0430994f3e800839579261d135f5c870.ppt
- Количество слайдов: 46
 Fundamentals of Fluorescence Microscopy E. D. Salmon University of North Carolina at Chapel Hill References: Murphy Book; http: //micro. magnet. fsu. edu/primer/techniques/ Fluorescence; and www. chroma. com
Fundamentals of Fluorescence Microscopy E. D. Salmon University of North Carolina at Chapel Hill References: Murphy Book; http: //micro. magnet. fsu. edu/primer/techniques/ Fluorescence; and www. chroma. com
 Basic Concept of Absorption and Emission
Basic Concept of Absorption and Emission
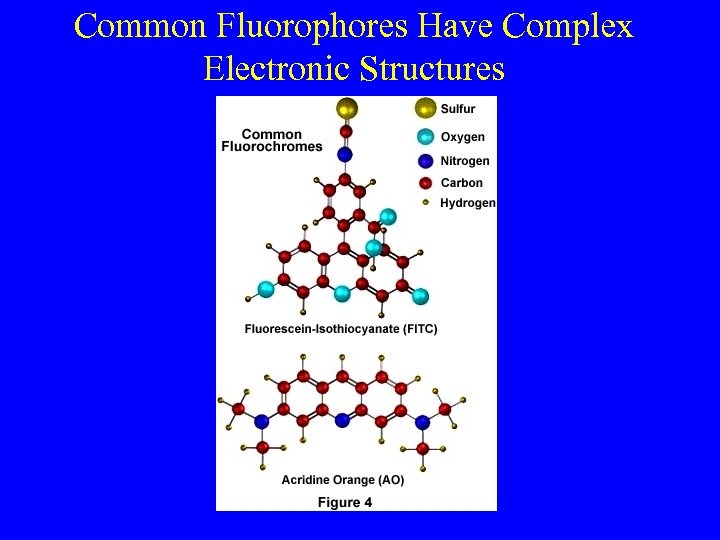 Common Fluorophores Have Complex Electronic Structures
Common Fluorophores Have Complex Electronic Structures
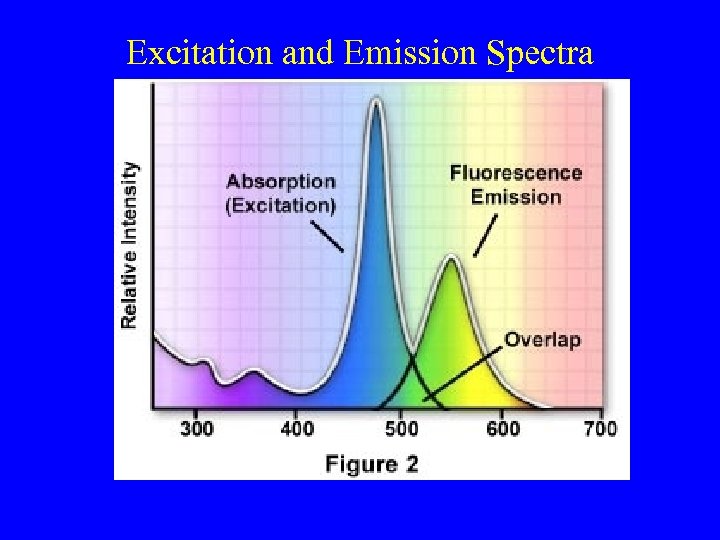 Excitation and Emission Spectra
Excitation and Emission Spectra
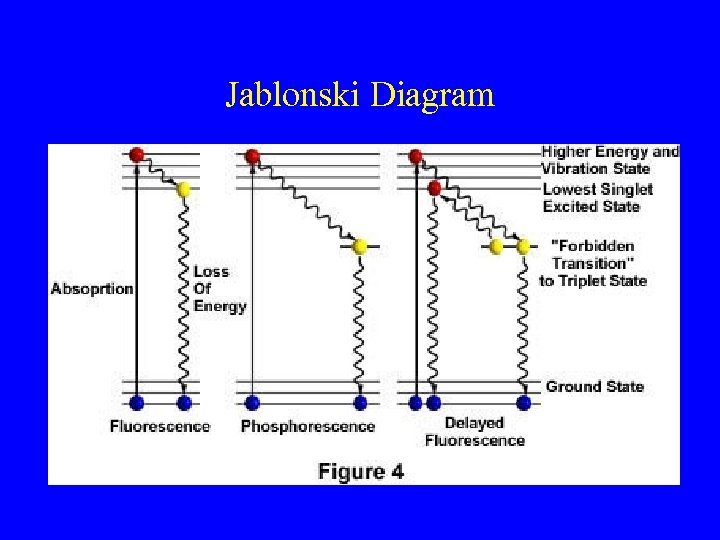 Jablonski Diagram
Jablonski Diagram
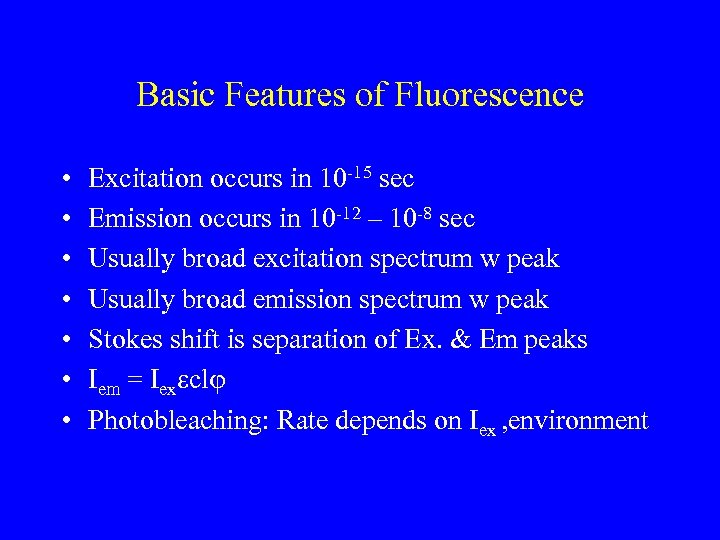 Basic Features of Fluorescence • • Excitation occurs in 10 -15 sec Emission occurs in 10 -12 – 10 -8 sec Usually broad excitation spectrum w peak Usually broad emission spectrum w peak Stokes shift is separation of Ex. & Em peaks Iem = Iexeclj Photobleaching: Rate depends on Iex , environment
Basic Features of Fluorescence • • Excitation occurs in 10 -15 sec Emission occurs in 10 -12 – 10 -8 sec Usually broad excitation spectrum w peak Usually broad emission spectrum w peak Stokes shift is separation of Ex. & Em peaks Iem = Iexeclj Photobleaching: Rate depends on Iex , environment
 Fluorophore Parameters • Absorption coefficient at peak absorption • Quantum efficiency at peak emission • Photostability (e. g. fluorescein has 10, 000 excitations before bleaching event) • Stokes Shift • Widths of excitation and emission spectra • Fluorescence is polarized: absorption and emission usually for E vector in plane of conjugated bonds
