041a408480530c8b6322771a4dc64029.ppt
- Количество слайдов: 19
 FT-IR spectroscopy for study of protein structure and protein temperature behaviour RNDr. Jaroslav Turánek, CSc. Výzkumný ústav veterinárního lékařství Brno Farmakologický ústav LF MU
FT-IR spectroscopy for study of protein structure and protein temperature behaviour RNDr. Jaroslav Turánek, CSc. Výzkumný ústav veterinárního lékařství Brno Farmakologický ústav LF MU
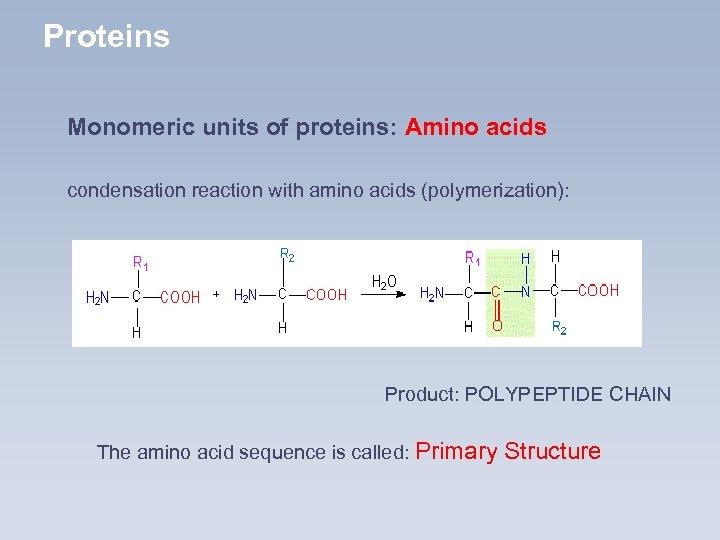 Proteins Monomeric units of proteins: Amino acids condensation reaction with amino acids (polymerization): Product: POLYPEPTIDE CHAIN The amino acid sequence is called: Primary Structure
Proteins Monomeric units of proteins: Amino acids condensation reaction with amino acids (polymerization): Product: POLYPEPTIDE CHAIN The amino acid sequence is called: Primary Structure
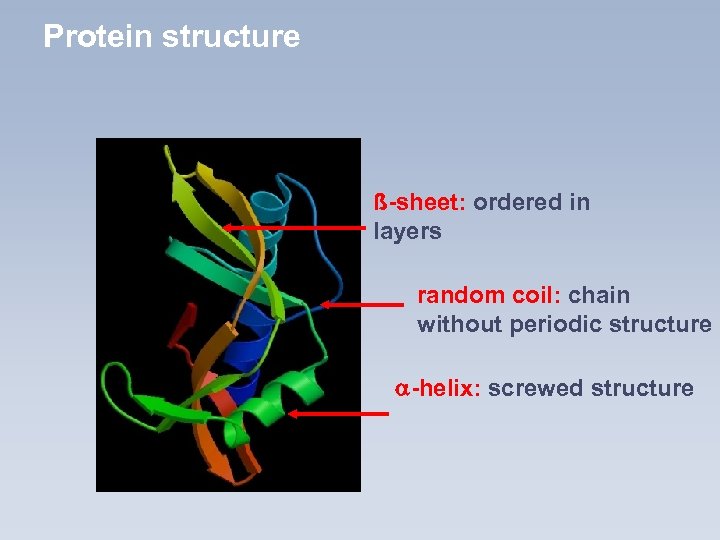 Protein structure ß-sheet: ordered in layers random coil: chain without periodic structure -helix: screwed structure
Protein structure ß-sheet: ordered in layers random coil: chain without periodic structure -helix: screwed structure
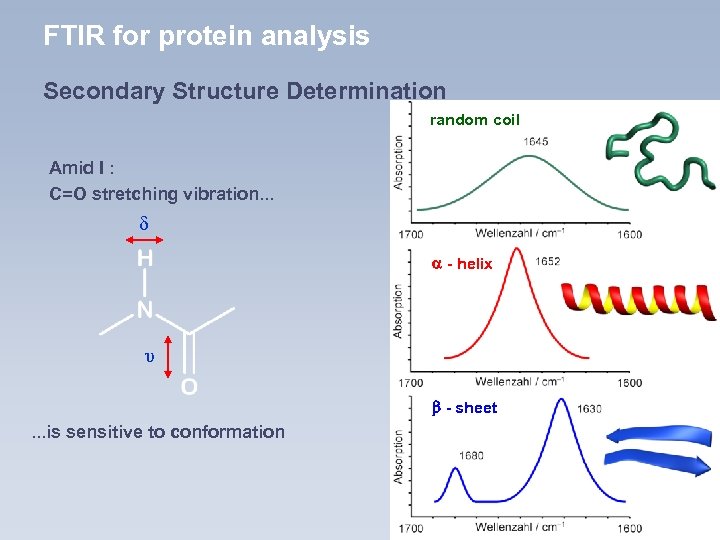 FTIR for protein analysis Secondary Structure Determination random coil Amid I : C=O stretching vibration. . . δ - helix υ - sheet . . . is sensitive to conformation
