f969bcc4fda80cf875d7343ad377babf.ppt
- Количество слайдов: 22
 Fuel Cells with no Precious Metals and no Liquid Electrolyte Shimshon Gottesfeld Cellera Technologies, Ltd Caesaria Industrial Park, Israel TAU, Feb 5, 2010
Fuel Cells with no Precious Metals and no Liquid Electrolyte Shimshon Gottesfeld Cellera Technologies, Ltd Caesaria Industrial Park, Israel TAU, Feb 5, 2010
 Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ?
Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ?
 Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ?
Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ?
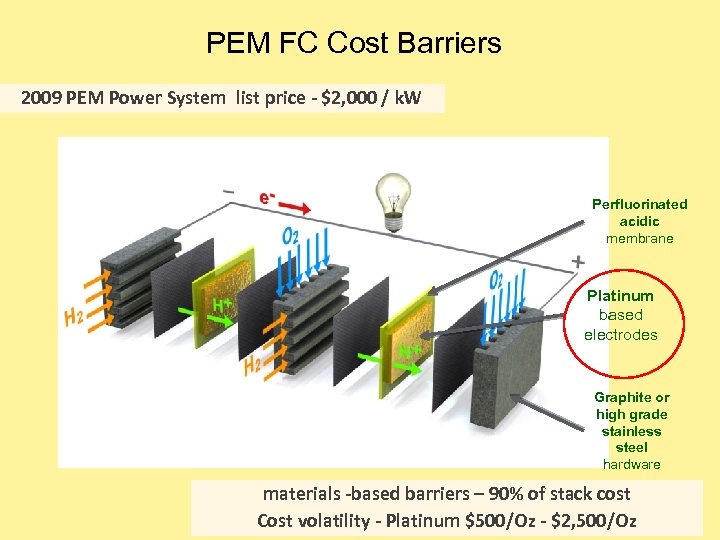 PEM FC Cost Barriers 2009 PEM Power System list price - $2, 000 / k. W Perfluorinated acidic membrane Platinum based electrodes Graphite or high grade stainless steel hardware materials -based barriers – 90% of stack cost Cost volatility - Platinum $500/Oz - $2, 500/Oz 4
PEM FC Cost Barriers 2009 PEM Power System list price - $2, 000 / k. W Perfluorinated acidic membrane Platinum based electrodes Graphite or high grade stainless steel hardware materials -based barriers – 90% of stack cost Cost volatility - Platinum $500/Oz - $2, 500/Oz 4
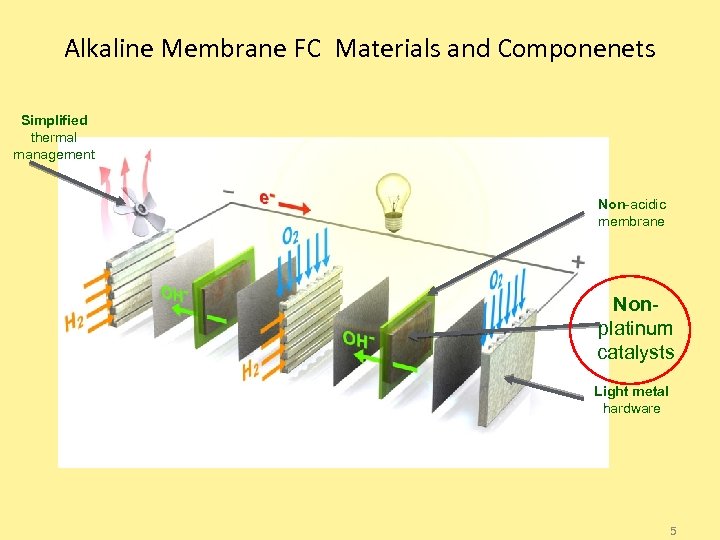 Alkaline Membrane FC Materials and Componenets Simplified thermal management Non-acidic membrane Nonplatinum catalysts Light metal hardware 5
Alkaline Membrane FC Materials and Componenets Simplified thermal management Non-acidic membrane Nonplatinum catalysts Light metal hardware 5
 Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ? metal catalysts & non-metal catalysts , in acid media & alkaline media , is there an activity-determining factor common to all ?
