996f1385ea547b71900f88613b8f0e8b.ppt
- Количество слайдов: 14
 Framework Programme 7 2012 Call Information Graham Hughes, FP 7 UK NCP for Health (Industry) Technology Strategy Board Driving Innovation
Framework Programme 7 2012 Call Information Graham Hughes, FP 7 UK NCP for Health (Industry) Technology Strategy Board Driving Innovation
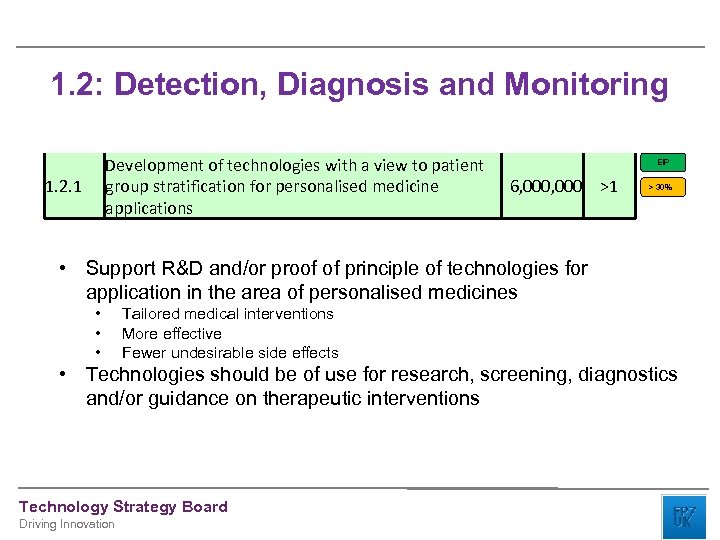 1. 2: Detection, Diagnosis and Monitoring Development of technologies with a view to patient group stratification for personalised medicine applications 1. 2. 1 EIP 6, 000 >1 > 30% • Support R&D and/or proof of principle of technologies for application in the area of personalised medicines • • • Tailored medical interventions More effective Fewer undesirable side effects • Technologies should be of use for research, screening, diagnostics and/or guidance on therapeutic interventions Technology Strategy Board Driving Innovation
1. 2: Detection, Diagnosis and Monitoring Development of technologies with a view to patient group stratification for personalised medicine applications 1. 2. 1 EIP 6, 000 >1 > 30% • Support R&D and/or proof of principle of technologies for application in the area of personalised medicines • • • Tailored medical interventions More effective Fewer undesirable side effects • Technologies should be of use for research, screening, diagnostics and/or guidance on therapeutic interventions Technology Strategy Board Driving Innovation
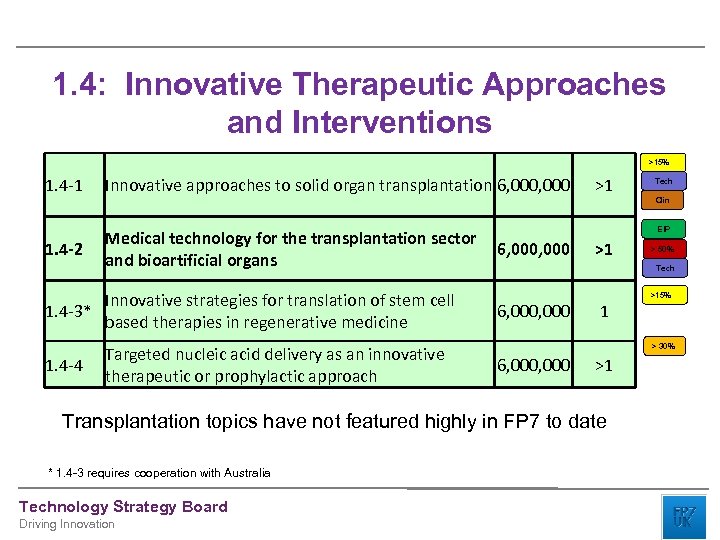 1. 4: Innovative Therapeutic Approaches and Interventions >15% 1. 4 -1 1. 4 -2 Innovative approaches to solid organ transplantation 6, 000 Medical technology for the transplantation sector and bioartificial organs Innovative strategies for translation of stem cell 1. 4 -3* based therapies in regenerative medicine 1. 4 -4 Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach >1 Technology Strategy Board Driving Innovation Clin EIP 6, 000 >1 > 50% Tech >15% 6, 000 1 > 30% 6, 000 >1 Transplantation topics have not featured highly in FP 7 to date * 1. 4 -3 requires cooperation with Australia Tech
