1a2aef3fd0c5c91f5fba1cdb18c864d3.ppt
- Количество слайдов: 31
 Fundamental Concepts of Thermodynamics • • First, second, and third law Entropy Heat capacity, enthalpy Reaction enthalpies and thermochemical cycles • Phase transitions • Calorimetry
Fundamental Concepts of Thermodynamics • • First, second, and third law Entropy Heat capacity, enthalpy Reaction enthalpies and thermochemical cycles • Phase transitions • Calorimetry

 Why I Count Calories for a Living • They are fascinating – Energetics whisper secrets of the strength of chemical bonds – Entropies sing of vibrating atoms, moving electrons, and structural disorder – Systematics have predictive power • They pay – thermodynamic data are essential to good materials processing – Environmental science needs thermodynamics, both for issues of stability and as a starting point for kinetics – Mineralogy, petrology, and deep Earth geophysics need thermodynamic data.
Why I Count Calories for a Living • They are fascinating – Energetics whisper secrets of the strength of chemical bonds – Entropies sing of vibrating atoms, moving electrons, and structural disorder – Systematics have predictive power • They pay – thermodynamic data are essential to good materials processing – Environmental science needs thermodynamics, both for issues of stability and as a starting point for kinetics – Mineralogy, petrology, and deep Earth geophysics need thermodynamic data.
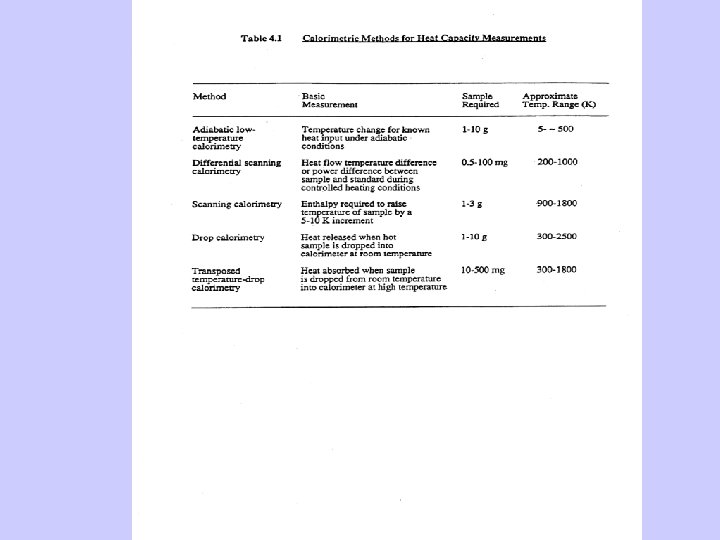
 Calorimetry Measures • Heat capacities • Heats of phase transitions’ • Heats of formation
Calorimetry Measures • Heat capacities • Heats of phase transitions’ • Heats of formation
 From these data one calculates • Entropies and free energies • Solubililities • Phase diagrams • Petrologic and geochemical processes • Materials synthesis and compatibility
From these data one calculates • Entropies and free energies • Solubililities • Phase diagrams • Petrologic and geochemical processes • Materials synthesis and compatibility
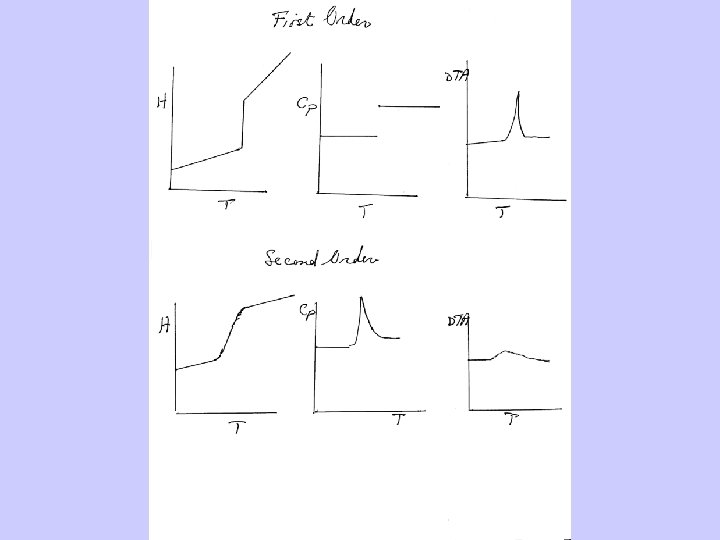
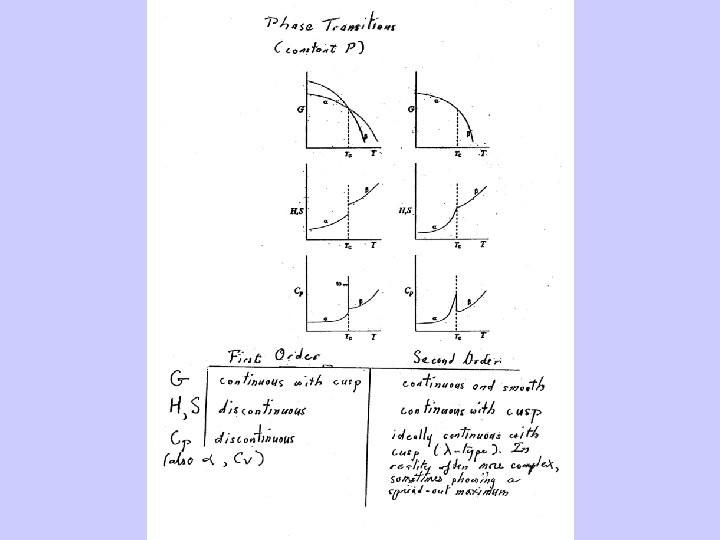
 Phase Transitions
Phase Transitions