Outline • Brief report on AMFC development at Cellera • Is there a common “activity yardstick” which applies to all fuel cell elecrtocatalysts ? metal catalysts & non-metal catalysts , in acid media & alkaline media , is there an activity-determining factor common to all ?
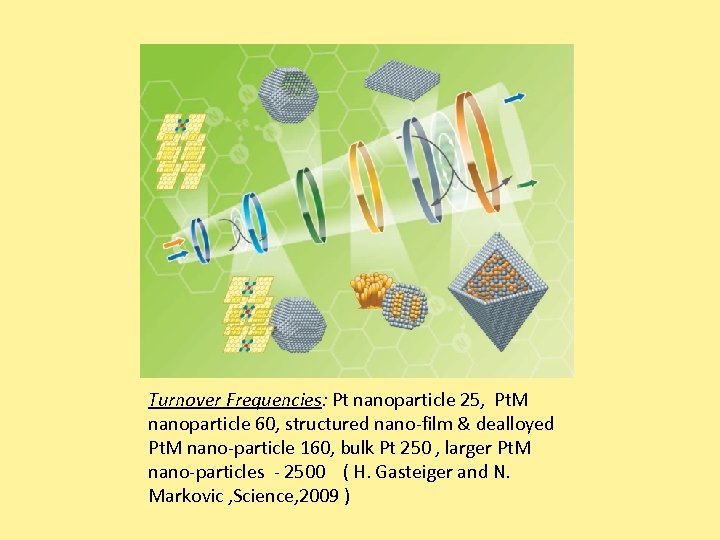 Turnover Frequencies: Pt nanoparticle 25, Pt. M nanoparticle 60, structured nano-film & dealloyed Pt. M nano-particle 160, bulk Pt 250 , larger Pt. M nano-particles - 2500 ( H. Gasteiger and N. Markovic , Science, 2009 )
Turnover Frequencies: Pt nanoparticle 25, Pt. M nanoparticle 60, structured nano-film & dealloyed Pt. M nano-particle 160, bulk Pt 250 , larger Pt. M nano-particles - 2500 ( H. Gasteiger and N. Markovic , Science, 2009 )
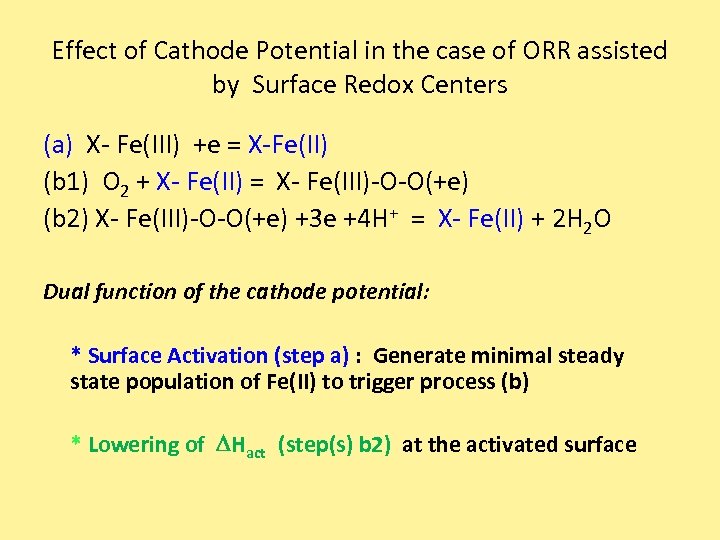 Effect of Cathode Potential in the case of ORR assisted by Surface Redox Centers (a) X- Fe(III) +e = X-Fe(II) (b 1) O 2 + X- Fe(II) = X- Fe(III)-O-O(+e) (b 2) X- Fe(III)-O-O(+e) +3 e +4 H+ = X- Fe(II) + 2 H 2 O Dual function of the cathode potential: * Surface Activation (step a) : Generate minimal steady state population of Fe(II) to trigger process (b) * Lowering of DHact (step(s) b 2) at the activated surface
