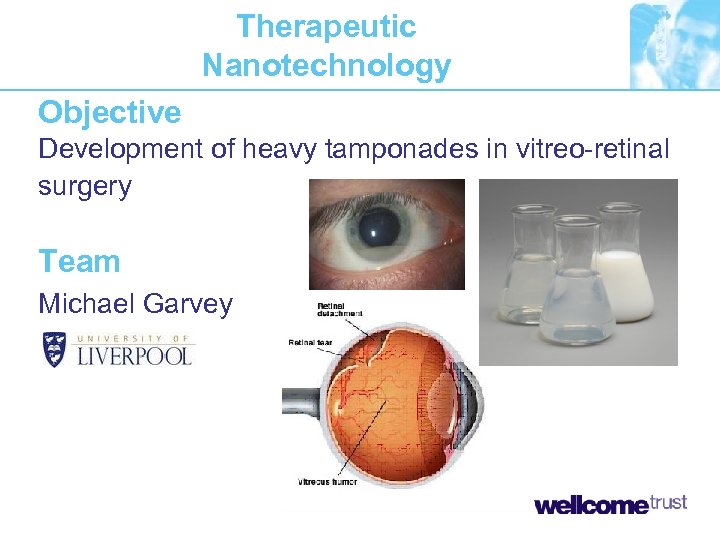 Therapeutic Nanotechnology Objective Development of heavy tamponades in vitreo-retinal surgery Team Michael Garvey
Therapeutic Nanotechnology Objective Development of heavy tamponades in vitreo-retinal surgery Team Michael Garvey
 Device Spider silk technology for meniscal repair Objective Development and evaluation of a bio-resorbable, load-bearing, tissue regenerative meniscal cartilage implant Team Nick Skaer, Orthox Ltd Silkworm silk fibres are dissolved in bulk. Super strong and resilient tissue scaffolds (Spidrex® can be made by emulating spiders spinning techniques. Prototype Orthox device matches target mechanical properties and supports cell growth, osteo-inductive and shows bone deposition 8 weeks in vivo
Device Spider silk technology for meniscal repair Objective Development and evaluation of a bio-resorbable, load-bearing, tissue regenerative meniscal cartilage implant Team Nick Skaer, Orthox Ltd Silkworm silk fibres are dissolved in bulk. Super strong and resilient tissue scaffolds (Spidrex® can be made by emulating spiders spinning techniques. Prototype Orthox device matches target mechanical properties and supports cell growth, osteo-inductive and shows bone deposition 8 weeks in vivo
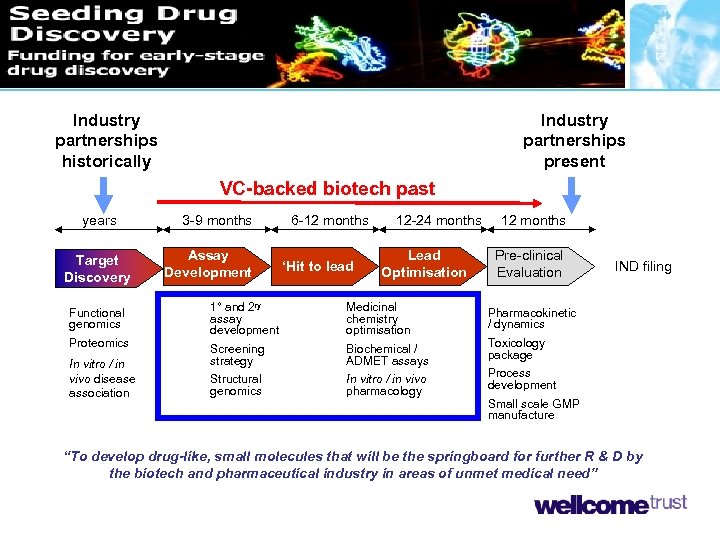 Industry partnerships historically Industry partnerships present VC-backed biotech past years Target Discovery Functional genomics Proteomics In vitro / in vivo disease association 3 -9 months Assay Development 6 -12 months ‘Hit to lead 12 -24 months Lead Optimisation 1° and 2 ry assay development Medicinal chemistry optimisation Screening strategy Biochemical / ADMET assays Structural genomics In vitro / in vivo pharmacology 12 months Pre-clinical Evaluation IND filing Pharmacokinetic / dynamics Toxicology package Process development Small scale GMP manufacture “To develop drug-like, small molecules that will be the springboard for further R & D by the biotech and pharmaceutical industry in areas of unmet medical need”