1. 4: Innovative Therapeutic Approaches and Interventions >15% 1. 4 -1 1. 4 -2 Innovative approaches to solid organ transplantation 6, 000 Medical technology for the transplantation sector and bioartificial organs Innovative strategies for translation of stem cell 1. 4 -3* based therapies in regenerative medicine 1. 4 -4 Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach >1 Technology Strategy Board Driving Innovation Clin EIP 6, 000 >1 > 50% Tech >15% 6, 000 1 > 30% 6, 000 >1 Transplantation topics have not featured highly in FP 7 to date * 1. 4 -3 requires cooperation with Australia Tech
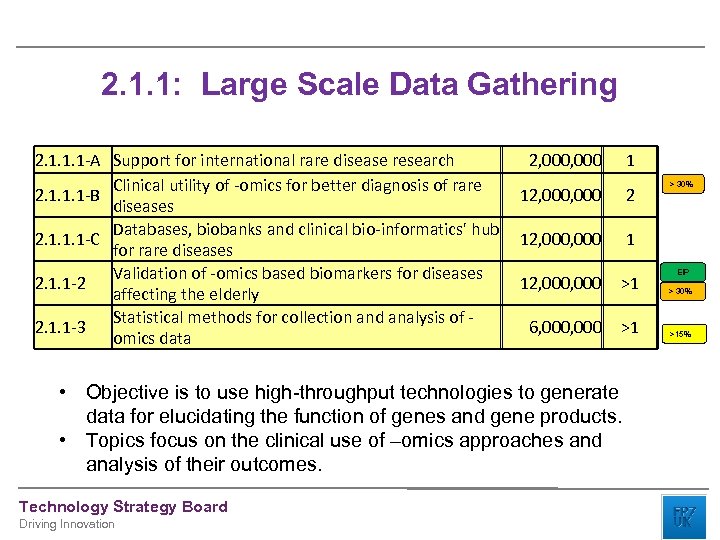 2. 1. 1: Large Scale Data Gathering 2. 1. 1. 1 -A Support for international rare disease research Clinical utility of -omics for better diagnosis of rare 2. 1. 1. 1 -B diseases Databases, biobanks and clinical bio-informatics' hub 2. 1. 1. 1 -C for rare diseases Validation of -omics based biomarkers for diseases 2. 1. 1 -2 affecting the elderly Statistical methods for collection and analysis of 2. 1. 1 -3 omics data 2, 000 1 12, 000 2 12, 000 1 Driving Innovation EIP 12, 000 >1 > 30% 6, 000 >1 >15% • Objective is to use high-throughput technologies to generate data for elucidating the function of genes and gene products. • Topics focus on the clinical use of –omics approaches and analysis of their outcomes. Technology Strategy Board > 30%
2. 1. 1: Large Scale Data Gathering 2. 1. 1. 1 -A Support for international rare disease research Clinical utility of -omics for better diagnosis of rare 2. 1. 1. 1 -B diseases Databases, biobanks and clinical bio-informatics' hub 2. 1. 1. 1 -C for rare diseases Validation of -omics based biomarkers for diseases 2. 1. 