Effect of Cathode Potential in the case of ORR assisted by Surface Redox Centers (a) X- Fe(III) +e = X-Fe(II) (b 1) O 2 + X- Fe(II) = X- Fe(III)-O-O(+e) (b 2) X- Fe(III)-O-O(+e) +3 e +4 H+ = X- Fe(II) + 2 H 2 O Dual function of the cathode potential: * Surface Activation (step a) : Generate minimal steady state population of Fe(II) to trigger process (b) * Lowering of DHact (step(s) b 2) at the activated surface
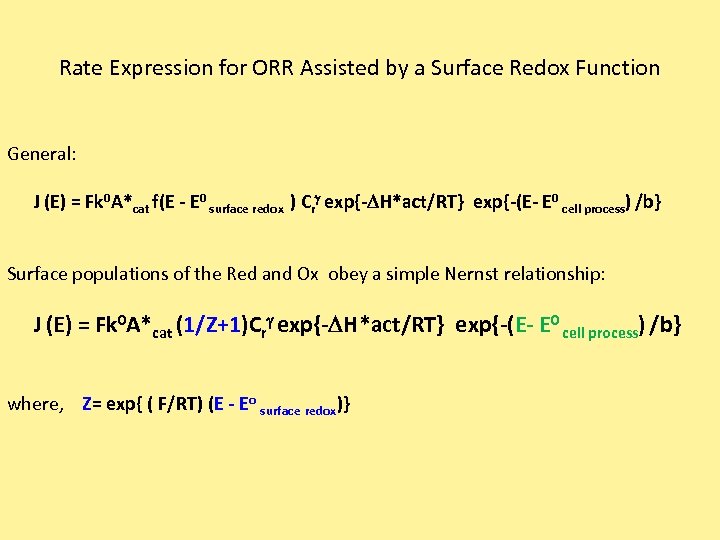 Rate Expression for ORR Assisted by a Surface Redox Function General: J (E) = Fk 0 A*cat f(E - E 0 surface redox ) Crg exp{-DH*act/RT} exp{-(E- E 0 cell process) /b} Surface populations of the Red and Ox obey a simple Nernst relationship: J (E) = Fk 0 A*cat (1/Z+1)Crg exp{-DH*act/RT} exp{-(E- E 0 cell process) /b} where, Z= exp{ ( F/RT) (E - Eo surface redox)}
Rate Expression for ORR Assisted by a Surface Redox Function General: J (E) = Fk 0 A*cat f(E - E 0 surface redox ) Crg exp{-DH*act/RT} exp{-(E- E 0 cell process) /b} Surface populations of the Red and Ox obey a simple Nernst relationship: J (E) = Fk 0 A*cat (1/Z+1)Crg exp{-DH*act/RT} exp{-(E- E 0 cell process) /b} where, Z= exp{ ( F/RT) (E - Eo surface redox)}
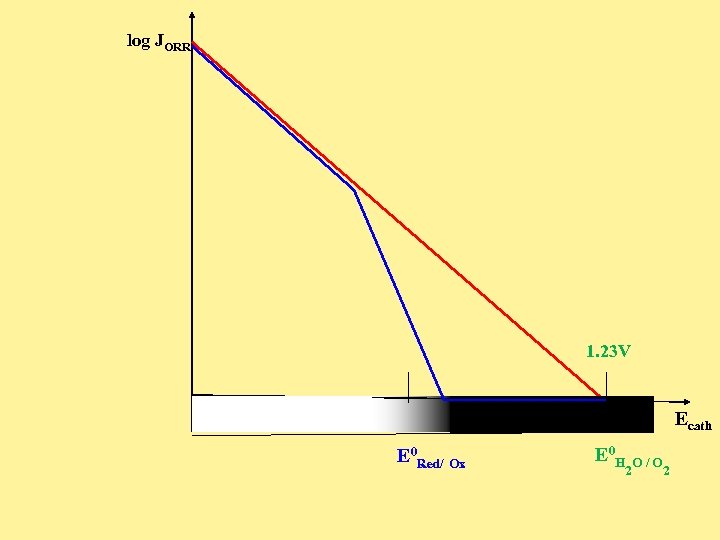 log JORR 1. 23 V Ecath E 0 Red/ Ox E 0 H 2 O/O 2
log JORR 1. 23 V Ecath E 0 Red/ Ox E 0 H 2 O/O 2
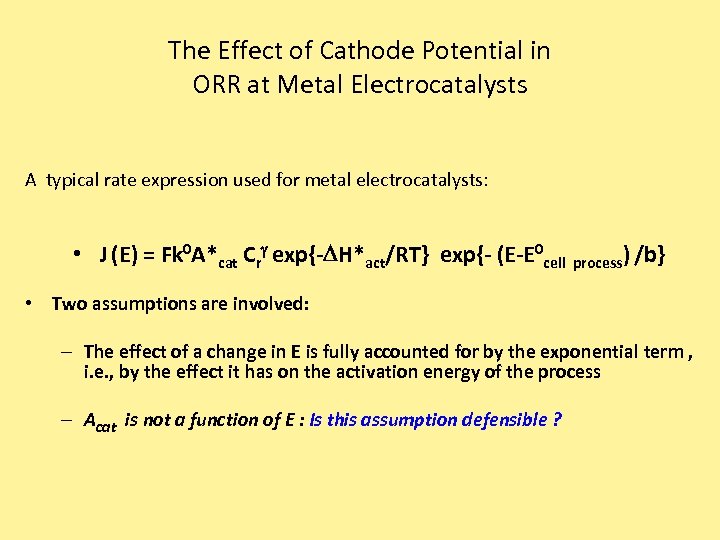 The Effect of Cathode Potential in ORR at Metal Electrocatalysts A typical rate expression used for metal electrocatalysts: • J (E) = Fk 0 A*cat Crg exp{-DH*act/RT} exp{- (E-E 0 cell process) /b} • Two assumptions are involved: – The effect of a change in E is fully accounted for by the exponential term , i. e. , by the effect it has on the activation energy of the process – Acat is not a function of E : Is this assumption defensible ?