FTIR for protein analysis Secondary Structure Determination random coil Amid I : C=O stretching vibration. . . δ - helix υ - sheet . . . is sensitive to conformation
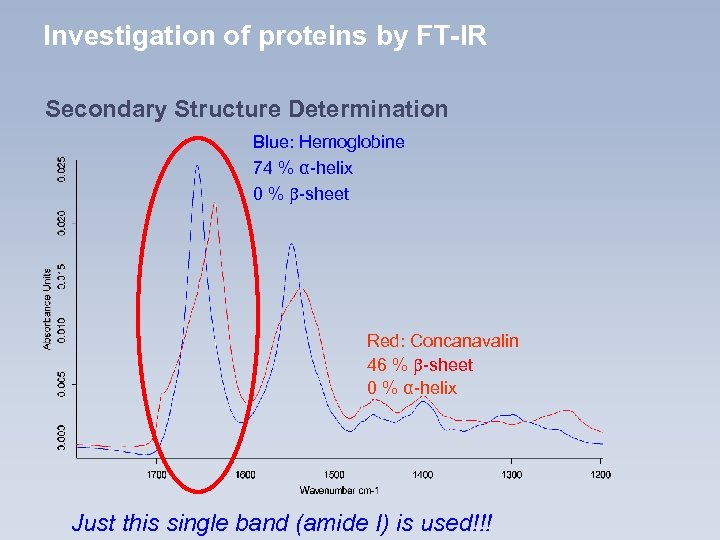 Investigation of proteins by FT-IR Secondary Structure Determination Blue: Hemoglobine 74 % α-helix 0 % -sheet Red: Concanavalin 46 % -sheet 0 % α-helix Just this single band (amide I) is used!!!
Investigation of proteins by FT-IR Secondary Structure Determination Blue: Hemoglobine 74 % α-helix 0 % -sheet Red: Concanavalin 46 % -sheet 0 % α-helix Just this single band (amide I) is used!!!
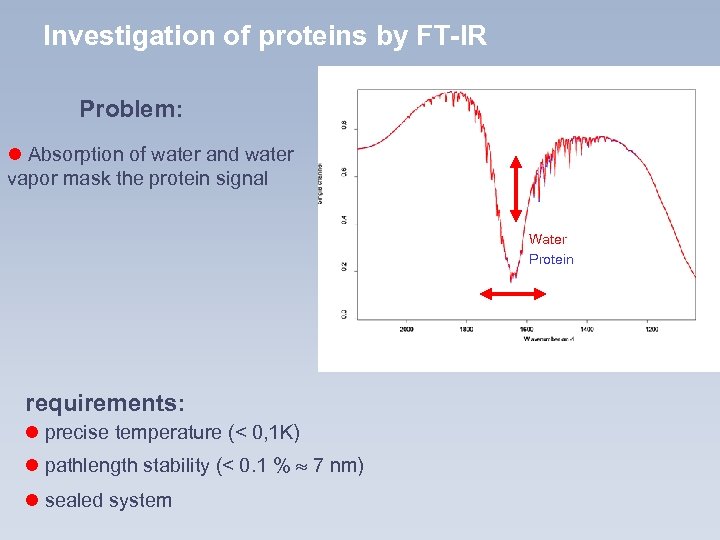 Investigation of proteins by FT-IR Problem: l Absorption of water and water vapor mask the protein signal Protein 20 mg/ml Wasser Water Proteinspektrum Proteinspektrum requirements: l precise temperature (< 0, 1 K) l pathlength stability (< 0. 1 % 7 nm) l sealed system
Investigation of proteins by FT-IR Problem: l Absorption of water and water vapor mask the protein signal Protein 20 mg/ml Wasser Water Proteinspektrum Proteinspektrum requirements: l precise temperature (< 0, 1 K) l pathlength stability (< 0. 1 % 7 nm) l sealed system
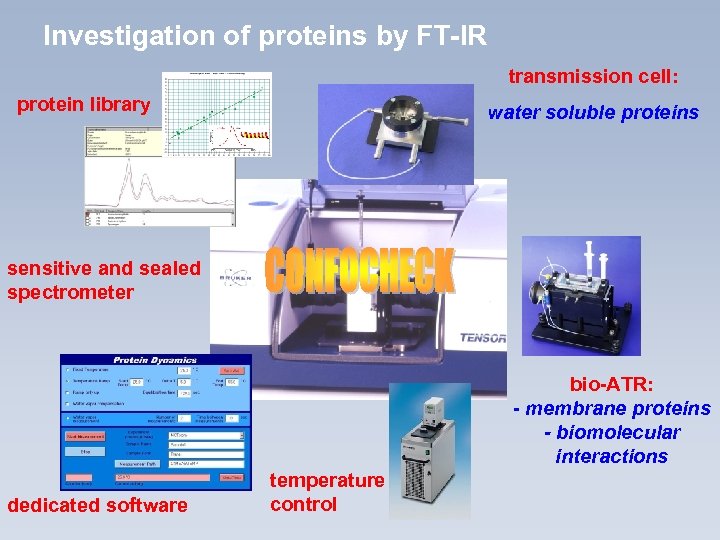 Investigation of proteins by FT-IR transmission cell: protein library water soluble proteins sensitive and sealed spectrometer bio-ATR: - membrane proteins - biomolecular interactions dedicated software temperature control