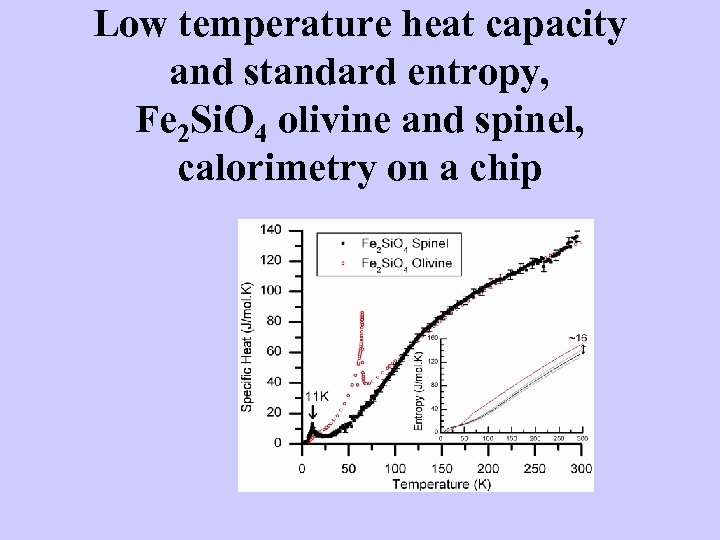 Low temperature heat capacity and standard entropy, Fe 2 Si. O 4 olivine and spinel, calorimetry on a chip
Low temperature heat capacity and standard entropy, Fe 2 Si. O 4 olivine and spinel, calorimetry on a chip
 Thermal Analysis and Scanning Calorimetry • Measure a signal (mass, heat, evolved gas, lemgth, X-ray pattern) at a variable heating (cooing) rate • Systems – Room temp to 600 o. C, common – 600 -1500 o. C, less common but we have – 1500 -2400 o. C, uncommon but we have
Thermal Analysis and Scanning Calorimetry • Measure a signal (mass, heat, evolved gas, lemgth, X-ray pattern) at a variable heating (cooing) rate • Systems – Room temp to 600 o. C, common – 600 -1500 o. C, less common but we have – 1500 -2400 o. C, uncommon but we have
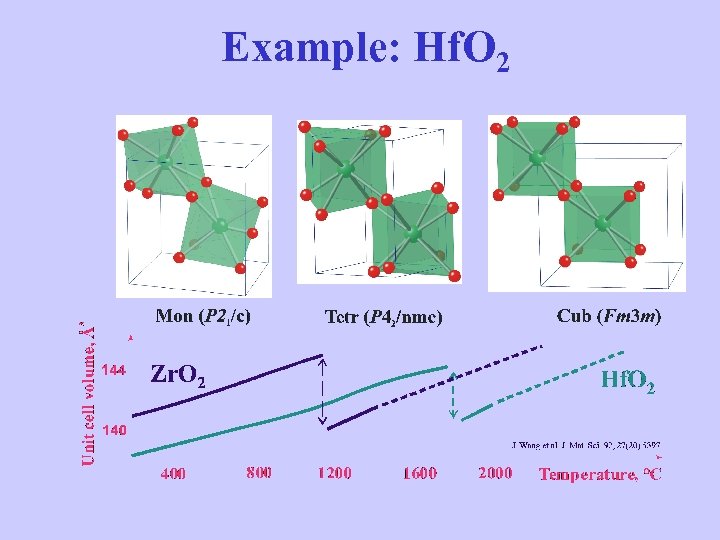 Example: Hf. O 2
Example: Hf. O 2
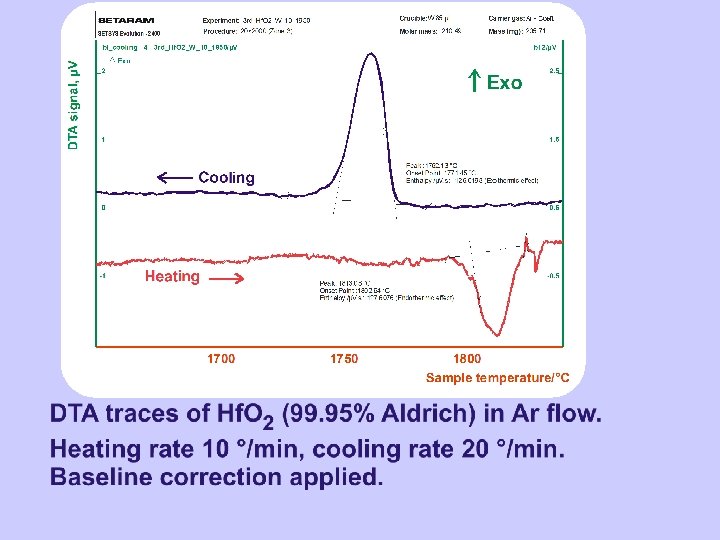
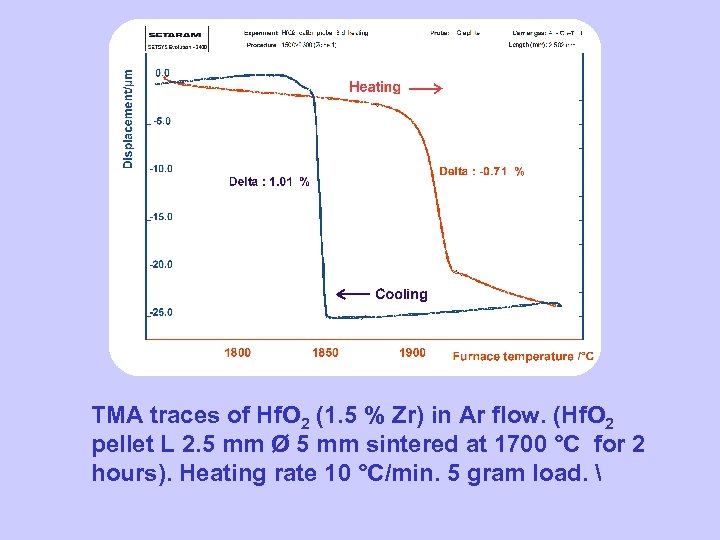 TMA on Hf. O 2 TMA traces of Hf. O 2 (1. 5 % Zr) in Ar flow. (Hf. O 2 pellet L 2. 5 mm Ø 5 mm sintered at 1700 °C for 2 hours). Heating rate 10 °C/min. 5 gram load.