The Effect of Cathode Potential in ORR at Metal Electrocatalysts A typical rate expression used for metal electrocatalysts: • J (E) = Fk 0 A*cat Crg exp{-DH*act/RT} exp{- (E-E 0 cell process) /b} • Two assumptions are involved: – The effect of a change in E is fully accounted for by the exponential term , i. e. , by the effect it has on the activation energy of the process – Acat is not a function of E : Is this assumption defensible ?
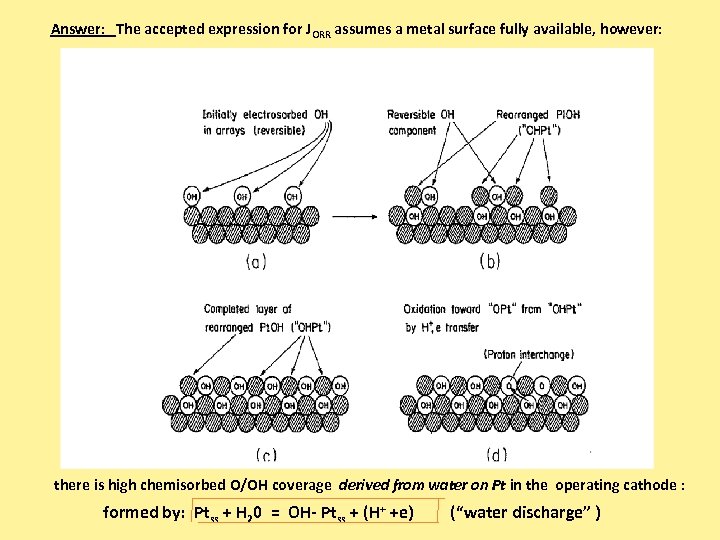 Answer: The accepted expression for JORR assumes a metal surface fully available, however: there is high chemisorbed O/OH coverage derived from water on Pt in the operating cathode : formed by: Ptss + H 20 = OH- Ptss + (H+ +e) (“water discharge” )
Answer: The accepted expression for JORR assumes a metal surface fully available, however: there is high chemisorbed O/OH coverage derived from water on Pt in the operating cathode : formed by: Ptss + H 20 = OH- Ptss + (H+ +e) (“water discharge” )
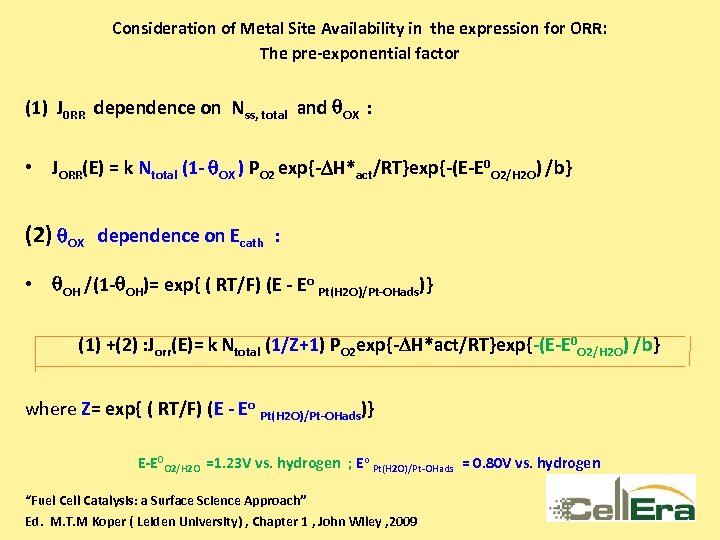 Consideration of Metal Site Availability in the expression for ORR: The pre-exponential factor (1) J 0 RR dependence on Nss, total and q. OX : • JORR(E) = k Ntotal (1 - q. OX ) PO 2 exp{-DH*act/RT}exp{-(E-E 