Fluorophore Parameters • Absorption coefficient at peak absorption • Quantum efficiency at peak emission • Photostability (e. g. fluorescein has 10, 000 excitations before bleaching event) • Stokes Shift • Widths of excitation and emission spectra • Fluorescence is polarized: absorption and emission usually for E vector in plane of conjugated bonds
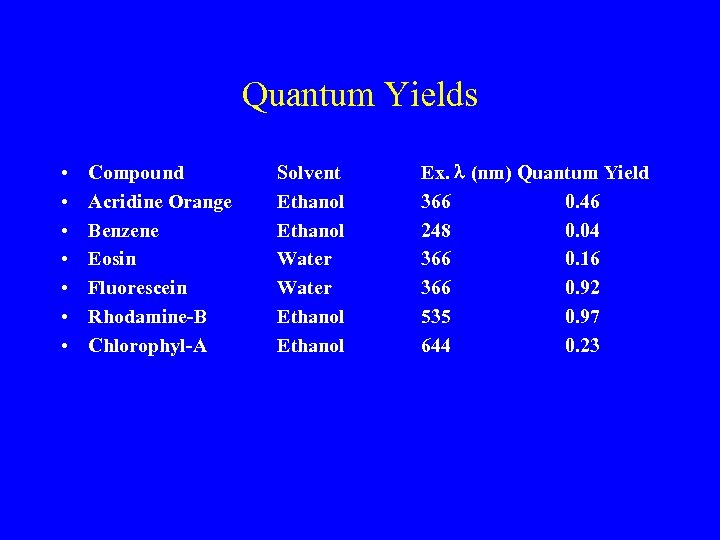 Quantum Yields • • Compound Acridine Orange Benzene Eosin Fluorescein Rhodamine-B Chlorophyl-A Solvent Ethanol Water Ethanol Ex. l (nm) Quantum Yield 366 0. 46 248 0. 04 366 0. 16 366 0. 92 535 0. 97 644 0. 23
Quantum Yields • • Compound Acridine Orange Benzene Eosin Fluorescein Rhodamine-B Chlorophyl-A Solvent Ethanol Water Ethanol Ex. l (nm) Quantum Yield 366 0. 46 248 0. 04 366 0. 16 366 0. 92 535 0. 97 644 0. 23
 Molecular Fluorescent Probes • Specific Fluorescent Dyes (e. g. DAPI) • Covalently bind fluorescent dye to purified protein • Fluorescent Antibodies (e. g immunofluorescent labeling with primary and fluorescent secondary antibodies) • Express in cells Green (C, Y, R) Fluorescent Protein (G, C, Y, R-FP) fused to protein of interest
Molecular Fluorescent Probes • Specific Fluorescent Dyes (e. g. DAPI) • Covalently bind fluorescent dye to purified protein • Fluorescent Antibodies (e. g immunofluorescent labeling with primary and fluorescent secondary antibodies) • Express in cells Green (C, Y, R) Fluorescent Protein (G, C, Y, R-FP) fused to protein of interest
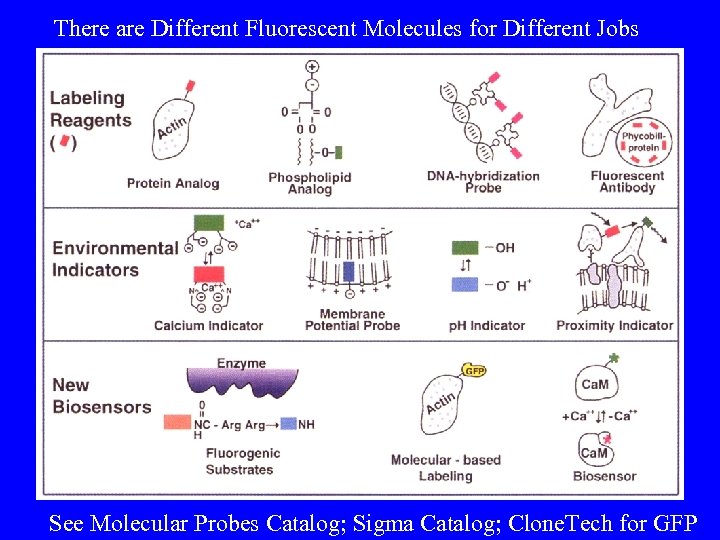 There are Different Fluorescent Molecules for Different Jobs See Molecular Probes Catalog; Sigma Catalog; Clone. Tech for GFP