TMA on Hf. O 2 TMA traces of Hf. O 2 (1. 5 % Zr) in Ar flow. (Hf. O 2 pellet L 2. 5 mm Ø 5 mm sintered at 1700 °C for 2 hours). Heating rate 10 °C/min. 5 gram load.

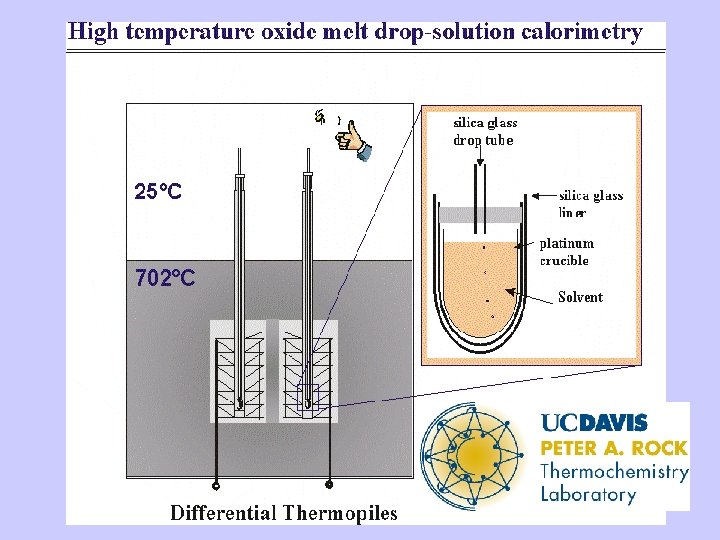
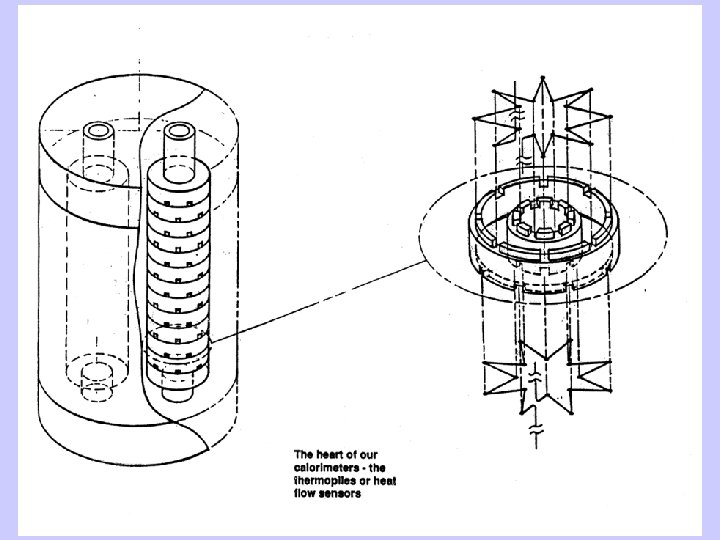
 High Temperature Oxide Melt Solution Calorimetry • Dissolve oxide samples (5 -15 mg) in a molten oxide solvent (20 g) at to form a dilute solution • Difference in heat of solution of reactants and products gives heat of reaction • Oxidative reactions for nitrides, sulfides, selenides, carbides • Needed for ceramic materials which do not dissolve in aqueous solvents
High Temperature Oxide Melt Solution Calorimetry • Dissolve oxide samples (5 -15 mg) in a molten oxide solvent (20 g) at to form a dilute solution • Difference in heat of solution of reactants and products gives heat of reaction • Oxidative reactions for nitrides, sulfides, selenides, carbides • Needed for ceramic materials which do not dissolve in aqueous solvents
 Solvents and Systems • Lead borate (2 Pb. O-4 B 2 O 3, sodium molybdate (3 Na 2 O-4 Mo. O 3), alkali borate • Oxides dissolve • H 2 O and CO 2 evolve as gases • Nitride oxidized to evolved N 2 • Sulfide oxidized to dissolved sulfate
