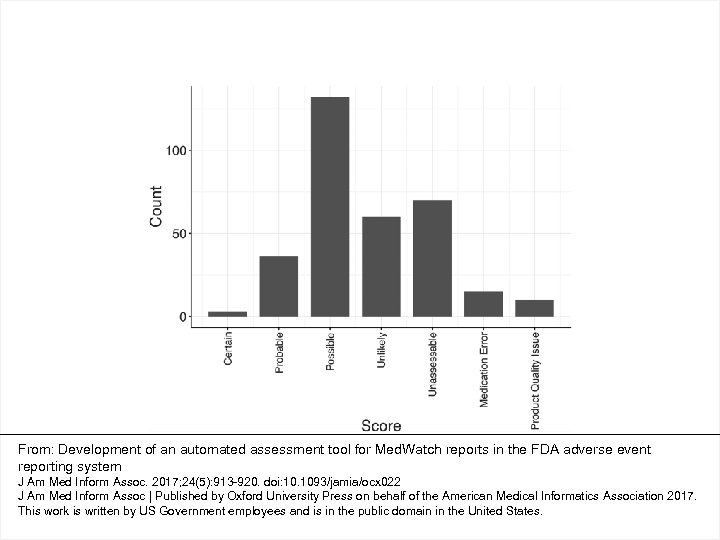
 From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
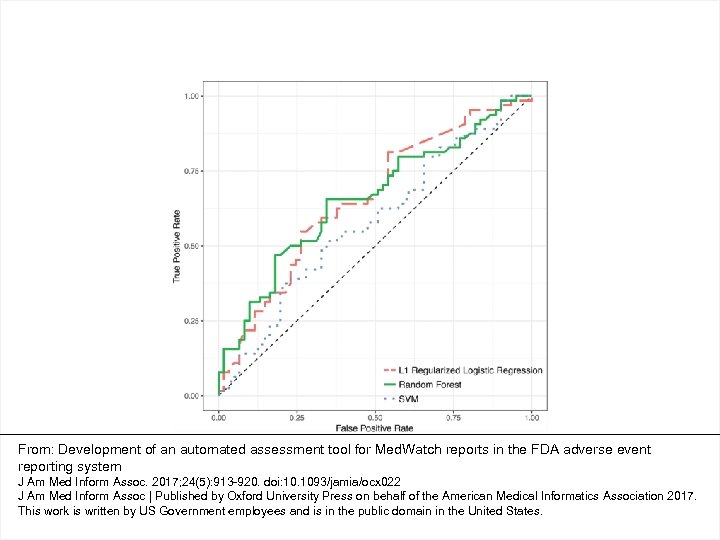 From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
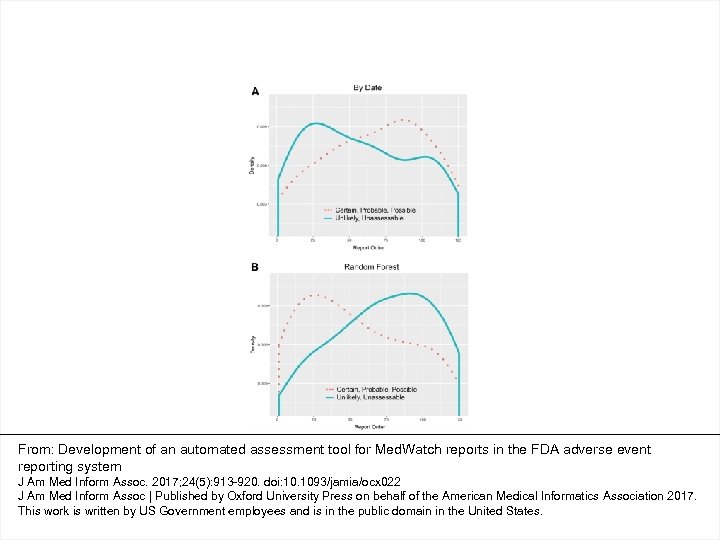 From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
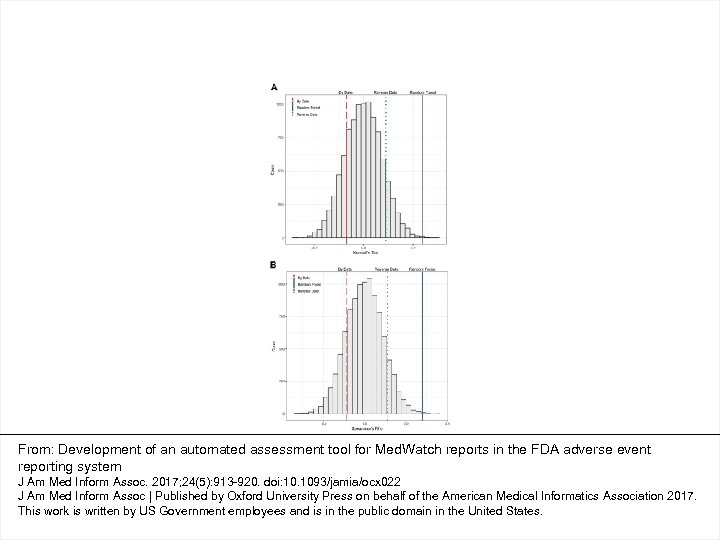 From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
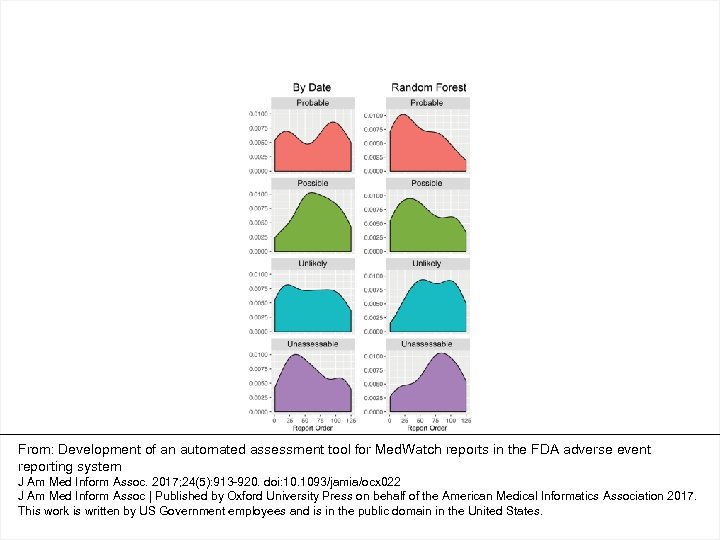 From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.
From: Development of an automated assessment tool for Med. Watch reports in the FDA adverse event reporting system J Am Med Inform Assoc. 2017; 24(5): 913 -920. doi: 10. 1093/jamia/ocx 022 J Am Med Inform Assoc | Published by Oxford University Press on behalf of the American Medical Informatics Association 2017. This work is written by US Government employees and is in the public domain in the United States.