0 O 2/H 2 O) /b} (2) q. OX dependence on Ecath : • q. OH /(1 -q. OH)= exp{ ( RT/F) (E - Eo Pt(H 2 O)/Pt-OHads)} (1) +(2) : Jorr(E)= k Ntotal (1/Z+1) PO 2 exp{-DH*act/RT}exp{-(E-E 0 O 2/H 2 O) /b} where Z= exp{ ( RT/F) (E - Eo Pt(H 2 O)/Pt-OHads)} E-E 0 O 2/H 2 O =1. 23 V vs. hydrogen ; Eo Pt(H 2 O)/Pt-OHads = 0. 80 V vs. hydrogen “Fuel Cell Catalysis: a Surface Science Approach” Ed. M. T. M Koper ( Leiden University) , Chapter 1 , John Wiley , 2009
Consideration of Metal Site Availability in the expression for ORR: The pre-exponential factor (1) J 0 RR dependence on Nss, total and q. OX : • JORR(E) = k Ntotal (1 - q. OX ) PO 2 exp{-DH*act/RT}exp{-(E-E 0 O 2/H 2 O) /b} (2) q. OX dependence on Ecath : • q. OH /(1 -q. OH)= exp{ ( RT/F) (E - Eo Pt(H 2 O)/Pt-OHads)} (1) +(2) : Jorr(E)= k Ntotal (1/Z+1) PO 2 exp{-DH*act/RT}exp{-(E-E 0 O 2/H 2 O) /b} where Z= exp{ ( RT/F) (E - Eo Pt(H 2 O)/Pt-OHads)} E-E 0 O 2/H 2 O =1. 23 V vs. hydrogen ; Eo Pt(H 2 O)/Pt-OHads = 0. 80 V vs. hydrogen “Fuel Cell Catalysis: a Surface Science Approach” Ed. M. T. M Koper ( Leiden University) , Chapter 1 , John Wiley , 2009
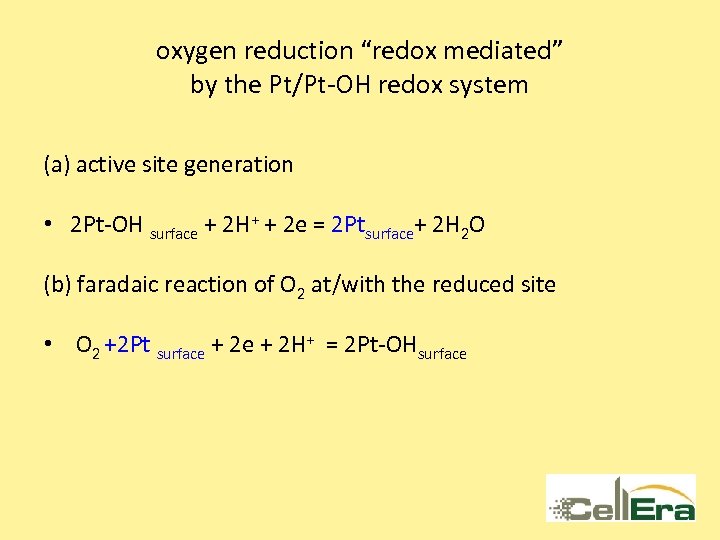 oxygen reduction “redox mediated” by the Pt/Pt-OH redox system (a) active site generation • 2 Pt-OH surface + 2 H+ + 2 e = 2 Ptsurface+ 2 H 2 O (b) faradaic reaction of O 2 at/with the reduced site • O 2 +2 Pt surface + 2 H+ = 2 Pt-OHsurface