Investigation of proteins by FT-IR transmission cell: protein library water soluble proteins sensitive and sealed spectrometer bio-ATR: - membrane proteins - biomolecular interactions dedicated software temperature control
 Protein Analysis Why FT-IR spectroscopy ? Structure (conformation) is correlated to function FT-IR spectroscopy is very sensitive to conformational changes !
Protein Analysis Why FT-IR spectroscopy ? Structure (conformation) is correlated to function FT-IR spectroscopy is very sensitive to conformational changes !
 CONFOCHECK: Applications 1) Fast determination of secondary structure 2) Detection of conformational changes (p. H, temperature) 3) Monitoring of biomolecular interactions (protein-ligand binding) +
CONFOCHECK: Applications 1) Fast determination of secondary structure 2) Detection of conformational changes (p. H, temperature) 3) Monitoring of biomolecular interactions (protein-ligand binding) +
 CONFOCHECK: Application Determination of protein secondary structure • fast (within minutes!) • in native state (solution) • many buffer systems allowed • low amount of protein required
CONFOCHECK: Application Determination of protein secondary structure • fast (within minutes!) • in native state (solution) • many buffer systems allowed • low amount of protein required
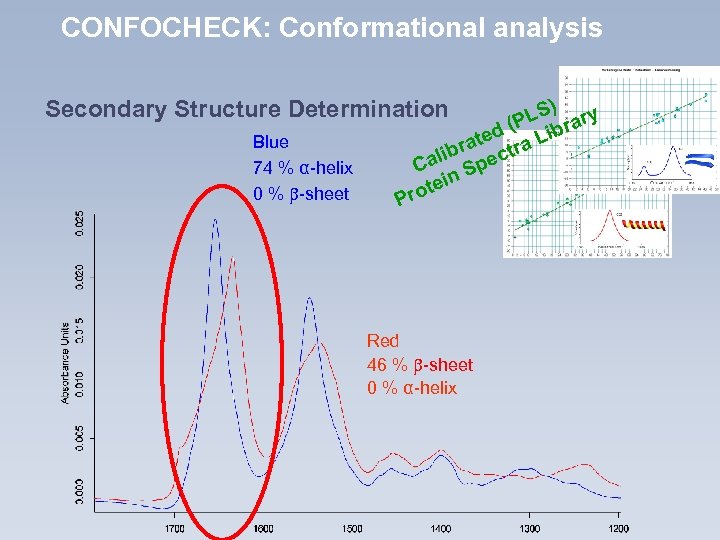 CONFOCHECK: Conformational analysis ) LS rary (P ed a Lib t bra ectr li Ca Sp in ote Pr Secondary Structure Determination Blue 74 % α-helix 0 % -sheet Red 46 % -sheet 0 % α-helix
CONFOCHECK: Conformational analysis ) LS rary (P ed a Lib t bra ectr li Ca Sp in ote Pr Secondary Structure Determination Blue 74 % α-helix 0 % -sheet Red 46 % -sheet 0 % α-helix
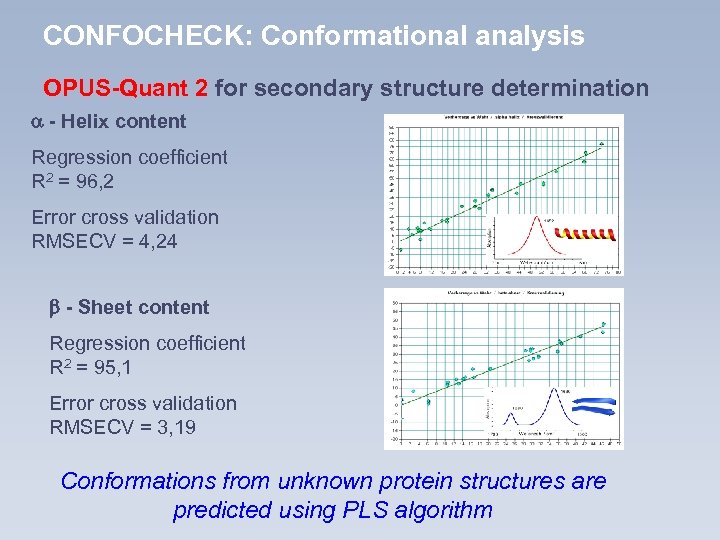 CONFOCHECK: Conformational analysis OPUS-Quant 2 for secondary structure determination - Helix content Regression coefficient R 2 = 96, 2 Error cross validation RMSECV = 4, 24 - Sheet content Regression coefficient R 2 = 95, 1 Error cross validation RMSECV = 3, 19 Conformations from unknown protein structures are predicted using PLS algorithm
CONFOCHECK: Conformational analysis OPUS-Quant 2 for secondary structure determination - Helix content Regression coefficient R 2 = 96, 2 Error cross validation RMSECV = 4, 24 - Sheet content Regression coefficient R 2 = 95, 1 Error cross validation RMSECV = 3, 19 Conformations from unknown protein structures are predicted using PLS algorithm
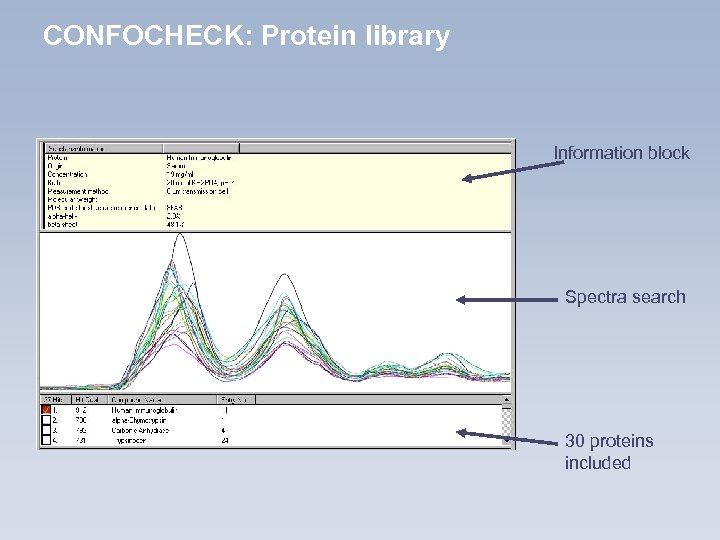 CONFOCHECK: Protein library Information block Spectra search 30 proteins included
CONFOCHECK: Protein library Information block Spectra search 30 proteins included
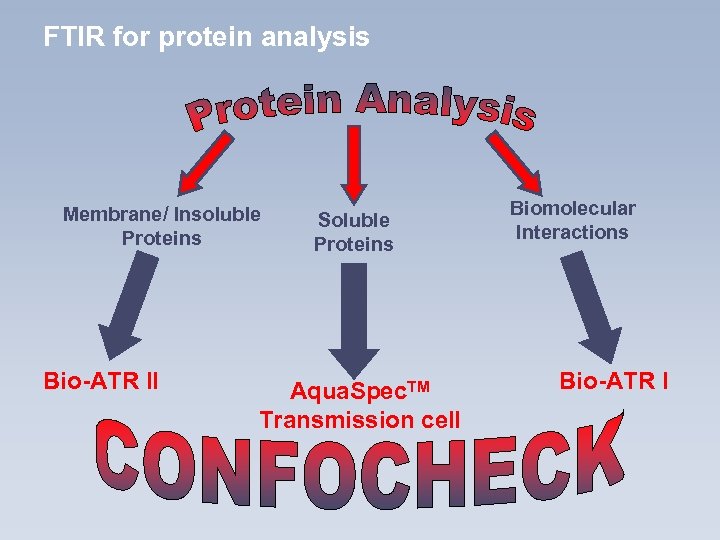 FTIR for protein analysis Membrane/ Insoluble Proteins Bio-ATR II Soluble Proteins Aqua. Spec. TM Transmission cell Biomolecular Interactions Bio-ATR I