Industry partnerships historically Industry partnerships present VC-backed biotech past years Target Discovery Functional genomics Proteomics In vitro / in vivo disease association 3 -9 months Assay Development 6 -12 months ‘Hit to lead 12 -24 months Lead Optimisation 1° and 2 ry assay development Medicinal chemistry optimisation Screening strategy Biochemical / ADMET assays Structural genomics In vitro / in vivo pharmacology 12 months Pre-clinical Evaluation IND filing Pharmacokinetic / dynamics Toxicology package Process development Small scale GMP manufacture “To develop drug-like, small molecules that will be the springboard for further R & D by the biotech and pharmaceutical industry in areas of unmet medical need”
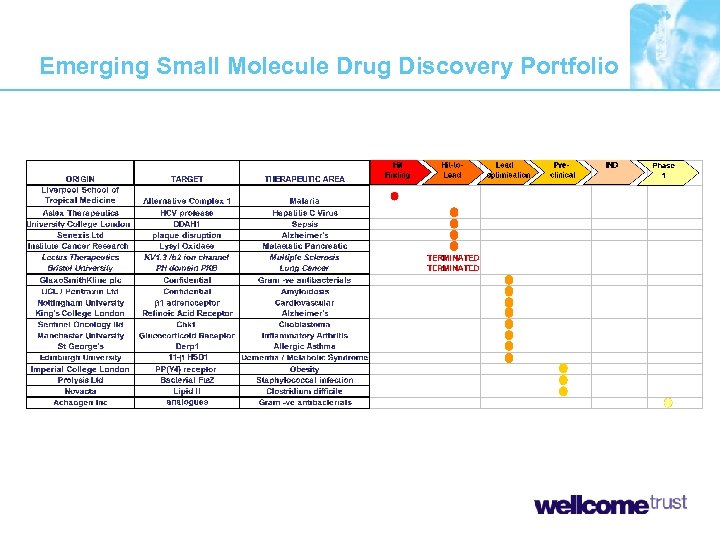 Seeding Drug Discovery Portfolio Emerging Small Molecule Drug Discovery Portfolio
Seeding Drug Discovery Portfolio Emerging Small Molecule Drug Discovery Portfolio
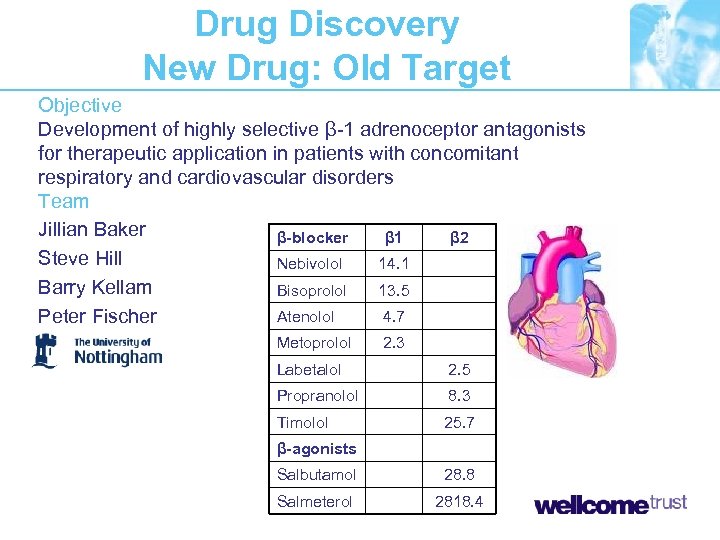 Drug Discovery New Drug: Old Target Objective Development of highly selective β-1 adrenoceptor antagonists for therapeutic application in patients with concomitant respiratory and cardiovascular disorders Team Jillian Baker β-blocker β 1 β 2 Steve Hill Nebivolol 14. 1 Barry Kellam Bisoprolol 13. 5 Atenolol 4. 7 Peter Fischer Metoprolol 2. 3 Labetalol 2. 5 Propranolol 8. 3 Timolol 25. 7 β-agonists Salbutamol 28. 8 Salmeterol 2818. 4
Drug Discovery New Drug: Old Target Objective Development of highly selective β-1 adrenoceptor antagonists for therapeutic application in patients with concomitant respiratory and cardiovascular disorders Team Jillian Baker β-blocker β 1 β 2 Steve Hill Nebivolol 14. 1 Barry Kellam Bisoprolol 13. 5 Atenolol 4. 7 Peter Fischer Metoprolol 2. 3 Labetalol 2. 5 Propranolol 8. 3 Timolol 25. 7 β-agonists Salbutamol 28. 8 Salmeterol 2818. 4
 The Problem and the Market “In 2004, total U. S. sales of beta blockers were approximately $1. 9 billion, a 20% increase over 2003 sales. The market leader is Astra. Zeneca’s Toprol XL (metoprolol). Total U. S. sales of Toprol XL in 2004 were approximately $1. 2 billion” If 1% population should be on a b-blocker but cannot be because of side effects = $ 190 million in sales (2003 data) BUT… Will market use the product? Pharma Longevity of safety and efficacy data