Solvents and Systems • Lead borate (2 Pb. O-4 B 2 O 3, sodium molybdate (3 Na 2 O-4 Mo. O 3), alkali borate • Oxides dissolve • H 2 O and CO 2 evolve as gases • Nitride oxidized to evolved N 2 • Sulfide oxidized to dissolved sulfate
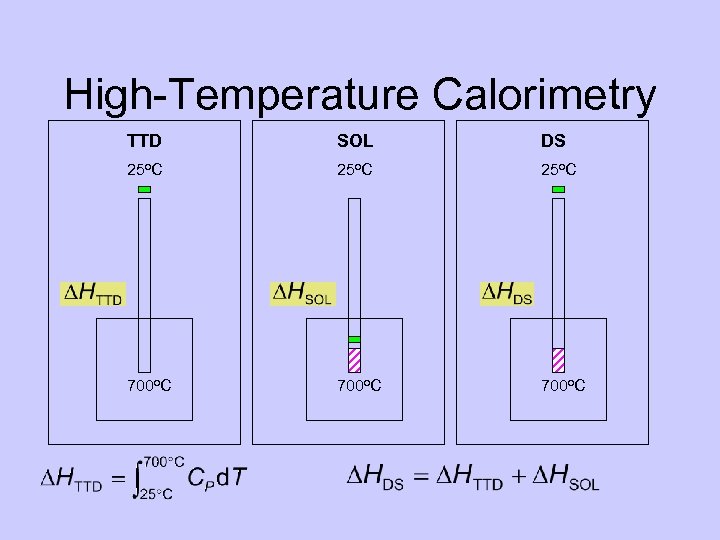 High-Temperature Calorimetry TTD SOL DS 25 o. C 700 o. C
High-Temperature Calorimetry TTD SOL DS 25 o. C 700 o. C
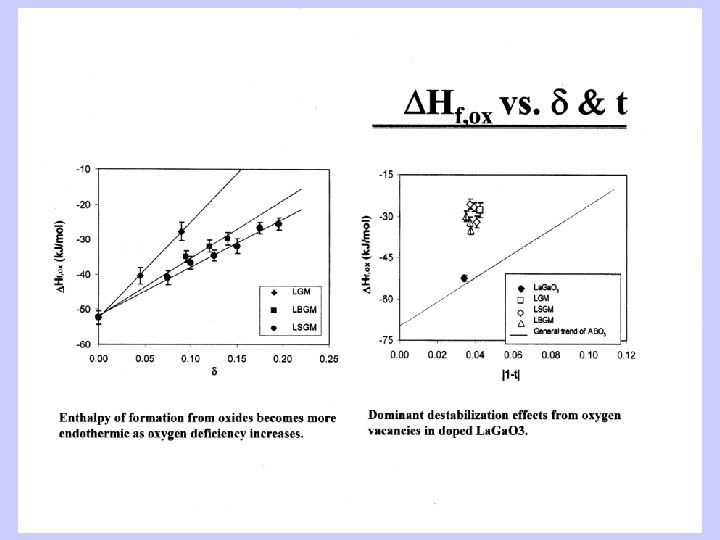
 Thermochemical Cycles Perovskites • 1. AO(xl, 298 K) = AO(dissolved, 973 K) • 2. BO 2(xl, 298 K) =BO 2(dissolved, 973 K) • 3. ABO 3(xl, 298 K) = ABO 3(dissolved, 973 K) _______________ • 4. AO(xl, 298 K) + BO 2(xl, 298 K) = ABO 3(xl, 298 K) , H 4 = H 1 + H 2 – H 3 • Tolerance factor t = d. AO/1. 414 d. BO
Thermochemical Cycles Perovskites • 1. AO(xl, 298 K) = AO(dissolved, 973 K) • 2. BO 2(xl, 298 K) =BO 2(dissolved, 973 K) • 3. ABO 3(xl, 298 K) = ABO 3(dissolved, 973 K) _______________ • 4. AO(xl, 298 K) + BO 2(xl, 298 K) = ABO 3(xl, 298 K) , H 4 = H 1 + H 2 – H 3 • Tolerance factor t = d. AO/1. 414 d. BO


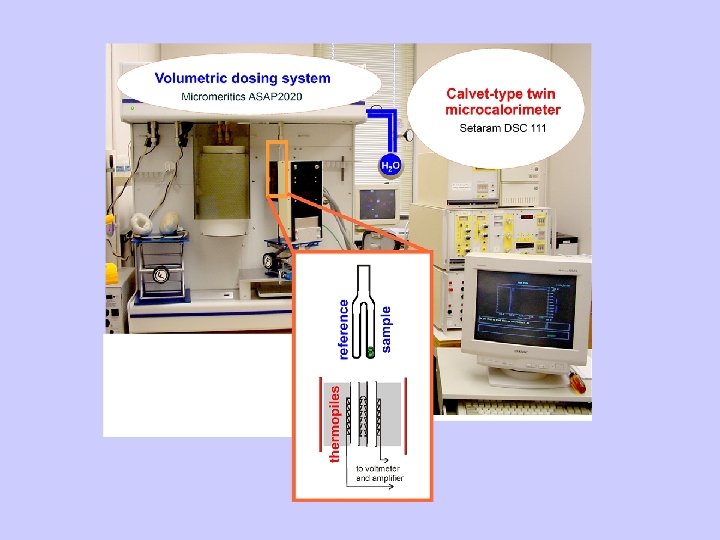