FTIR for protein analysis Membrane/ Insoluble Proteins Bio-ATR II Soluble Proteins Aqua. Spec. TM Transmission cell Biomolecular Interactions Bio-ATR I
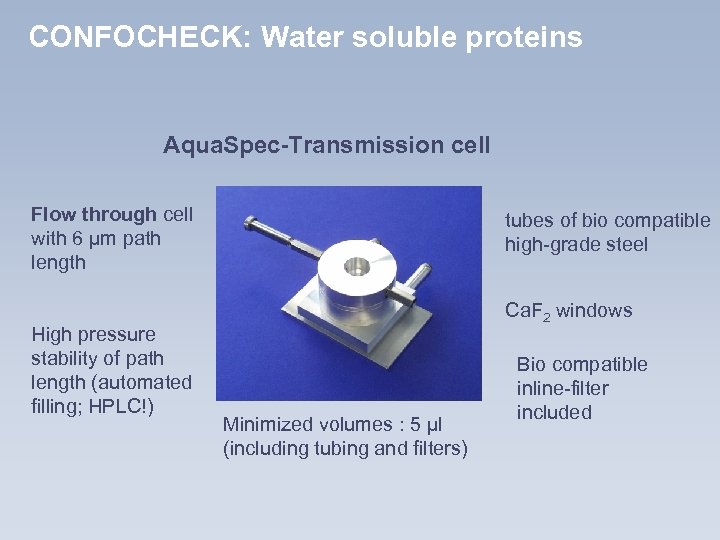 CONFOCHECK: Water soluble proteins Aqua. Spec-Transmission cell Flow through cell with 6 µm path length High pressure stability of path length (automated filling; HPLC!) tubes of bio compatible high-grade steel Ca. F 2 windows Minimized volumes : 5 µl (including tubing and filters) Bio compatible inline-filter included
CONFOCHECK: Water soluble proteins Aqua. Spec-Transmission cell Flow through cell with 6 µm path length High pressure stability of path length (automated filling; HPLC!) tubes of bio compatible high-grade steel Ca. F 2 windows Minimized volumes : 5 µl (including tubing and filters) Bio compatible inline-filter included
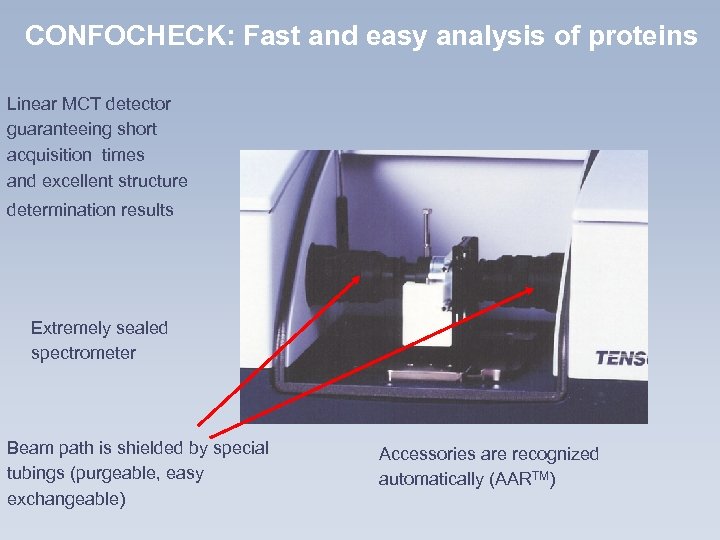 CONFOCHECK: Fast and easy analysis of proteins Linear MCT detector guaranteeing short acquisition times and excellent structure determination results Extremely sealed spectrometer Beam path is shielded by special tubings (purgeable, easy exchangeable) Accessories are recognized automatically (AARTM)
CONFOCHECK: Fast and easy analysis of proteins Linear MCT detector guaranteeing short acquisition times and excellent structure determination results Extremely sealed spectrometer Beam path is shielded by special tubings (purgeable, easy exchangeable) Accessories are recognized automatically (AARTM)