There are Different Fluorescent Molecules for Different Jobs See Molecular Probes Catalog; Sigma Catalog; Clone. Tech for GFP
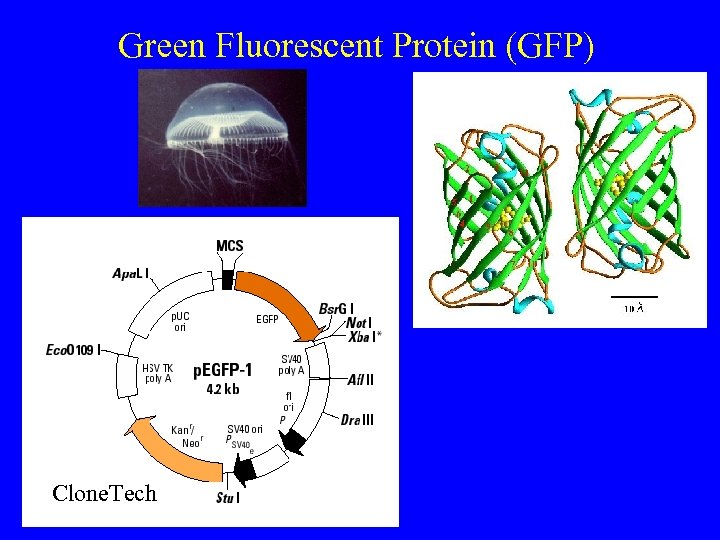 Green Fluorescent Protein (GFP) Clone. Tech
Green Fluorescent Protein (GFP) Clone. Tech
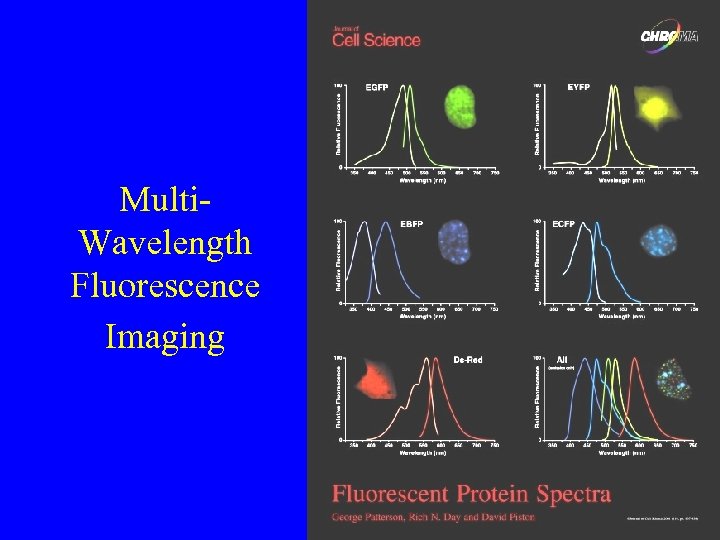 Multi. Wavelength Fluorescence Imaging
Multi. Wavelength Fluorescence Imaging
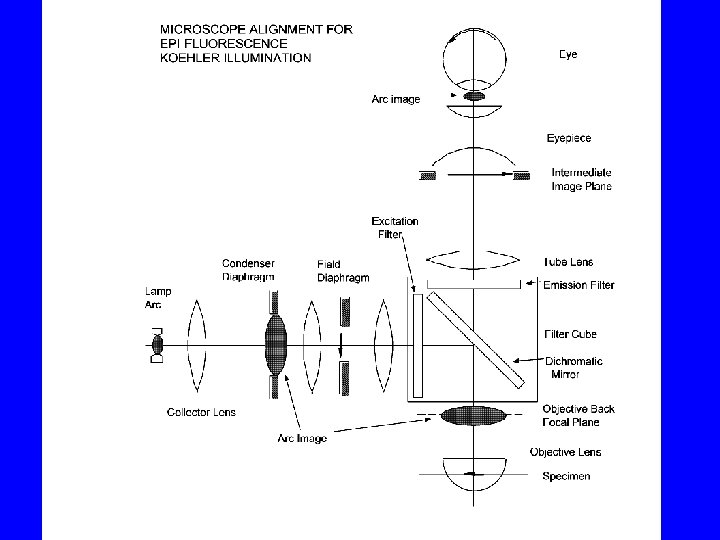
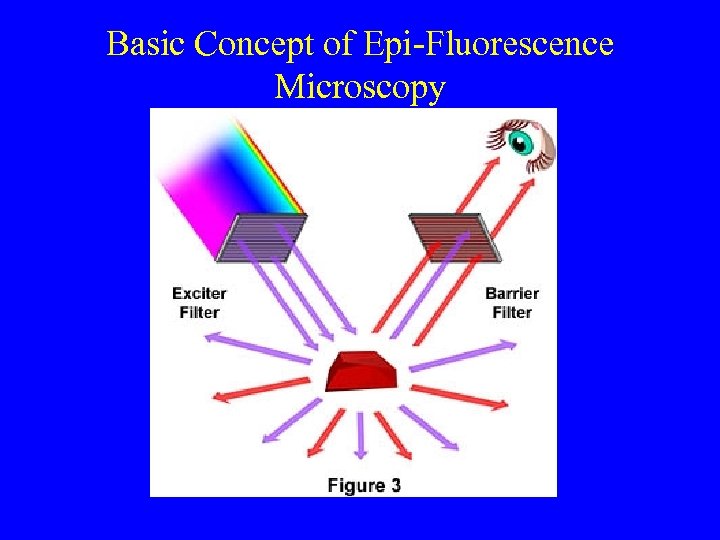 Basic Concept of Epi-Fluorescence Microscopy
Basic Concept of Epi-Fluorescence Microscopy
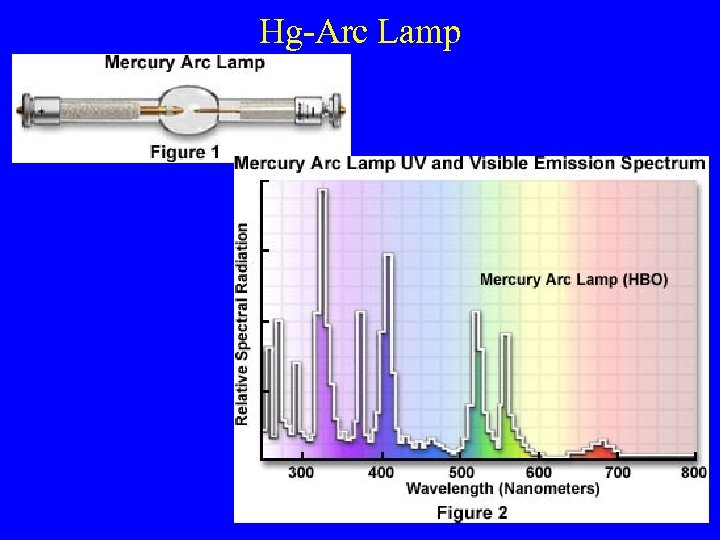 Hg-Arc Lamp
Hg-Arc Lamp
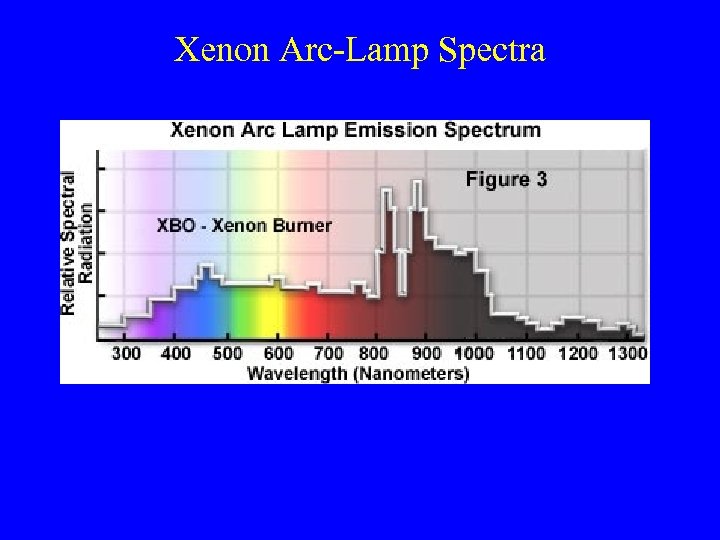 Xenon Arc-Lamp Spectra
Xenon Arc-Lamp Spectra
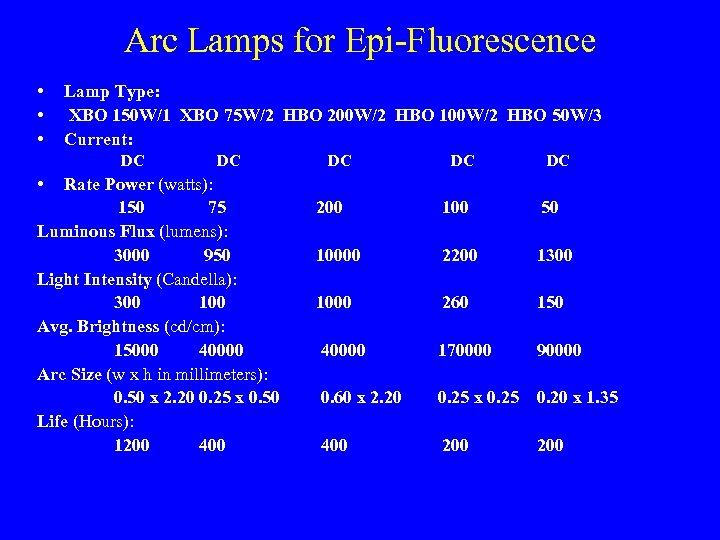 Arc Lamps for Epi-Fluorescence • • • Lamp Type: XBO 150 W/1 XBO 75 W/2 HBO 200 W/2 HBO 100 W/2 HBO 50 W/3 Current: DC DC DC • Rate Power (watts): 150 75 Luminous Flux (lumens): 3000 950 Light Intensity (Candella): 300 100 Avg. Brightness (cd/cm): 15000 40000 Arc Size (w x h in millimeters): 0. 50 x 2. 20 0. 25 x 0. 50 Life (Hours): 1200 400 200 100 50 10000 2200 1300 1000 260 150 40000 170000 90000 0. 60 x 2. 20 0. 25 x 0. 25 0. 20 x 1. 35 400 200
Arc Lamps for Epi-Fluorescence • • • Lamp Type: XBO 150 W/1 XBO 75 W/2 HBO 200 W/2 HBO 100 W/2 HBO 50 W/3 Current: DC DC DC • Rate Power (watts): 150 75 Luminous Flux (lumens): 3000 950 Light Intensity (Candella): 300 100 Avg. Brightness (cd/cm): 15000 40000 Arc Size (w x h in millimeters): 0. 50 x 2. 20 0. 25 x 0. 50 Life (Hours): 1200 400 200 100 50 10000 2200 1300 1000 260 150 40000 170000 90000 0. 60 x 2. 20 0. 25 x 0. 25 0. 20 x 1. 35 400 200
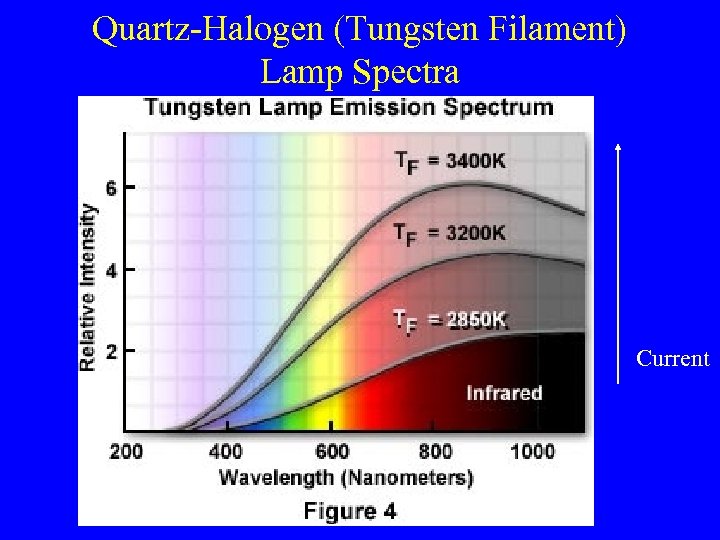 Quartz-Halogen (Tungsten Filament) Lamp Spectra Current
Quartz-Halogen (Tungsten Filament) Lamp Spectra Current
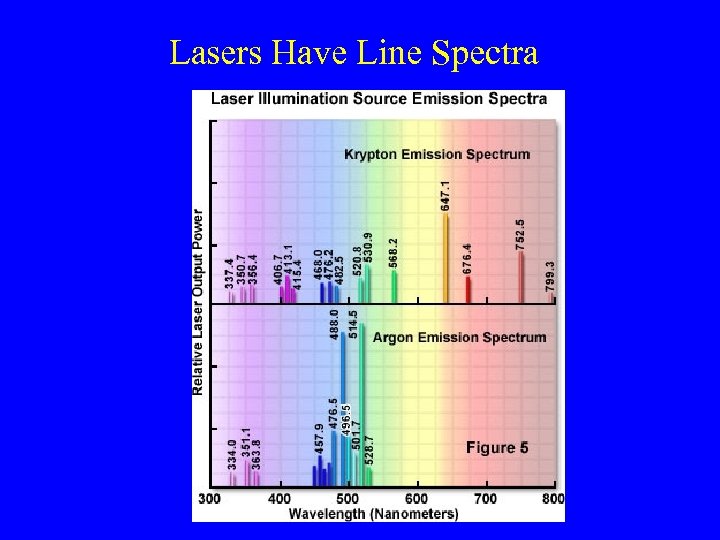 Lasers Have Line Spectra