oxygen reduction “redox mediated” by the Pt/Pt-OH redox system (a) active site generation • 2 Pt-OH surface + 2 H+ + 2 e = 2 Ptsurface+ 2 H 2 O (b) faradaic reaction of O 2 at/with the reduced site • O 2 +2 Pt surface + 2 H+ = 2 Pt-OHsurface
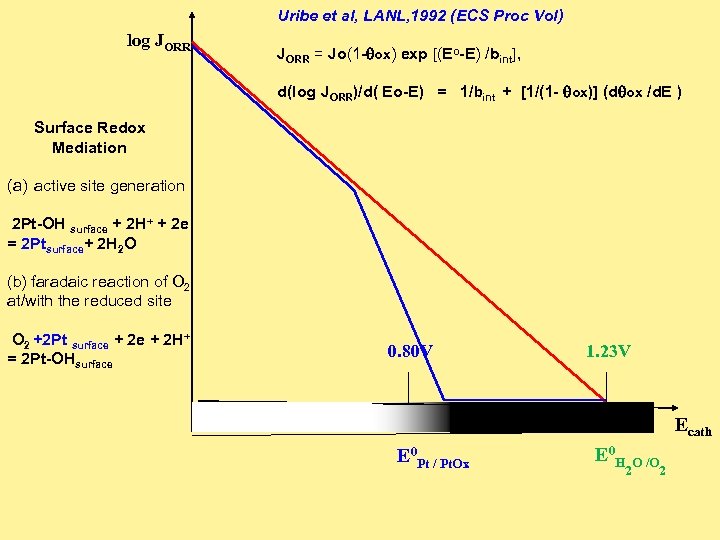 Uribe et al, LANL, 1992 (ECS Proc Vol) log JORR Surface Redox Mediation JORR = Jo(1 -qox) exp [(Eo-E) /bint], d(log JORR)/d( Eo-E) = 1/bint + [1/(1 - qox)] (dqox /d. E ) (a) active site generation 2 Pt-OH surface + 2 H+ + 2 e = 2 Ptsurface+ 2 H 2 O (b) faradaic reaction of O 2 at/with the reduced site O 2 +2 Pt surface + 2 H+ = 2 Pt-OHsurface 0. 80 V 1. 23 V Ecath E 0 Pt / Pt. Ox E 0 H 2 O /O 2
Uribe et al, LANL, 1992 (ECS Proc Vol) log JORR Surface Redox Mediation JORR = Jo(1 -qox) exp [(Eo-E) /bint], d(log JORR)/d( Eo-E) = 1/bint + [1/(1 - qox)] (dqox /d. E ) (a) active site generation 2 Pt-OH surface + 2 H+ + 2 e = 2 Ptsurface+ 2 H 2 O (b) faradaic reaction of O 2 at/with the reduced site O 2 +2 Pt surface + 2 H+ = 2 Pt-OHsurface 0. 80 V 1. 23 V Ecath E 0 Pt / Pt. Ox E 0 H 2 O /O 2
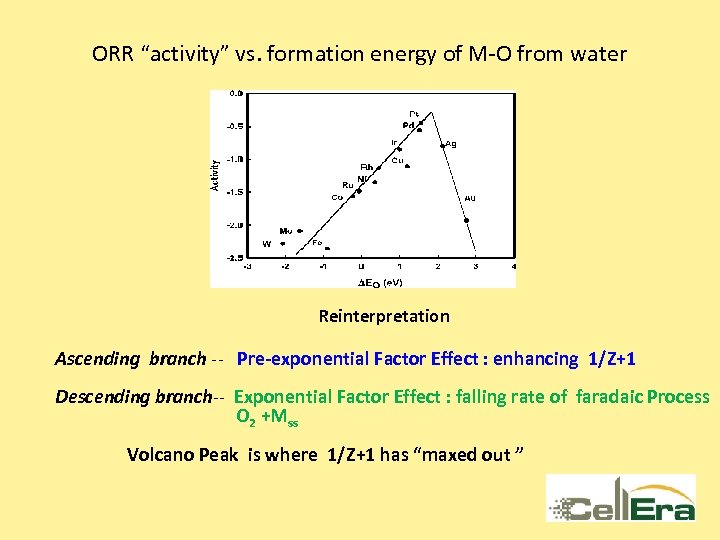 ORR “activity” vs. formation energy of M-O from water Reinterpretation Ascending branch -- Pre-exponential Factor Effect : enhancing 1/Z+1 Descending branch-- Exponential Factor Effect : falling rate of faradaic Process O 2 +Mss Volcano Peak is where 1/Z+1 has “maxed out ”
