dce383d2723e2f4cb94b4806ccc8116c.ppt
- Количество слайдов: 20
 Fun with Mechanical Engineering: 1. Engineering scrutiny 2. History of internal combustion engines http: //ronney. usc. edu/AME 436 S 05 Paul D. Ronney University of Southern California, USA National Central University Jhong-Li, Taiwan, October 3, 2005 Travel supported by the Combustion Institute
Fun with Mechanical Engineering: 1. Engineering scrutiny 2. History of internal combustion engines http: //ronney. usc. edu/AME 436 S 05 Paul D. Ronney University of Southern California, USA National Central University Jhong-Li, Taiwan, October 3, 2005 Travel supported by the Combustion Institute
 University of Southern California Ø Established 125 years ago this week! Ø …jointly by a Catholic, a Protestant and a Jew - USC has always been a multi-ethnic, multi-cultural, coeducational university Ø Today: 32, 000 students, 3000 faculty Ø 2 main campuses: University Park and Health Sciences Ø USC Trojans football team ranked #1 in USA last 2 years
University of Southern California Ø Established 125 years ago this week! Ø …jointly by a Catholic, a Protestant and a Jew - USC has always been a multi-ethnic, multi-cultural, coeducational university Ø Today: 32, 000 students, 3000 faculty Ø 2 main campuses: University Park and Health Sciences Ø USC Trojans football team ranked #1 in USA last 2 years
 USC Viterbi School of Engineering Ø Ø Ø Naming gift by Andrew & Erma Viterbi Andrew Viterbi: co-founder of Qualcomm, co-inventor of CDMA 1900 undergraduates, 3300 graduate students, 165 faculty, 30 degree options Ø $135 million external research funding Ø Distance Education Network (DEN): 900 students in 28 M. S. degree programs; 171 MS degrees awarded in 2005 Ø More info: http: //viterbi. usc. edu
USC Viterbi School of Engineering Ø Ø Ø Naming gift by Andrew & Erma Viterbi Andrew Viterbi: co-founder of Qualcomm, co-inventor of CDMA 1900 undergraduates, 3300 graduate students, 165 faculty, 30 degree options Ø $135 million external research funding Ø Distance Education Network (DEN): 900 students in 28 M. S. degree programs; 171 MS degrees awarded in 2005 Ø More info: http: //viterbi. usc. edu
 Paul Ronney Ø Ø B. S. in Mechanical Engineering, UC Berkeley M. S. in Aeronautics, Caltech Ph. D. in Aeronautics & Astronautics, MIT Postdocs: NASA Glenn, Cleveland; US Naval Research Lab, Washington DC Ø Assistant Professor, Princeton University Ø Associate/Full Professor, USC Ø Research interests Ø Microscale combustion and power generation (10/4, INER; 10/5 NCKU) Ø Microgravity combustion and fluid mechanics (10/4, NCU) Ø Turbulent combustion (10/7, NTHU) Ø Internal combustion engines Ø Ignition, flammability, extinction limits of flames (10/3, NCU) Ø Flame spread over solid fuel beds Ø Biophysics and biofilms (10/6, NCKU)
Paul Ronney Ø Ø B. S. in Mechanical Engineering, UC Berkeley M. S. in Aeronautics, Caltech Ph. D. in Aeronautics & Astronautics, MIT Postdocs: NASA Glenn, Cleveland; US Naval Research Lab, Washington DC Ø Assistant Professor, Princeton University Ø Associate/Full Professor, USC Ø Research interests Ø Microscale combustion and power generation (10/4, INER; 10/5 NCKU) Ø Microgravity combustion and fluid mechanics (10/4, NCU) Ø Turbulent combustion (10/7, NTHU) Ø Internal combustion engines Ø Ignition, flammability, extinction limits of flames (10/3, NCU) Ø Flame spread over solid fuel beds Ø Biophysics and biofilms (10/6, NCKU)