Lasers Have Line Spectra
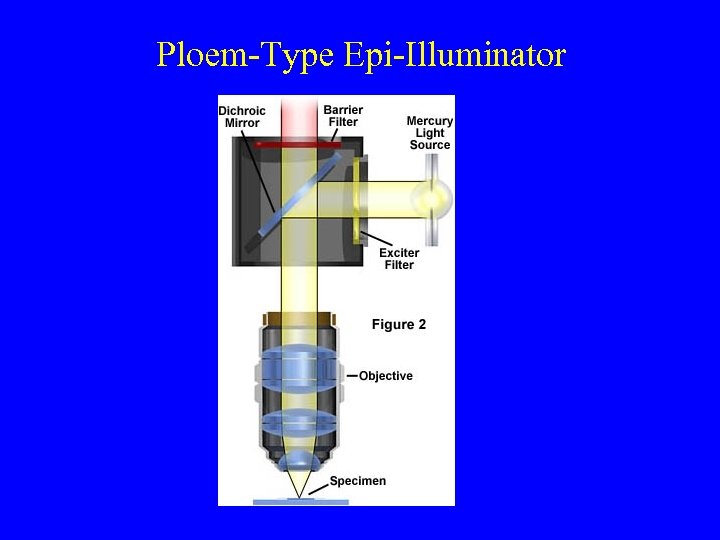 Ploem-Type Epi-Illuminator
Ploem-Type Epi-Illuminator
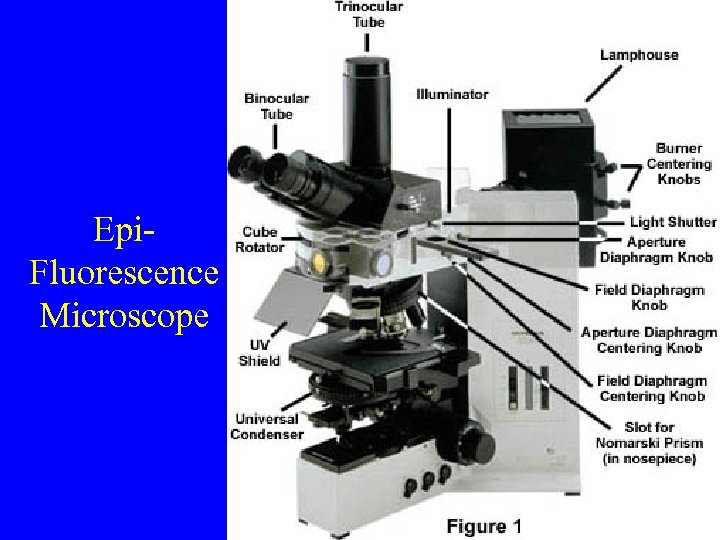 Epi. Fluorescence Microscope
Epi. Fluorescence Microscope
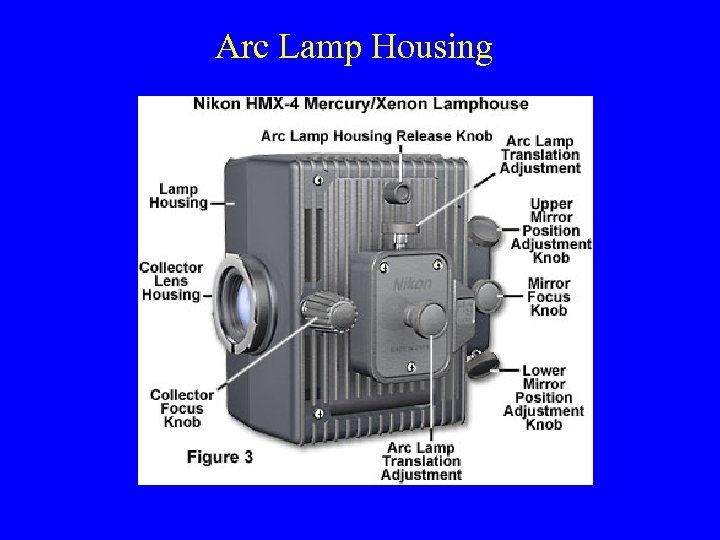 Arc Lamp Housing
Arc Lamp Housing
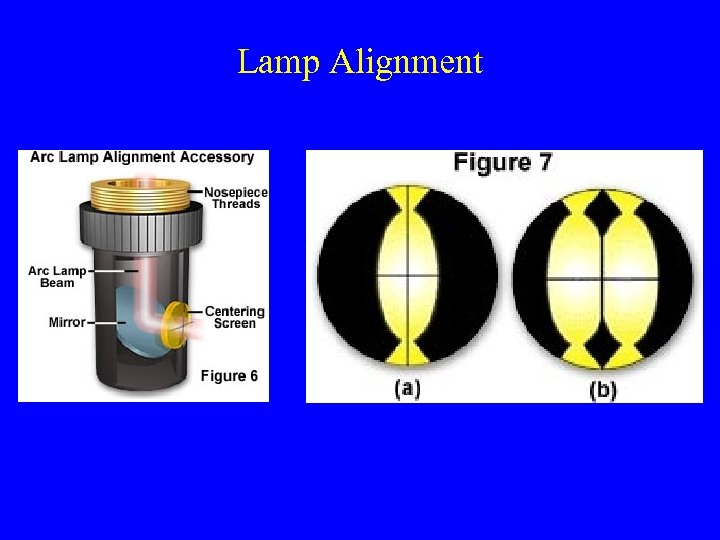 Lamp Alignment
Lamp Alignment
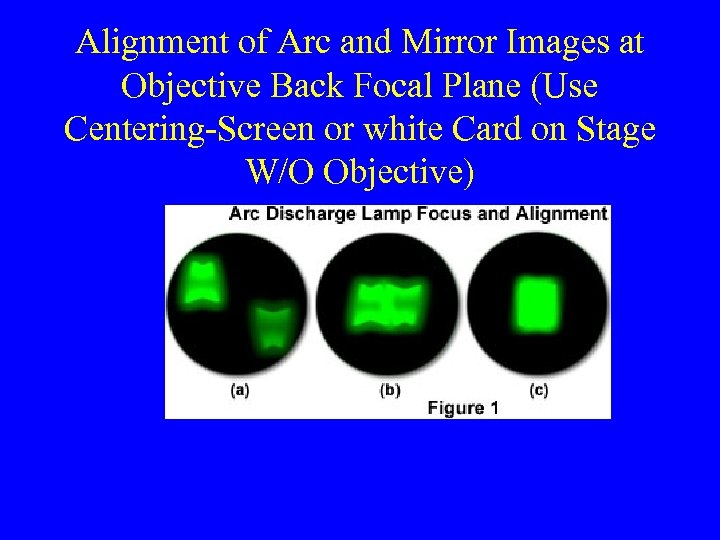 Alignment of Arc and Mirror Images at Objective Back Focal Plane (Use Centering-Screen or white Card on Stage W/O Objective)
Alignment of Arc and Mirror Images at Objective Back Focal Plane (Use Centering-Screen or white Card on Stage W/O Objective)
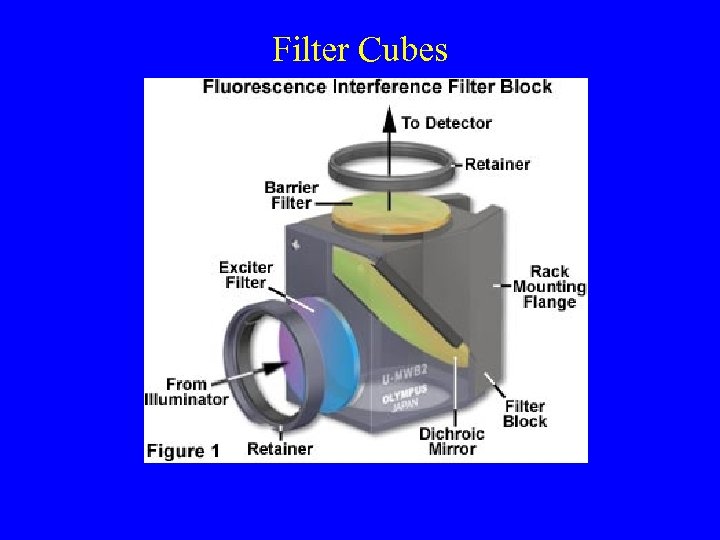 Filter Cubes
Filter Cubes
 Filter Cubes Are Not Inter-Changeable Between Different Manufactures
Filter Cubes Are Not Inter-Changeable Between Different Manufactures
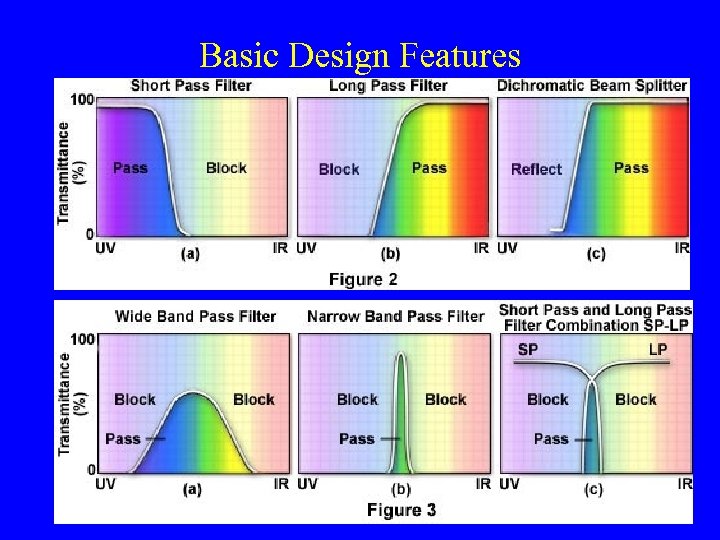 Basic Design Features
Basic Design Features
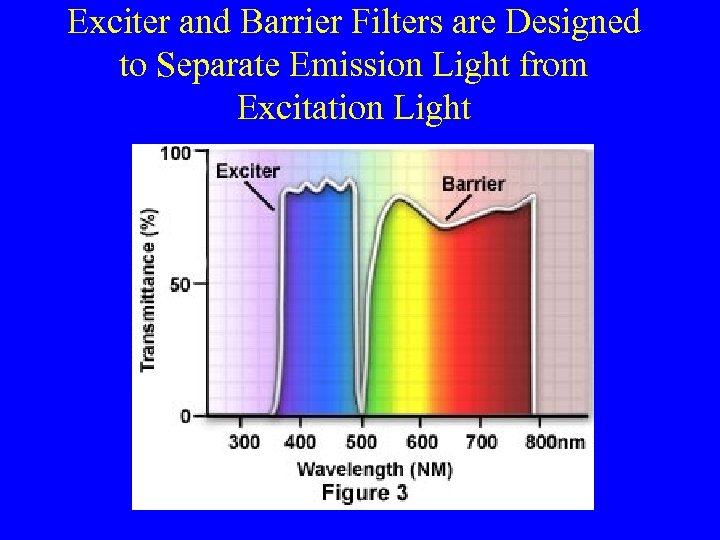 Exciter and Barrier Filters are Designed to Separate Emission Light from Excitation Light
Exciter and Barrier Filters are Designed to Separate Emission Light from Excitation Light
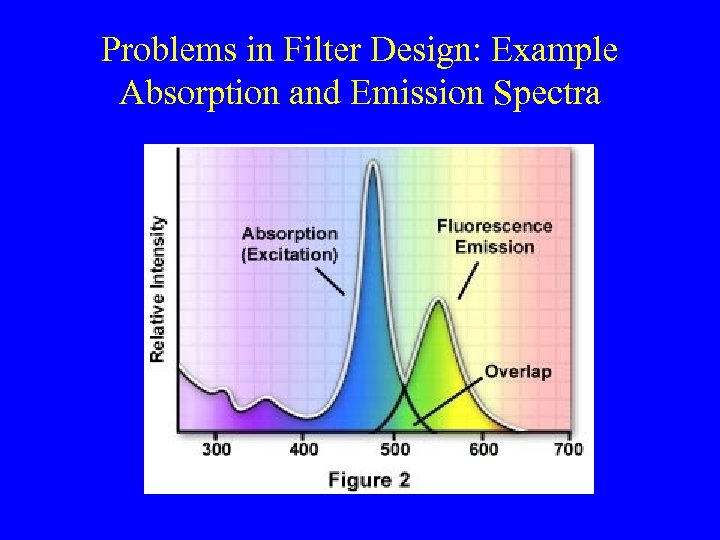 Problems in Filter Design: Example Absorption and Emission Spectra
Problems in Filter Design: Example Absorption and Emission Spectra
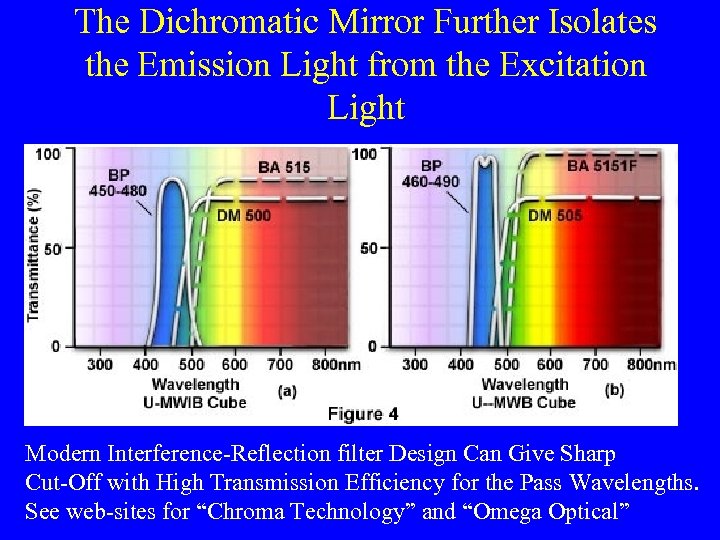 The Dichromatic Mirror Further Isolates the Emission Light from the Excitation Light Modern Interference-Reflection filter Design Can Give Sharp Cut-Off with High Transmission Efficiency for the Pass Wavelengths. See web-sites for “Chroma Technology” and “Omega Optical”