 CONFOCHECK: Application Detection of conformational changes temperature p. H mutation chemical influences
CONFOCHECK: Application Detection of conformational changes temperature p. H mutation chemical influences
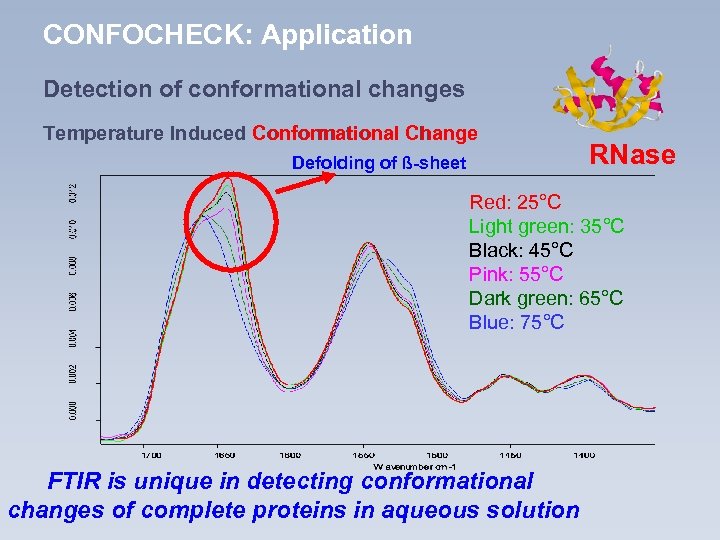 CONFOCHECK: Application Detection of conformational changes Temperature Induced Conformational Change Defolding of ß-sheet RNase Red: 25°C Light green: 35°C Black: 45°C Pink: 55°C Dark green: 65°C Blue: 75°C FTIR is unique in detecting conformational changes of complete proteins in aqueous solution
CONFOCHECK: Application Detection of conformational changes Temperature Induced Conformational Change Defolding of ß-sheet RNase Red: 25°C Light green: 35°C Black: 45°C Pink: 55°C Dark green: 65°C Blue: 75°C FTIR is unique in detecting conformational changes of complete proteins in aqueous solution
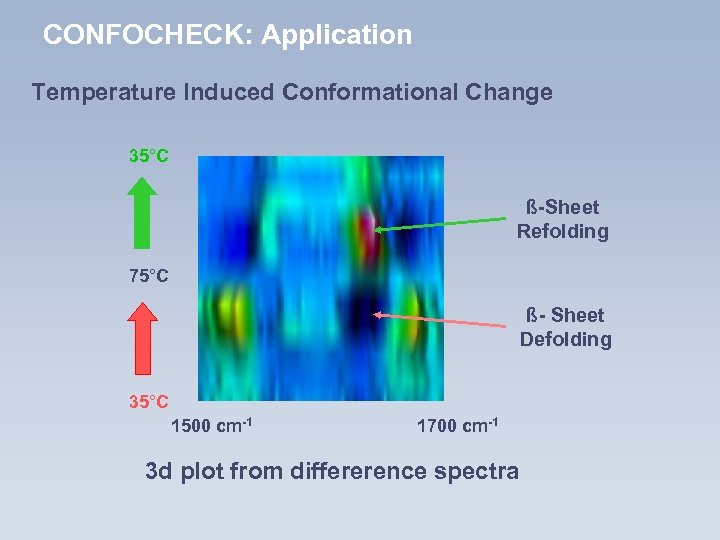 CONFOCHECK: Application Temperature Induced Conformational Change 35°C ß-Sheet Refolding 75°C ß- Sheet Defolding 35°C 1500 cm-1 1700 cm-1 3 d plot from differerence spectra
CONFOCHECK: Application Temperature Induced Conformational Change 35°C ß-Sheet Refolding 75°C ß- Sheet Defolding 35°C 1500 cm-1 1700 cm-1 3 d plot from differerence spectra