 Paul Ronney
Paul Ronney
 “Engineering scrutiny” 1. Smoke test Ø Equivalent in building electronics: turn the power switch on and see if it smokes Ø For analysis: check the units - this will catch 90% of your mistakes Ø Example: I just derived the ideal gas law as Pv = R/T, obviously units are wrong Ø Other rules Ø Anything inside a square root, cube root, etc. must have units that is a square (e. g. m 2/sec 2) or cube, etc. Ø Anything inside a log, exponent, trigonometric function, etc. , must be dimensionless Ø Any two quantities that are added together must have the same units
“Engineering scrutiny” 1. Smoke test Ø Equivalent in building electronics: turn the power switch on and see if it smokes Ø For analysis: check the units - this will catch 90% of your mistakes Ø Example: I just derived the ideal gas law as Pv = R/T, obviously units are wrong Ø Other rules Ø Anything inside a square root, cube root, etc. must have units that is a square (e. g. m 2/sec 2) or cube, etc. Ø Anything inside a log, exponent, trigonometric function, etc. , must be dimensionless Ø Any two quantities that are added together must have the same units
 “Engineering scrutiny” 2. Function test Ø Equivalent in building electronics: does the device do what it was designed it to do, e. g. the red light blinks when I flip switch on, the bell rings when I push the button, etc. Ø For analysis: does the result gives sensible predictions? Ø Determine if sign (+ or -) of result is reasonable, e. g. if predicted absolute temperature is – 72 K, obviously it’s wrong Ø Determine whether what happens to y as x goes up or down is reasonable or not. For example, in the ideal gas law, Pv = RT: Ø At fixed v, as T increases then P increases – reasonable Ø At fixed T, as v increases then P decreases – reasonable Ø Etc.
“Engineering scrutiny” 2. Function test Ø Equivalent in building electronics: does the device do what it was designed it to do, e. g. the red light blinks when I flip switch on, the bell rings when I push the button, etc. Ø For analysis: does the result gives sensible predictions? Ø Determine if sign (+ or -) of result is reasonable, e. g. if predicted absolute temperature is – 72 K, obviously it’s wrong Ø Determine whether what happens to y as x goes up or down is reasonable or not. For example, in the ideal gas law, Pv = RT: Ø At fixed v, as T increases then P increases – reasonable Ø At fixed T, as v increases then P decreases – reasonable Ø Etc.
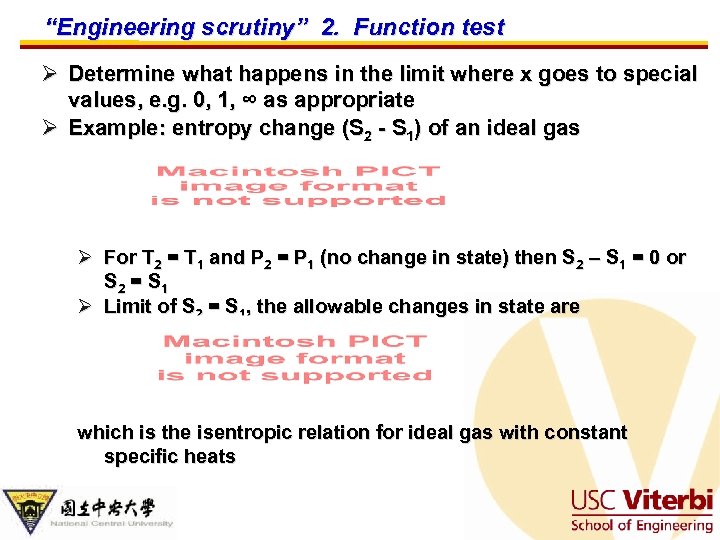 “Engineering scrutiny” 2. Function test Ø Determine what happens in the limit where x goes to special values, e. g. 0, 1, ∞ as appropriate Ø Example: entropy change (S 2 - S 1) of an ideal gas Ø For T 2 = T 1 and P 2 = P 1 (no change in state) then S 2 – S 1 = 0 or S 2 = S 1 Ø Limit of S 2 = S 1, the allowable changes in state are which is the isentropic relation for ideal gas with constant specific heats