The Dichromatic Mirror Further Isolates the Emission Light from the Excitation Light Modern Interference-Reflection filter Design Can Give Sharp Cut-Off with High Transmission Efficiency for the Pass Wavelengths. See web-sites for “Chroma Technology” and “Omega Optical”
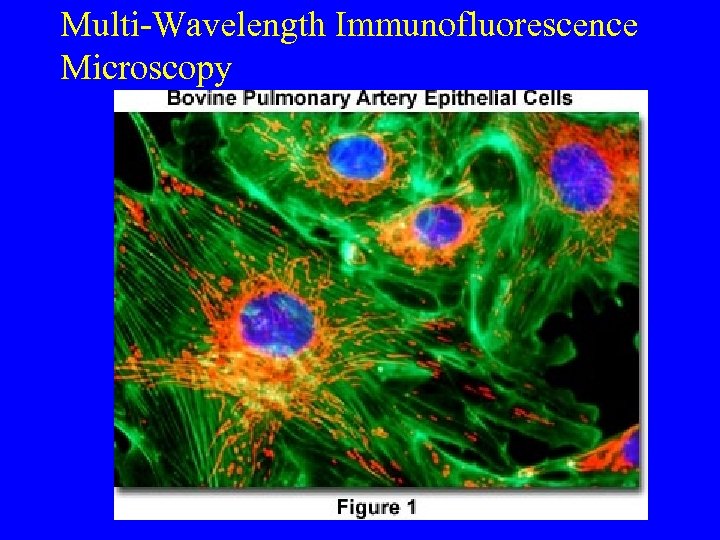 Multi-Wavelength Immunofluorescence Microscopy
Multi-Wavelength Immunofluorescence Microscopy
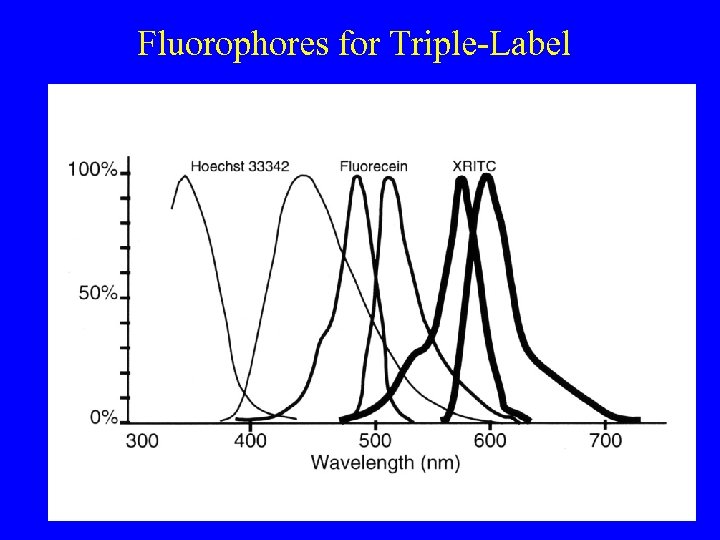 Fluorophores for Triple-Label
Fluorophores for Triple-Label
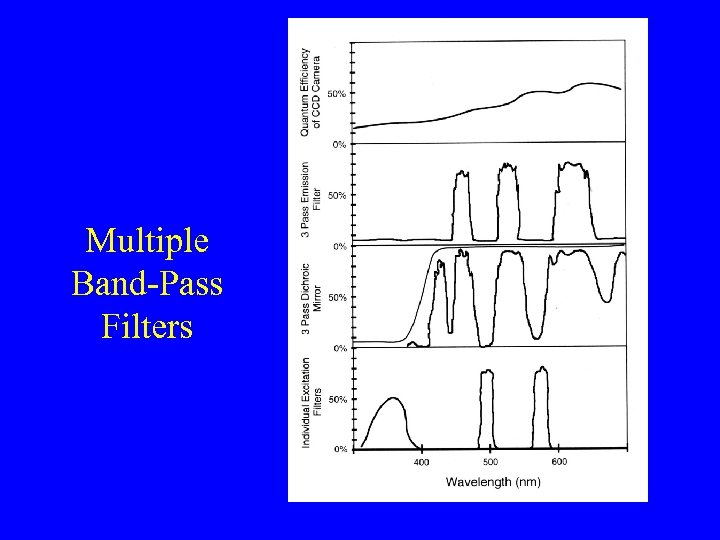 Multiple Band-Pass Filters
Multiple Band-Pass Filters
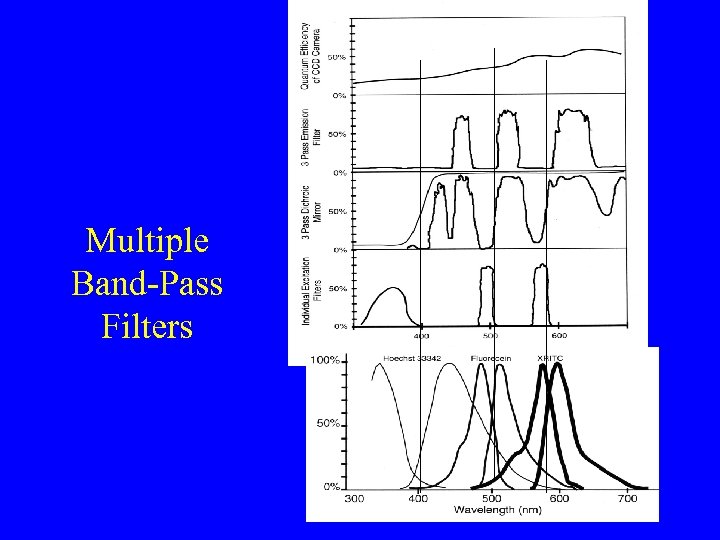 Multiple Band-Pass Filters
Multiple Band-Pass Filters
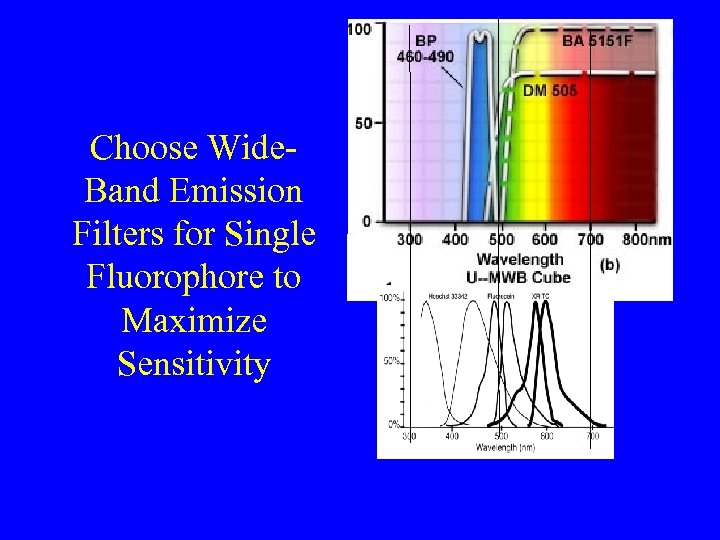 Choose Wide. Band Emission Filters for Single Fluorophore to Maximize Sensitivity
Choose Wide. Band Emission Filters for Single Fluorophore to Maximize Sensitivity
 Chroma Technology Corp. is an employee- owned company that produces the world's finest optical filters and filter sets. The company specializes in the design and manufacture of optical filters and coatings for applications which require the greatest precision in color separation, optical quality and signal purity. For more about us, see our About Chroma page. Welcome to our new website! This site is under construction, so if you don't find what you need please give us a call at (800) 824 -7662. Handbook of Optical Filters for Fluorescence Microscopy: Download a copy of our "Handbook of Optical Filters for Fluorescence Microscopy" in Adobe Acrobat PDF format. www. chroma. com
Chroma Technology Corp. is an employee- owned company that produces the world's finest optical filters and filter sets. The company specializes in the design and manufacture of optical filters and coatings for applications which require the greatest precision in color separation, optical quality and signal purity. For