d307321fa2ac1d54c310f2cca287ec03.ppt
- Количество слайдов: 16
 Fuel Cells ME 252 Thermal-Fluid Systems G. Kallio 1
Fuel Cells ME 252 Thermal-Fluid Systems G. Kallio 1
 Introduction • Fuel cells directly convert chemical energy into electricity • Battery: fixed quantity of fuel used as a energy source or storage device (may be recharged) • Fuel Cell: continuous supply of fuel and outflow of products, used solely as an energy source (no storage or recharging function) • 1839 - concept first demonstrated • 1950 s - developed as a useful device 2
Introduction • Fuel cells directly convert chemical energy into electricity • Battery: fixed quantity of fuel used as a energy source or storage device (may be recharged) • Fuel Cell: continuous supply of fuel and outflow of products, used solely as an energy source (no storage or recharging function) • 1839 - concept first demonstrated • 1950 s - developed as a useful device 2
 Characteristics of Fuel Cells • Oxidation-Reduction reaction: < sketch > • No thermal-to-mechanical energy conversion; therefore, operation is not limited by Second Law or Carnot thermal efficiency 3
Characteristics of Fuel Cells • Oxidation-Reduction reaction: < sketch > • No thermal-to-mechanical energy conversion; therefore, operation is not limited by Second Law or Carnot thermal efficiency 3
 Applications of Fuel Cells • Stationary power – Large systems: hospitals, schools, hotels, office buildings, airports – Residential • Transportation – Major auto manufacturers – Trains, buses, boats, planes, scooters, bicycles • Portable power – Small electronics, power tools • Landfill/Wastewater Treatment – Methane H 2 electricity 4
Applications of Fuel Cells • Stationary power – Large systems: hospitals, schools, hotels, office buildings, airports – Residential • Transportation – Major auto manufacturers – Trains, buses, boats, planes, scooters, bicycles • Portable power – Small electronics, power tools • Landfill/Wastewater Treatment – Methane H 2 electricity 4
 Characteristics of Fuel Cells, cont. • Fuel cell power plants produce very low levels of environmental pollution • Pollution estimated to be a factor of 10 less than fossil-fuel plants with best available pollution control • Hydrogen fuel can be extracted from fossil-fuels by reforming • Off-peak electricity can be “stored” by electrolysis, generating H 2 and O 2 that are recombined by fuel cells during peak hours 5