“Engineering scrutiny” 2. Function test Ø Determine what happens in the limit where x goes to special values, e. g. 0, 1, ∞ as appropriate Ø Example: entropy change (S 2 - S 1) of an ideal gas Ø For T 2 = T 1 and P 2 = P 1 (no change in state) then S 2 – S 1 = 0 or S 2 = S 1 Ø Limit of S 2 = S 1, the allowable changes in state are which is the isentropic relation for ideal gas with constant specific heats
 “Engineering scrutiny” 3. Performance test Ø Equivalent in building electronics: how fast, how accurate, etc. is the device Ø For analysis: how accurate is the result? Ø Need to compare result to something else, e. g. a “careful” experiment, more sophisticated analysis, trusted published result, etc. Ø Example, I derived the ideal gas law and predicted Pv = 7 RT - passes smoke and function tests, but fails the performance test miserably (by a factor of 7)
“Engineering scrutiny” 3. Performance test Ø Equivalent in building electronics: how fast, how accurate, etc. is the device Ø For analysis: how accurate is the result? Ø Need to compare result to something else, e. g. a “careful” experiment, more sophisticated analysis, trusted published result, etc. Ø Example, I derived the ideal gas law and predicted Pv = 7 RT - passes smoke and function tests, but fails the performance test miserably (by a factor of 7)
 Why internal combustion engines? Ø Alternatives - external combustion - "steam engine, " "Stirling cycle” Ø Heat transfer, gasoline engine Ø Heat transfer per unit area (q/A) = k(d. T/dx) Ø Turbulent mixture inside engine: k ≈ 100 kno turbulence ≈ 2. 5 W/m. K Ø d. T/dx ≈ T/ x ≈ 1500 K / 0. 02 m Ø q/A ≈ 187, 500 W/m 2 Ø Combustion: q/A = Yf. QRST = (10 kg/m 3) x 0. 067 x (4. 5 x 107 J/kg) x 2 m/s = 60. 3 x 106 W/m 2 - 321 x higher! Ø CONCLUSION: HEAT TRANSFER IS TOO SLOW!!! Ø That’s why 10 Boeing 747 engines ≈ large (1 gigawatt) coal-fueled electric power plant k = gas thermal conductivity, T = temperature, x = distance, = density, Yf = fuel mass fraction, QR = fuel heating value, ST = turbulent flame speed in engine
Why internal combustion engines? Ø Alternatives - external combustion - "steam engine, " "Stirling cycle” Ø Heat transfer, gasoline engine Ø Heat transfer per unit area (q/A) = k(d. T/dx) Ø Turbulent mixture inside engine: k ≈ 100 kno turbulence ≈ 2. 5 W/m. K Ø d. T/dx ≈ T/ x ≈ 1500 K / 0. 02 m Ø q/A ≈ 187, 500 W/m 2 Ø Combustion: q/A = Yf. QRST = (10 kg/m 3) x 0. 067 x (4. 5 x 107 J/kg) x 2 m/s = 60. 3 x 106 W/m 2 - 321 x higher! Ø CONCLUSION: HEAT TRANSFER IS TOO SLOW!!! Ø That’s why 10 Boeing 747 engines ≈ large (1 gigawatt) coal-fueled electric power plant k = gas thermal conductivity, T = temperature, x = distance, = density, Yf = fuel mass fraction, QR = fuel heating value, ST = turbulent flame speed in engine
 Why internal combustion engines? Ø Alternatives - electric vehicles Ø Why not generate electricity in a large central power plant ( ≈ 40%), distribute to charge batteries to power electric motors ( ≈ 80%)? Ø Car battery, lead acid: 100 amp-hours, 12 volts, 20 kg; energy/mass = 100 A * 12 V * 3600 sec / 20 kg = 2 x 105 J/kg Ø Gasoline (and other hydrocarbons): 4. 5 x 107 J/kg Ø Batteries are heavy ≈ 1000 lbs/gal of gasoline equivalent Ø Fuel cell systems better, but still nowhere near gasoline Ø "Zero emissions" myth - EVs export pollution Ø Environmental cost of battery materials Ø Possible advantage: makes smaller, lighter, more streamlined cars acceptable to consumers Ø Prediction: eventual conversion of electric vehicles to gasoline power (>100 miles per gallon)