more about us, see our About Chroma page. Welcome to our new website! This site is under construction, so if you don't find what you need please give us a call at (800) 824 -7662. Handbook of Optical Filters for Fluorescence Microscopy: Download a copy of our "Handbook of Optical Filters for Fluorescence Microscopy" in Adobe Acrobat PDF format. www. chroma. com
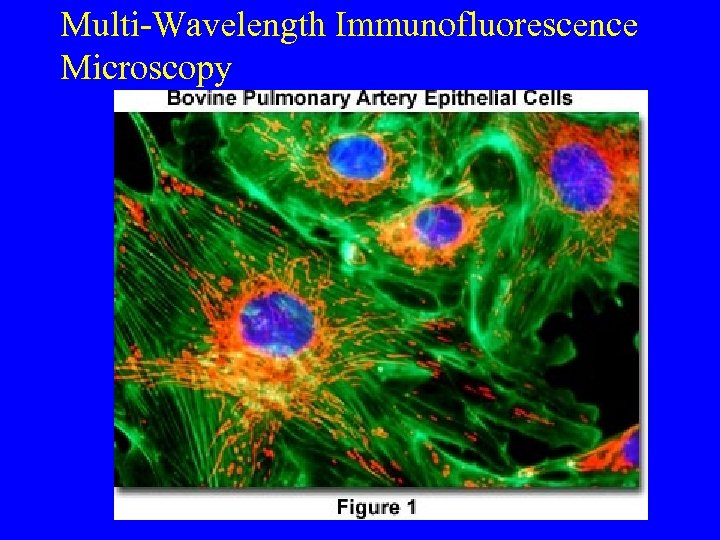 Multi-Wavelength Immunofluorescence Microscopy
Multi-Wavelength Immunofluorescence Microscopy
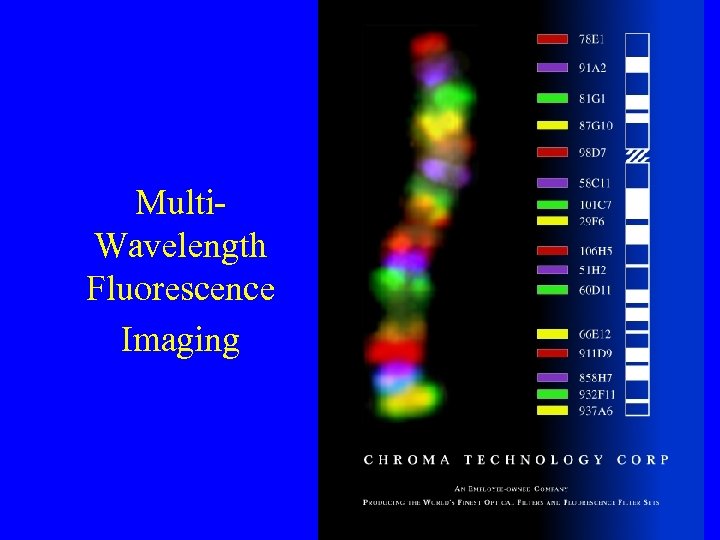 Multi. Wavelength Fluorescence Imaging
Multi. Wavelength Fluorescence Imaging
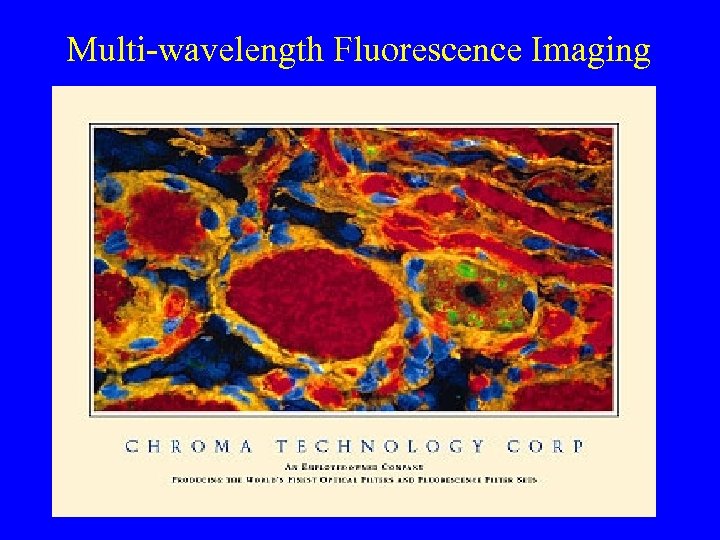 Multi-wavelength Fluorescence Imaging
Multi-wavelength Fluorescence Imaging
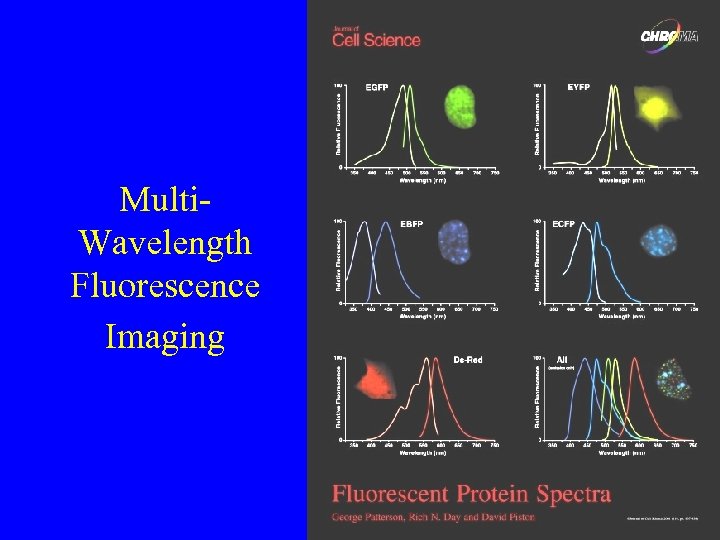 Multi. Wavelength Fluorescence Imaging
Multi. Wavelength Fluorescence Imaging
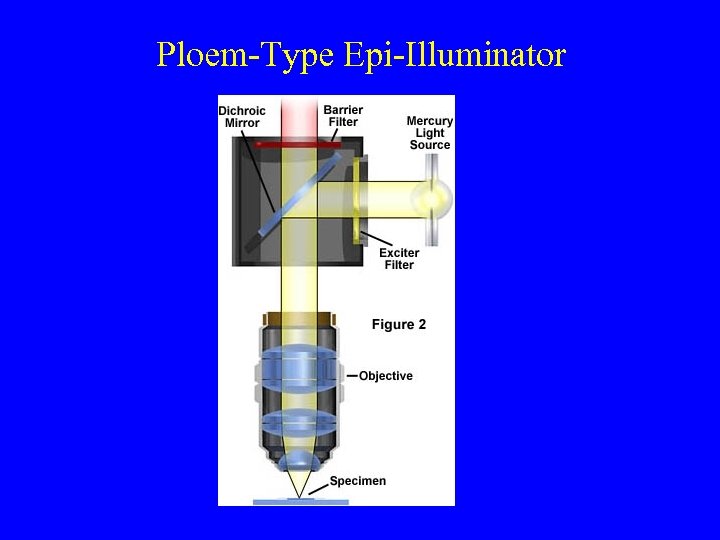 Ploem-Type Epi-Illuminator
Ploem-Type Epi-Illuminator
 Parameters for Maximizing Sensitivity • Use High Objective NA and Lowest Magnification: Ifl ~ Iil. NAobj 4/Mtot 2 • Use high efficiency filters • Use as few optical components as possible • Close Field Diaphragm down as far as possible • Buy the newest objective: select for best efficiency • Match magnification to camera resolution: MMax = 3*Pixel Size of Detector/Optical Resolution E. g. : 3*7 mm/[0. 6 *520 nm/1. 4] = 91 X • Reduce Photobleaching • Use High Quantum Efficiency Detector in Camera
Parameters for Maximizing Sensitivity • Use High Objective NA and Lowest Magnification: Ifl ~ Iil. NAobj 4/Mtot 2 • Use high efficiency filters • Use as few optical components as possible • Close Field Diaphragm down as far as possible • Buy the newest objective: select for best efficiency • Match magnification to camera resolution: MMax = 3*Pixel Size of Detector/Optical Resolution E. g. : 3*7 mm/[0. 6 *520 nm/1. 4] = 91 X • Reduce Photobleaching • Use High Quantum Efficiency Detector in Camera