Characteristics of Fuel Cells, cont. • Fuel cell power plants produce very low levels of environmental pollution • Pollution estimated to be a factor of 10 less than fossil-fuel plants with best available pollution control • Hydrogen fuel can be extracted from fossil-fuels by reforming • Off-peak electricity can be “stored” by electrolysis, generating H 2 and O 2 that are recombined by fuel cells during peak hours 5
 Characteristics of Fuel Cells, cont. • Fuel cells are built from a large number of unit cells, forming a stack • Electrode characteristics – catalytic: platinum or sintered nickel used to accelerate ion formation – high porosity: allows fuel and electrolyte to penetrate, presenting large surface area for catalytic reaction – conductive: allow efficient electron migration to terminal • Electrolyte - provide high rate of ion transport between anode to cathode 6
Characteristics of Fuel Cells, cont. • Fuel cells are built from a large number of unit cells, forming a stack • Electrode characteristics – catalytic: platinum or sintered nickel used to accelerate ion formation – high porosity: allows fuel and electrolyte to penetrate, presenting large surface area for catalytic reaction – conductive: allow efficient electron migration to terminal • Electrolyte - provide high rate of ion transport between anode to cathode 6
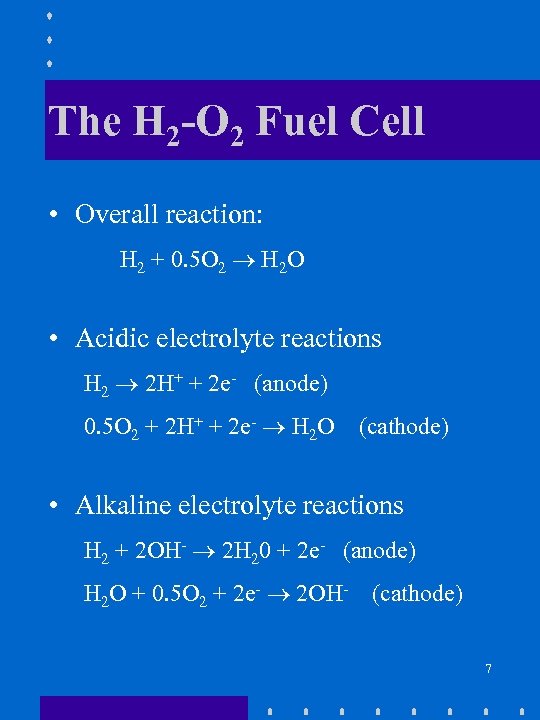 The H 2 -O 2 Fuel Cell • Overall reaction: H 2 + 0. 5 O 2 H 2 O • Acidic electrolyte reactions H 2 2 H+ + 2 e- (anode) 0. 5 O 2 + 2 H+ + 2 e- H 2 O (cathode) • Alkaline electrolyte reactions H 2 + 2 OH- 2 H 20 + 2 e- (anode) H 2 O + 0. 5 O 2 + 2 e- 2 OH- (cathode) 7
The H 2 -O 2 Fuel Cell • Overall reaction: H 2 + 0. 5 O 2 H 2 O • Acidic electrolyte reactions H 2 2 H+ + 2 e- (anode) 0. 5 O 2 + 2 H+ + 2 e- H 2 O (cathode) • Alkaline electrolyte reactions H 2 + 2 OH- 2 H 20 + 2 e- (anode) H 2 O + 0. 5 O 2 + 2 e- 2 OH- (cathode) 7
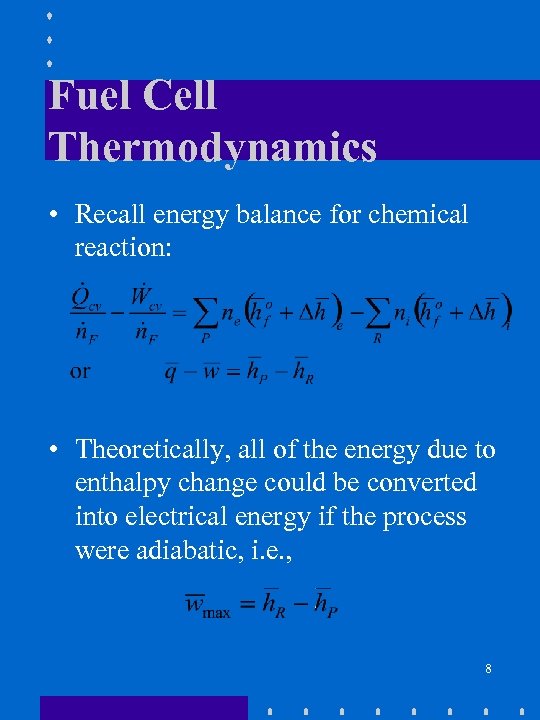 Fuel Cell Thermodynamics • Recall energy balance for chemical reaction: • Theoretically, all of the energy due to enthalpy change could be converted into electrical energy if the process were adiabatic, i. e. , 8
Fuel Cell Thermodynamics • Recall energy balance for chemical reaction: • Theoretically, all of the energy due to enthalpy change could be converted into electrical energy if the process were adiabatic, i. e. , 8
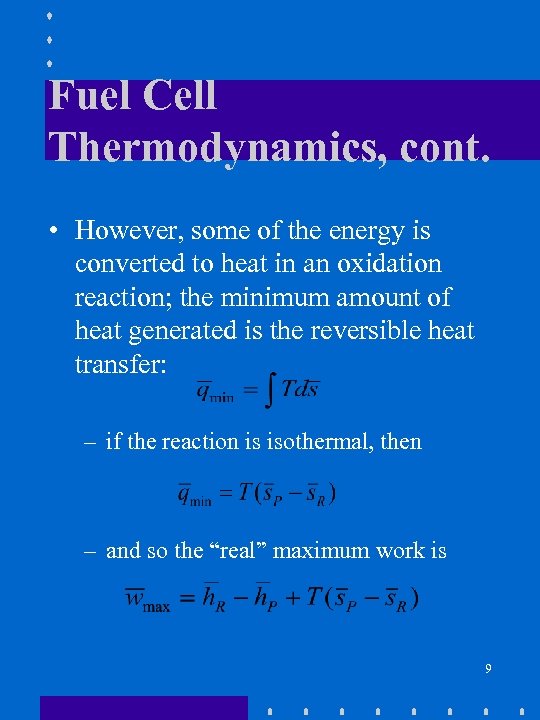 Fuel Cell Thermodynamics, cont. • However, some of the energy is converted to heat in an oxidation reaction; the minimum amount of heat generated is the reversible heat transfer: – if the reaction is isothermal, then – and so the “real” maximum work is 9