Why internal combustion engines? Ø Alternatives - electric vehicles Ø Why not generate electricity in a large central power plant ( ≈ 40%), distribute to charge batteries to power electric motors ( ≈ 80%)? Ø Car battery, lead acid: 100 amp-hours, 12 volts, 20 kg; energy/mass = 100 A * 12 V * 3600 sec / 20 kg = 2 x 105 J/kg Ø Gasoline (and other hydrocarbons): 4. 5 x 107 J/kg Ø Batteries are heavy ≈ 1000 lbs/gal of gasoline equivalent Ø Fuel cell systems better, but still nowhere near gasoline Ø "Zero emissions" myth - EVs export pollution Ø Environmental cost of battery materials Ø Possible advantage: makes smaller, lighter, more streamlined cars acceptable to consumers Ø Prediction: eventual conversion of electric vehicles to gasoline power (>100 miles per gallon)
 “Zero emission” electric vehicles
“Zero emission” electric vehicles
 Why internal combustion engines? Ø Alternatives - solar Ø Arizona, high noon, mid summer: solar flux ≈ 1000 W/m 2 Ø Gasoline engine, 20 mi/gal, 60 mi/hr, thermal power = (60 mi/hr / 20 mi/gal) x (6 lb/gal) x (kg / 2. 2 lb) x (4. 5 x 107 J/kg) x (hr / 3600 sec) = 102 k. W Ø Need ≈ 100 m 2 collector ≈ 32 ft x 32 ft - lots of air drag, what about underpasses, nighttime, bad weather, northern/southern latitudes, etc. ?
Why internal combustion engines? Ø Alternatives - solar Ø Arizona, high noon, mid summer: solar flux ≈ 1000 W/m 2 Ø Gasoline engine, 20 mi/gal, 60 mi/hr, thermal power = (60 mi/hr / 20 mi/gal) x (6 lb/gal) x (kg / 2. 2 lb) x (4. 5 x 107 J/kg) x (hr / 3600 sec) = 102 k. W Ø Need ≈ 100 m 2 collector ≈ 32 ft x 32 ft - lots of air drag, what about underpasses, nighttime, bad weather, northern/southern latitudes, etc. ?
 Why internal combustion engines? Ø Alternatives - nuclear Ø Who are we kidding ? ? ? Ø Higher energy density though » U 235 fission: 3. 2 x 10 -11 J/atom * (6. 02 x 1023 atom / 0. 235 kg) = 8. 2 x 1013 J/kg ≈ 2 million x hydrocarbons! » Radioactive decay less, but still much higher than hydrocarbon fuel Ø Moral - hard to beat liquid-fueled internal combustion engines for Ø Power/weight & power/volume of engine Ø Energy/weight (4. 5 x 107 J/kg assuming only fuel, not air, is carried) & energy/volume of liquid hydrocarbon fuel Ø Distribution & handling convenience of liquids Ø Conclusion: IC engines are the worst form of vehicle propulsion, except for all the other forms
Why internal combustion engines? Ø Alternatives - nuclear Ø Who are we kidding ? ? ? Ø Higher energy density though » U 235 fission: 3. 2 x 10 -11 J/atom * (6. 02 x 1023 atom / 0. 235 kg) = 8. 2 x 1013 J/kg ≈ 2 million x hydrocarbons! » Radioactive decay less, but still much higher than hydrocarbon fuel Ø Moral - hard to beat liquid-fueled internal combustion engines for Ø Power/weight & power/volume of engine Ø Energy/weight (4. 5 x 107 J/kg assuming only fuel, not air, is carried) & energy/volume of liquid hydrocarbon fuel Ø Distribution & handling convenience of liquids Ø Conclusion: IC engines are the worst form of vehicle propulsion, except for all the other forms