 Reducing Photobleaching • For fixed specimens use anti-fade compounds: These reduce oxygen effects • 95% glycerol works quite well • For live specimens, reduce oxygen with: - Oxyrase - Catalase + glucose-oxidase
Reducing Photobleaching • For fixed specimens use anti-fade compounds: These reduce oxygen effects • 95% glycerol works quite well • For live specimens, reduce oxygen with: - Oxyrase - Catalase + glucose-oxidase
 Reducing Photobleaching: Anti-Fade Reagents for Fixed Specimens • p-phenylenediamine: The most effective reagent for FITC. Also effective for Rhodamine. Should be adjusted to 0. 1% pphenylenediamine in glycerol/PBS for use. Reagent blackens when subjected to light exposure so it should be stored in a dark place. Skin contact is extremely dangerous. G. D. Johnson & G. M. Araujo (1981) J. Immunol. Methods, 43: 349 -350 • DABCO (1, 4 -diazabi-cyclo-2, 2, 2 -octane): Highly effective for FITC. Although its effect is slightly lower than p-phenylenediamine, it is more resistant to light and features a higher level of safety. G. D. Johnson et. al. , (1982) J. Immunol. Methods, 55: 231 -242. • n-propylgallate: The most effective reagent for Rhodamine, also effective for FITC. Should be adjusted to 1% propylgallate in glycerol/PBS for use. H. Giloh & J. W. Sedat (1982), Science, 217: 1252 -12552. • mercapto-ethylamine: Used to observe chromosome and DNA specimens stained with propidium iodide, acridine orange, or Chromomysin A 3. Should be adjusted to 0. 1 m. M 2 mercaptotheylamine in Tris-EDTAS. Fujita & T. Minamikawa (1990), Experimental Medicine, 8: 75 -82