The Problem and the Market “In 2004, total U. S. sales of beta blockers were approximately $1. 9 billion, a 20% increase over 2003 sales. The market leader is Astra. Zeneca’s Toprol XL (metoprolol). Total U. S. sales of Toprol XL in 2004 were approximately $1. 2 billion” If 1% population should be on a b-blocker but cannot be because of side effects = $ 190 million in sales (2003 data) BUT… Will market use the product? Pharma Longevity of safety and efficacy data
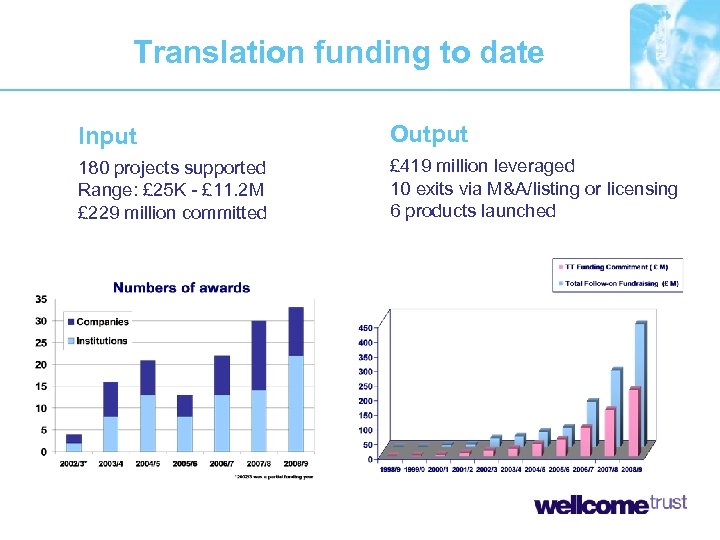 Translation funding to date Input Output 180 projects supported Range: £ 25 K - £ 11. 2 M £ 229 million committed £ 419 million leveraged 10 exits via M&A/listing or licensing 6 products launched
Translation funding to date Input Output 180 projects supported Range: £ 25 K - £ 11. 2 M £ 229 million committed £ 419 million leveraged 10 exits via M&A/listing or licensing 6 products launched
 Dr Fiona Mac. Laughlin Senior Business Analyst Technology Transfer Division (t) +44 (0)20 7611 8310 (e) f. maclaughlin@wellcome. ac. uk
Dr Fiona Mac. Laughlin Senior Business Analyst Technology Transfer Division (t) +44 (0)20 7611 8310 (e) f. maclaughlin@wellcome. ac. uk
 Funding Mechanisms Managing Wellcome Trust-funded Intellectual Property: Revenue/Equity Sharing
Funding Mechanisms Managing Wellcome Trust-funded Intellectual Property: Revenue/Equity Sharing
 Funding Terms and Conditions • ACADEMIA – Funding Agreement aligning with principles of Grant conditions – Trust leads on exploitation of commercialisable assets • SME – Unsecured Convertible loan (Equity conversion at 20% discount to next professional investment round) – Repayment options at Trust discretion (LIBOR + 2%) • PHARMA – Milestone / royalties based deals – Step in rights to ensure achievement of charitable mission • OTHER (based on circumstance) – Bespoke
Funding Terms and Conditions • ACADEMIA – Funding Agreement aligning with principles of Grant conditions – Trust leads on exploitation of commercialisable assets • SME – Unsecured Convertible loan (Equity conversion at 20% discount to next professional investment round) – Repayment options at Trust discretion (LIBOR + 2%) • PHARMA – Milestone / royalties based deals – Step in rights to ensure achievement of charitable mission • OTHER (based on circumstance) – Bespoke
 Key Aims of IP Management • Achieve health care benefits • Promote & Maintain supportive environment for future biomedical research - Encourage open & innovative approach - Partnerships of funders, scientists, institutions and companies