 History of automotive engines Ø 1859 - Oil discovered in Pennsylvania Ø 1876 - Premixed-charge 4 -stroke engine - Otto Ø 1 st practical IC engine Ø Power: 2 hp; Weight: 1250 pounds Ø Comp. ratio = 4 (knock limited), 14% efficiency (theory 38%) Ø Today CR = 8 (still knock limited), 30% efficiency (theory 52%) Ø 1897 - Nonpremixed-charge engine - Diesel - higher efficiency due to Ø Higher compression ratio (no knock problem) Ø No throttling loss - use fuel/air ratio to control power
History of automotive engines Ø 1859 - Oil discovered in Pennsylvania Ø 1876 - Premixed-charge 4 -stroke engine - Otto Ø 1 st practical IC engine Ø Power: 2 hp; Weight: 1250 pounds Ø Comp. ratio = 4 (knock limited), 14% efficiency (theory 38%) Ø Today CR = 8 (still knock limited), 30% efficiency (theory 52%) Ø 1897 - Nonpremixed-charge engine - Diesel - higher efficiency due to Ø Higher compression ratio (no knock problem) Ø No throttling loss - use fuel/air ratio to control power
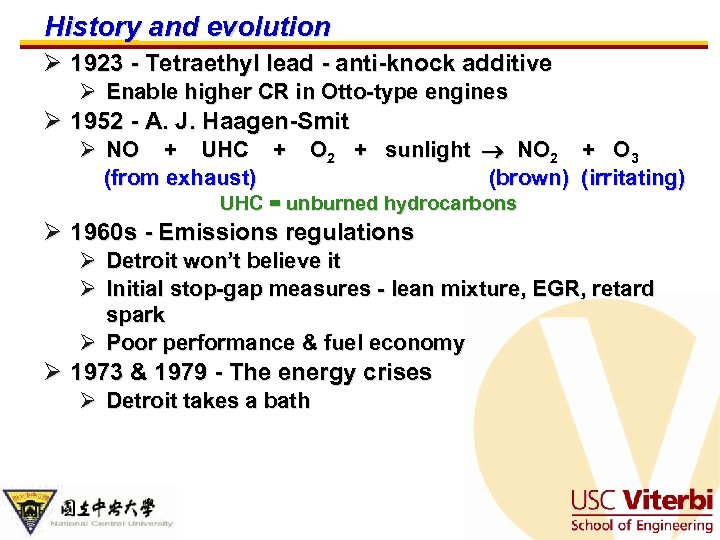 History and evolution Ø 1923 - Tetraethyl lead - anti-knock additive Ø Enable higher CR in Otto-type engines Ø 1952 - A. J. Haagen-Smit Ø NO + UHC + O 2 + sunlight NO 2 + O 3 (from exhaust) (brown) (irritating) UHC = unburned hydrocarbons Ø 1960 s - Emissions regulations Ø Ø Detroit won’t believe it Initial stop-gap measures - lean mixture, EGR, retard spark Ø Poor performance & fuel economy Ø 1973 & 1979 - The energy crises Ø Detroit takes a bath
History and evolution Ø 1923 - Tetraethyl lead - anti-knock additive Ø Enable higher CR in Otto-type engines Ø 1952 - A. J. Haagen-Smit Ø NO + UHC + O 2 + sunlight NO 2 + O 3 (from exhaust) (brown) (irritating) UHC = unburned hydrocarbons Ø 1960 s - Emissions regulations Ø Ø Detroit won’t believe it Initial stop-gap measures - lean mixture, EGR, retard spark Ø Poor performance & fuel economy Ø 1973 & 1979 - The energy crises Ø Detroit takes a bath
 History and evolution Ø 1975 - Catalytic converters, unleaded fuel Ø Detroit forced to buy technology Ø More “aromatics” (e. g. , benzene) in gasoline - high octane but carcinogenic, soot-producing Ø 1980 s - Microcomputer control of engines Ø Tailor operation for best emissions, efficiency, . . . Ø 1990 s - Reformulated gasoline Ø Ø Reduced need for aromatics, cleaner(? ). . . but higher cost, lower miles per gallon Now we find MTBE pollutes groundwater!!! Alternative “oxygenated” fuel additive - ethanol - very attractive to powerful senators from farm states Ø 2000’s - hybrid vehicles Ø Use small gasoline engine operating at maximum power (most efficient way to operate) or turned off if not needed Ø Use generator/batteries/motors to make/store/use surplus power from gasoline engine Ø More efficient, but much more equipment on board - not clear if fuel savings justify extra cost