Reducing Photobleaching: Anti-Fade Reagents for Fixed Specimens • p-phenylenediamine: The most effective reagent for FITC. Also effective for Rhodamine. Should be adjusted to 0. 1% pphenylenediamine in glycerol/PBS for use. Reagent blackens when subjected to light exposure so it should be stored in a dark place. Skin contact is extremely dangerous. G. D. Johnson & G. M. Araujo (1981) J. Immunol. Methods, 43: 349 -350 • DABCO (1, 4 -diazabi-cyclo-2, 2, 2 -octane): Highly effective for FITC. Although its effect is slightly lower than p-phenylenediamine, it is more resistant to light and features a higher level of safety. G. D. Johnson et. al. , (1982) J. Immunol. Methods, 55: 231 -242. • n-propylgallate: The most effective reagent for Rhodamine, also effective for FITC. Should be adjusted to 1% propylgallate in glycerol/PBS for use. H. Giloh & J. W. Sedat (1982), Science, 217: 1252 -12552. • mercapto-ethylamine: Used to observe chromosome and DNA specimens stained with propidium iodide, acridine orange, or Chromomysin A 3. Should be adjusted to 0. 1 m. M 2 mercaptotheylamine in Tris-EDTAS. Fujita & T. Minamikawa (1990), Experimental Medicine, 8: 75 -82
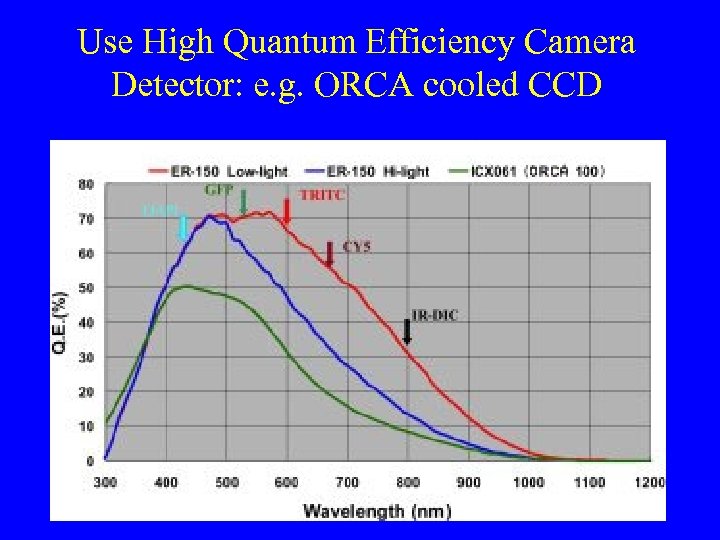 Use High Quantum Efficiency Camera Detector: e. g. ORCA cooled CCD
Use High Quantum Efficiency Camera Detector: e. g. ORCA cooled CCD
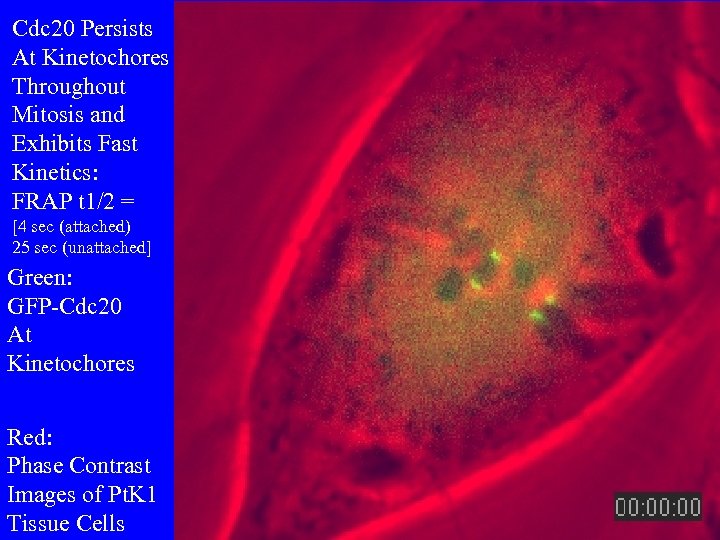 Cdc 20 Persists At Kinetochores Throughout Mitosis and Exhibits Fast Kinetics: FRAP t 1/2 = [4 sec (attached) 25 sec (unattached] Green: GFP-Cdc 20 At Kinetochores Red: Phase Contrast Images of Pt. K 1 Tissue Cells
Cdc 20 Persists At Kinetochores Throughout Mitosis and Exhibits Fast Kinetics: FRAP t 1/2 = [4 sec (attached) 25 sec (unattached] Green: GFP-Cdc 20 At Kinetochores Red: Phase Contrast Images of Pt. K 1 Tissue Cells