Key Aims of IP Management • Achieve health care benefits • Promote & Maintain supportive environment for future biomedical research - Encourage open & innovative approach - Partnerships of funders, scientists, institutions and companies
 Managing IP from Trust Grants • Wellcome Trust IP Policy & Grant Conditions • IP to vest in Host-Institution / University • Systems for identifying and managing IP • Incidental private benefit only • Appropriate way to achieve public benefit? – Case-by-case – IP protection? : Patent filing – Unprotected access? : Publication of research results – Need to attract follow-on R&D funding? (VCs; Pharma) – No reputational risk to Trust
Managing IP from Trust Grants • Wellcome Trust IP Policy & Grant Conditions • IP to vest in Host-Institution / University • Systems for identifying and managing IP • Incidental private benefit only • Appropriate way to achieve public benefit? – Case-by-case – IP protection? : Patent filing – Unprotected access? : Publication of research results – Need to attract follow-on R&D funding? (VCs; Pharma) – No reputational risk to Trust
 Revenue / Equity Sharing • Trust sole funder: revenue / equity share applies to the total gross income / equity received • Trust not sole funder: revenue / equity share applies pro-rata to gross income / equity received taking into account: – Inventive contribution of inventors – Proportionate funding contributions of Trust, Institution and third party funders – Importance / value of respective funding contributions to the deal
Revenue / Equity Sharing • Trust sole funder: revenue / equity share applies to the total gross income / equity received • Trust not sole funder: revenue / equity share applies pro-rata to gross income / equity received taking into account: – Inventive contribution of inventors – Proportionate funding contributions of Trust, Institution and third party funders – Importance / value of respective funding contributions to the deal
 Dr Fiona Mac. Laughlin Senior Business Analyst Technology Transfer Division (t) +44 (0)20 7611 8310 (e) f. maclaughlin@wellcome. ac. uk
Dr Fiona Mac. Laughlin Senior Business Analyst Technology Transfer Division (t) +44 (0)20 7611 8310 (e) f. maclaughlin@wellcome. ac. uk