History and evolution Ø 1975 - Catalytic converters, unleaded fuel Ø Detroit forced to buy technology Ø More “aromatics” (e. g. , benzene) in gasoline - high octane but carcinogenic, soot-producing Ø 1980 s - Microcomputer control of engines Ø Tailor operation for best emissions, efficiency, . . . Ø 1990 s - Reformulated gasoline Ø Ø Reduced need for aromatics, cleaner(? ). . . but higher cost, lower miles per gallon Now we find MTBE pollutes groundwater!!! Alternative “oxygenated” fuel additive - ethanol - very attractive to powerful senators from farm states Ø 2000’s - hybrid vehicles Ø Use small gasoline engine operating at maximum power (most efficient way to operate) or turned off if not needed Ø Use generator/batteries/motors to make/store/use surplus power from gasoline engine Ø More efficient, but much more equipment on board - not clear if fuel savings justify extra cost
 Things you need to understand before. . . …you invent the zero-emission, 100 mpg 1000 hp engine, revolutionize the automotive industry and shop for your retirement home on the French Riviera Ø Room for improvement - factor of 2 in efficiency Ø Ø Ø Ideal Otto cycle engine with CR = 8: 52% Real engine: 25 - 30% Differences because of » Throttling losses » Heat losses » Friction losses » Slow burning » Incomplete combustion is a very minor effect
Things you need to understand before. . . …you invent the zero-emission, 100 mpg 1000 hp engine, revolutionize the automotive industry and shop for your retirement home on the French Riviera Ø Room for improvement - factor of 2 in efficiency Ø Ø Ø Ideal Otto cycle engine with CR = 8: 52% Real engine: 25 - 30% Differences because of » Throttling losses » Heat losses » Friction losses » Slow burning » Incomplete combustion is a very minor effect
 Things you need to understand before. . . Ø Room for improvement - infinite in pollutants Ø Pollutants are a non-equilibrium effect » Burn: Fuel + O 2 + N 2 H 2 O + CO 2 + N 2 + CO + UHC + NO OK OK Bad » Expand: CO + UHC + NO “frozen” at high levels » With slow expansion, no heat loss: CO + UHC + NO H 2 O + CO 2 + N 2 . . . but how to slow the expansion and eliminate heat loss? Ø Worst problems: cold start, transients, old or out-oftune vehicles - 90% of pollution generated by 10% of vehicles
Things you need to understand before. . . Ø Room for improvement - infinite in pollutants Ø Pollutants are a non-equilibrium effect » Burn: Fuel + O 2 + N 2 H 2 O + CO 2 + N 2 + CO + UHC + NO OK OK Bad » Expand: CO + UHC + NO “frozen” at high levels » With slow expansion, no heat loss: CO + UHC + NO H 2 O + CO 2 + N 2 . . . but how to slow the expansion and eliminate heat loss? Ø Worst problems: cold start, transients, old or out-oftune vehicles - 90% of pollution generated by 10% of vehicles
 Things you need to understand before. . . Ø Room for improvement - very little in power Ø IC engines are air processors » Fuel takes up little space » Air flow = power » Limitation on air flow due to • “Choked” flow past intake valves • Friction loss, mechanical strength - limits RPM • Slow burn Ø Majority of power is used to overcome air resistance - smaller, more aerodynamic vehicles beneficial
Things you need to understand before. . . Ø Room for improvement - very little in power Ø IC engines are air processors » Fuel takes up little space » Air flow = power » Limitation on air flow due to • “Choked” flow past intake valves • Friction loss, mechanical strength - limits RPM • Slow burn Ø Majority of power is used to overcome air resistance - smaller, more aerodynamic vehicles beneficial