ORR “activity” vs. formation energy of M-O from water Reinterpretation Ascending branch -- Pre-exponential Factor Effect : enhancing 1/Z+1 Descending branch-- Exponential Factor Effect : falling rate of faradaic Process O 2 +Mss Volcano Peak is where 1/Z+1 has “maxed out ”
 Good general principle for searching an active ORR catalyst “A Pourbaix guide for electrocatalysis galaxy travel”: match the target electrode potential in the operating fuel cell, with : the M/M-OHads standard potential of the metal , or metal alloy catalyst, or with: the M+n/+(n+1) standard potential of the active surface redox couple
Good general principle for searching an active ORR catalyst “A Pourbaix guide for electrocatalysis galaxy travel”: match the target electrode potential in the operating fuel cell, with : the M/M-OHads standard potential of the metal , or metal alloy catalyst, or with: the M+n/+(n+1) standard potential of the active surface redox couple
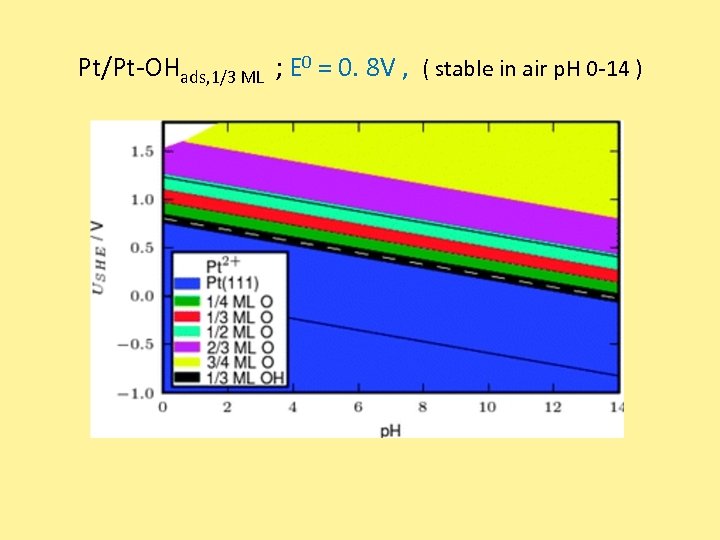 Pt/Pt-OHads, 1/3 ML ; E 0 = 0. 8 V , ( stable in air p. H 0 -14 )
Pt/Pt-OHads, 1/3 ML ; E 0 = 0. 8 V , ( stable in air p. H 0 -14 )
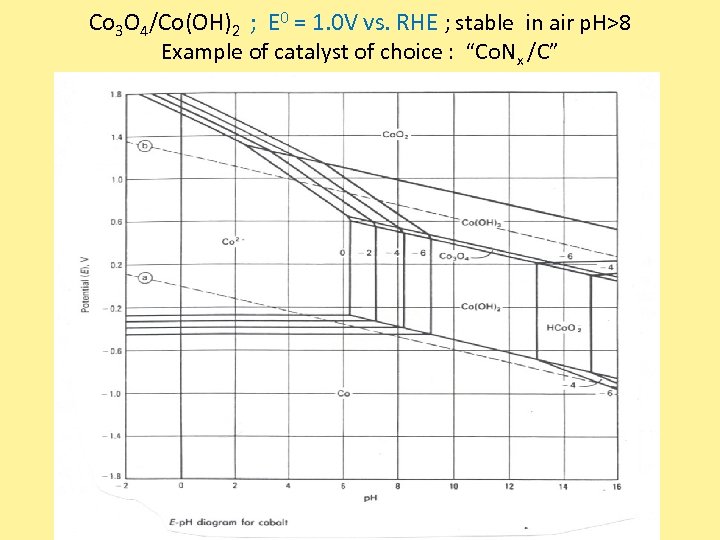 Co 3 O 4/Co(OH)2 ; E 0 = 1. 0 V vs. RHE ; stable in air p. H>8 Example of catalyst of choice : “Co. Nx /C”
Co 3 O 4/Co(OH)2 ; E 0 = 1. 0 V vs. RHE ; stable in air p. H>8 Example of catalyst of choice : “Co. Nx /C”
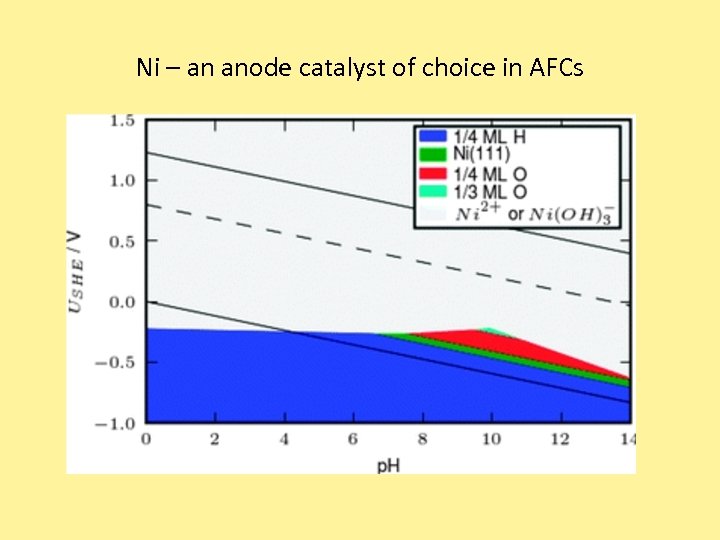 Ni – an anode catalyst of choice in AFCs