Fuel Cell Thermodynamics, cont. • However, some of the energy is converted to heat in an oxidation reaction; the minimum amount of heat generated is the reversible heat transfer: – if the reaction is isothermal, then – and so the “real” maximum work is 9
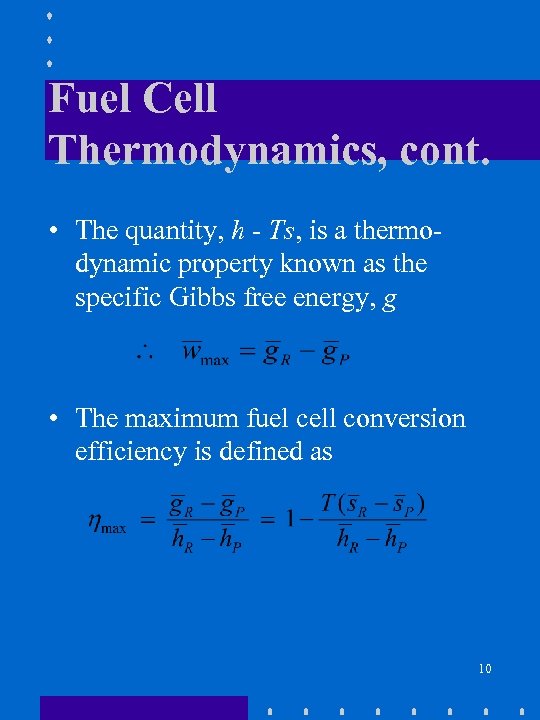 Fuel Cell Thermodynamics, cont. • The quantity, h - Ts, is a thermodynamic property known as the specific Gibbs free energy, g • The maximum fuel cell conversion efficiency is defined as 10
Fuel Cell Thermodynamics, cont. • The quantity, h - Ts, is a thermodynamic property known as the specific Gibbs free energy, g • The maximum fuel cell conversion efficiency is defined as 10
 Maximum Efficiency for the H 2 -O 2 Fuel Cell • Assuming 25°C isothermal conditions, • Actual fuel cells have efficiencies of 40 -60% due to various losses and inefficiencies 11
Maximum Efficiency for the H 2 -O 2 Fuel Cell • Assuming 25°C isothermal conditions, • Actual fuel cells have efficiencies of 40 -60% due to various losses and inefficiencies 11
 Fuel Cell Electrical Inefficiencies • Ohmic polarization - internal resistance to motion of electrons through electrodes and ions through electrolyte • Concentration polarization - mass transfer rate losses related to diffusion through porous electrodes and solubility of reactants/products • Activation polarization - activation energy barriers in the oxidationreduction reaction 12
Fuel Cell Electrical Inefficiencies • Ohmic polarization - internal resistance to motion of electrons through electrodes and ions through electrolyte • Concentration polarization - mass transfer rate losses related to diffusion through porous electrodes and solubility of reactants/products • Activation polarization - activation energy barriers in the oxidationreduction reaction 12
 Fuel Cell Performance Trade-offs • Theoretical conversion efficiency of fuel cells increases with decreasing temperature • Low temperature fuel cells require expensive catalysts to maintain high reaction rates • High temperature fuel cells can achieve high reaction rates w/o catalysts but typically have reduced lifetimes due to corrosion and other effects • Cogeneration or a combined cycle can improve the overall efficiency of high temperature fuel cells 13
