 Frogs Legs, Peas and Potatoes
Frogs Legs, Peas and Potatoes
 Alternative to Batteries Introduction: We have been looking at different ways to make batteries from several different food substances. We have looked at five different substances and have carried out an experiment to see if they can carry an electrical current. The food and drink we have chosen are the following: Carrot, potato, vinegar, salty water, banana and mashed potato. We also experimented by using the same electrodes.
Alternative to Batteries Introduction: We have been looking at different ways to make batteries from several different food substances. We have looked at five different substances and have carried out an experiment to see if they can carry an electrical current. The food and drink we have chosen are the following: Carrot, potato, vinegar, salty water, banana and mashed potato. We also experimented by using the same electrodes.
 Aim of the Experiment The aim of this experiment is to find substances which would make a homemade battery and follow out an experiment to collect the data. Our main plan was to find the substance with the highest and lowest voltage.
Aim of the Experiment The aim of this experiment is to find substances which would make a homemade battery and follow out an experiment to collect the data. Our main plan was to find the substance with the highest and lowest voltage.
 Equipment Ø Ø Ø Ø Ø Voltmeter Carrot Potato 2. 6 Vinegar Salty water Banana Voltmeter Two Different metals (Iron, Copper) Beaker Wires
Equipment Ø Ø Ø Ø Ø Voltmeter Carrot Potato 2. 6 Vinegar Salty water Banana Voltmeter Two Different metals (Iron, Copper) Beaker Wires
 Method Take your substance and place your two different metals in it. Connect the wires to the metals and then connect the other end of the wires to the voltmeter. Then see if the voltmeter recognises that a current is passing through it. If so the substance you have chosen has passed as a homemade battery. If not there must be something blocking the current and it’s a good idea to find out what.
Method Take your substance and place your two different metals in it. Connect the wires to the metals and then connect the other end of the wires to the voltmeter. Then see if the voltmeter recognises that a current is passing through it. If so the substance you have chosen has passed as a homemade battery. If not there must be something blocking the current and it’s a good idea to find out what.
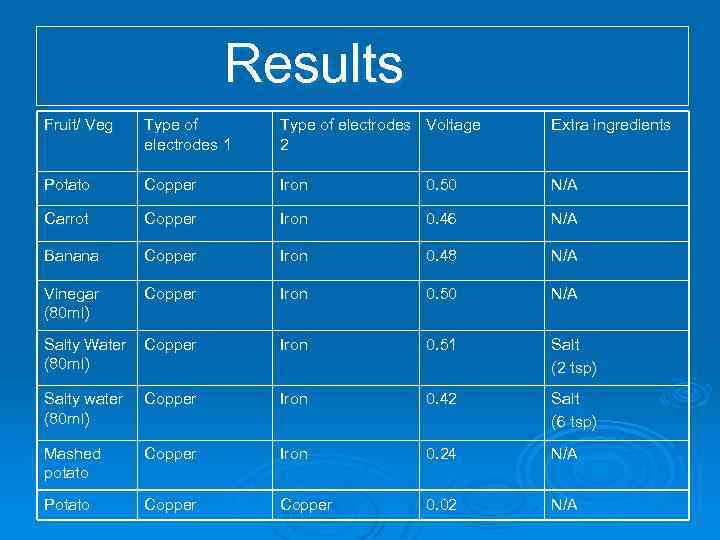 Results Fruit/ Veg Type of electrodes 1 Type of electrodes Voltage 2 Extra ingredients Potato Copper Iron 0. 50 N/A Carrot Copper Iron 0. 46 N/A Banana Copper Iron 0. 48 N/A Vinegar (80 ml) Copper Iron 0. 50 N/A Salty Water (80 ml) Copper Iron 0. 51 Salt (2 tsp) Salty water (80 ml) Copper Iron 0. 42 Salt (6 tsp) Mashed potato Copper Iron 0. 24 N/A Potato Copper 0. 02 N/A
Results Fruit/ Veg Type of electrodes 1 Type of electrodes Voltage 2 Extra ingredients Potato Copper Iron 0. 50 N/A Carrot Copper Iron 0. 46 N/A Banana Copper Iron 0. 48 N/A Vinegar (80 ml) Copper Iron 0. 50 N/A Salty Water (80 ml) Copper Iron 0. 51 Salt (2 tsp) Salty water (80 ml) Copper Iron 0. 42 Salt (6 tsp) Mashed potato Copper Iron 0. 24 N/A Potato Copper 0. 02 N/A
 Conclusion We tried experimenting with several different substances but in our results the best was the salty water with only 2 tsp. On the other hand the potato using the same electrodes was the worst. We also experimented with mashed potatoes, unfortunately the results were twice as bad as a raw potato. All the results varied from 0. 02 – 0. 51. Voltmeter 2. 6
Conclusion We tried experimenting with several different substances but in our results the best was the salty water with only 2 tsp. On the other hand the potato using the same electrodes was the worst. We also experimented with mashed potatoes, unfortunately the results were twice as bad as a raw potato. All the results varied from 0. 02 – 0. 51. Voltmeter 2. 6