Ni – an anode catalyst of choice in AFCs
 A Yardstick for Electrocatalytic Activity • a wide variety of electrocatalytic processes , taking place at either redoxfunctionalized or metal surfaces, are “surface redox mediated” • optimum value for E 0 cell process - E 0 surface redox (DE 0 ) is a guideline for maximizing the electrocatalytic activity , because • An optimized DE 0 addresses conflicting demands of (1) minimum overpotential for surface activation and (2) high rate of the faradaic process at the activated surface • Active ORR electrocatalysts are all associated with DE 0 of 0. 3 V-0. 4 V
A Yardstick for Electrocatalytic Activity • a wide variety of electrocatalytic processes , taking place at either redoxfunctionalized or metal surfaces, are “surface redox mediated” • optimum value for E 0 cell process - E 0 surface redox (DE 0 ) is a guideline for maximizing the electrocatalytic activity , because • An optimized DE 0 addresses conflicting demands of (1) minimum overpotential for surface activation and (2) high rate of the faradaic process at the activated surface • Active ORR electrocatalysts are all associated with DE 0 of 0. 3 V-0. 4 V
 Acknowledgements of Support *Israel Cleantech Ventures *Office of the Chief Scientist Ministry of Commerce & Industry
Acknowledgements of Support *Israel Cleantech Ventures *Office of the Chief Scientist Ministry of Commerce & Industry