 Gas Adsorption Calorimetry • Combine sensitive microcalorimeter with automated gas dosing system • Measure heat of adsorption and adsorption isotherm simultaneously • Apply to high surface area and microporous materials
Gas Adsorption Calorimetry • Combine sensitive microcalorimeter with automated gas dosing system • Measure heat of adsorption and adsorption isotherm simultaneously • Apply to high surface area and microporous materials
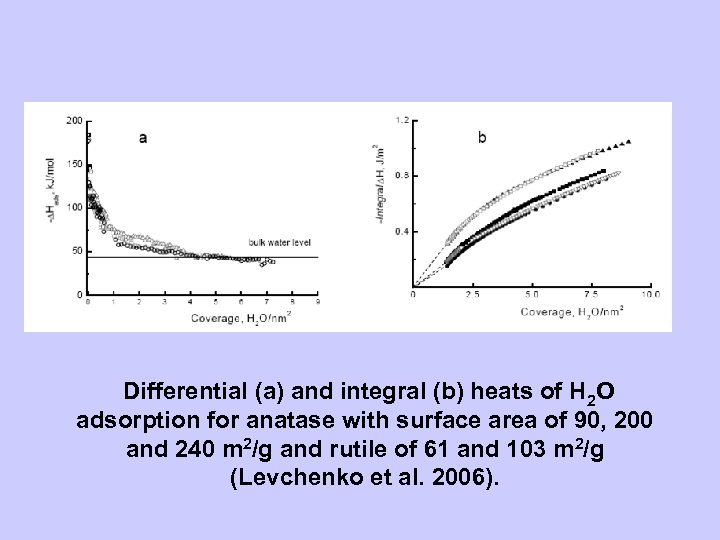 Differential (a) and integral (b) heats of H 2 O adsorption for anatase with surface area of 90, 200 and 240 m 2/g and rutile of 61 and 103 m 2/g (Levchenko et al. 2006).
Differential (a) and integral (b) heats of H 2 O adsorption for anatase with surface area of 90, 200 and 240 m 2/g and rutile of 61 and 103 m 2/g (Levchenko et al. 2006).
 The Peter A. Rock Thermochemistry Laboratory • A unique suite of equipment and expertise • Can design a calorimetric experiment to suit almost any material and problem
The Peter A. Rock Thermochemistry Laboratory • A unique suite of equipment and expertise • Can design a calorimetric experiment to suit almost any material and problem