Fuel Cell Performance Trade-offs • Theoretical conversion efficiency of fuel cells increases with decreasing temperature • Low temperature fuel cells require expensive catalysts to maintain high reaction rates • High temperature fuel cells can achieve high reaction rates w/o catalysts but typically have reduced lifetimes due to corrosion and other effects • Cogeneration or a combined cycle can improve the overall efficiency of high temperature fuel cells 13
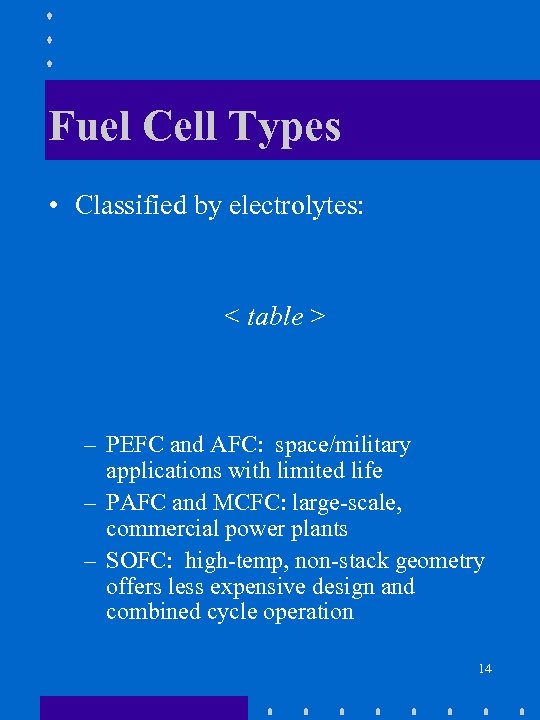 Fuel Cell Types • Classified by electrolytes: < table > – PEFC and AFC: space/military applications with limited life – PAFC and MCFC: large-scale, commercial power plants – SOFC: high-temp, non-stack geometry offers less expensive design and combined cycle operation 14
Fuel Cell Types • Classified by electrolytes: < table > – PEFC and AFC: space/military applications with limited life – PAFC and MCFC: large-scale, commercial power plants – SOFC: high-temp, non-stack geometry offers less expensive design and combined cycle operation 14
 Fuel Cell Types: New Developments • Proton Exchange Membrane (PEM): lowtemperature (80°C), platinum-coated, plastic membrane electrode and solid organic polymer electrolyte; high power density, suitable for light-duty vehicles • Direct Methanol Fuel Cells (DMFC): similar to PEM where H 2 is drawn directly from liquid methanol which eliminates reformer; operates at 50 -100°C; suitable for small applications such as cell phones, laptops, and military electronics. • Protonic Ceramic Fuel Cell (PCFC): hightemperature (700°C), high-protonic conductivity ceramic electrolyte cell; hydrocarbon fuel directly oxidizes at anode to produce H 2, eliminating need for reformer. 15
Fuel Cell Types: New Developments • Proton Exchange Membrane (PEM): lowtemperature (80°C), platinum-coated, plastic membrane electrode and solid organic polymer electrolyte; high power density, suitable for light-duty vehicles • Direct Methanol Fuel Cells (DMFC): similar to PEM where H 2 is drawn directly from liquid methanol which eliminates reformer; operates at 50 -100°C; suitable for small applications such as cell phones, laptops, and military electronics. • Protonic Ceramic Fuel Cell (PCFC): hightemperature (700°C), high-protonic conductivity ceramic electrolyte cell; hydrocarbon fuel directly oxidizes at anode to produce H 2, eliminating need for reformer. 15
 Hydrogen Fuel Reforming • Natural gas partial oxidation reaction: CH 4 + a. O 2 b. H 2 + c. CO +d. CO 2 + e. H 2 O + f. C • Shift reaction: CO + H 2 O CO 2 + H 2 16
Hydrogen Fuel Reforming • Natural gas partial oxidation reaction: CH 4 + a. O 2 b. H 2 + c. CO +d. CO 2 + e. H 2 O + f. C • Shift reaction: CO + H 2 O CO 2 + H 2 16