1 -2 affecting the elderly Statistical methods for collection and analysis of 2. 1. 1 -3 omics data 2, 000 1 12, 000 2 12, 000 1 Driving Innovation EIP 12, 000 >1 > 30% 6, 000 >1 >15% • Objective is to use high-throughput technologies to generate data for elucidating the function of genes and gene products. • Topics focus on the clinical use of –omics approaches and analysis of their outcomes. Technology Strategy Board > 30%
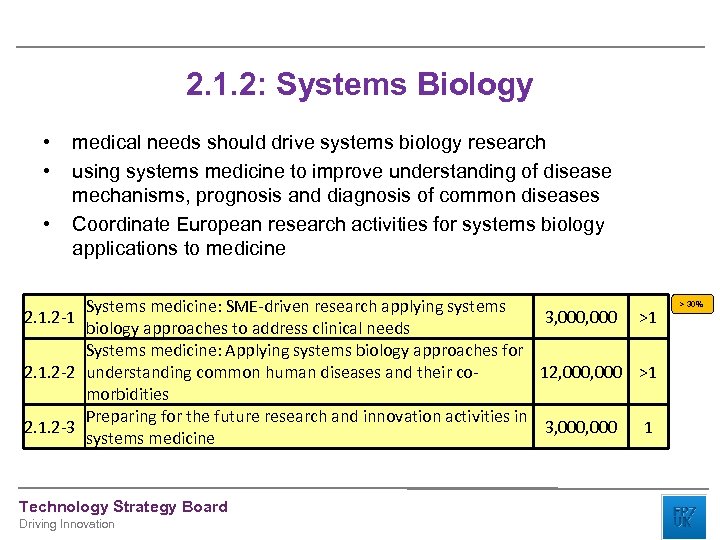 2. 1. 2: Systems Biology • • • medical needs should drive systems biology research using systems medicine to improve understanding of disease mechanisms, prognosis and diagnosis of common diseases Coordinate European research activities for systems biology applications to medicine Systems medicine: SME-driven research applying systems 2. 1. 2 -1 3, 000 >1 biology approaches to address clinical needs Systems medicine: Applying systems biology approaches for 2. 1. 2 -2 understanding common human diseases and their co 12, 000 >1 morbidities Preparing for the future research and innovation activities in 2. 1. 2 -3 3, 000 1 systems medicine Technology Strategy Board Driving Innovation > 30%
2. 1. 2: Systems Biology • • • medical needs should drive systems biology research using systems medicine to improve understanding of disease mechanisms, prognosis and diagnosis of common diseases Coordinate European research activities for systems biology applications to medicine Systems medicine: SME-driven research applying systems 2. 1. 2 -1 3, 000 >1 biology approaches to address clinical needs Systems medicine: Applying systems biology approaches for 2. 1. 2 -2 understanding common human diseases and their co 12, 000 >1 morbidities Preparing for the future research and innovation activities in 2. 1. 2 -3 3, 000 1 systems medicine Technology Strategy Board Driving Innovation > 30%
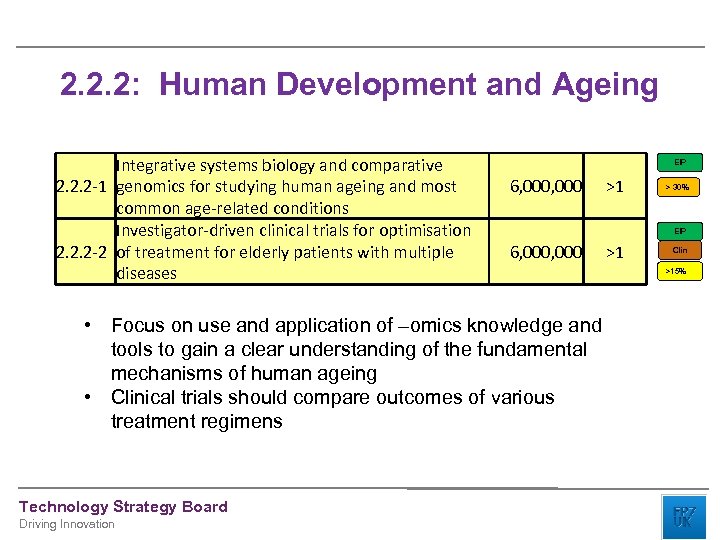 2. 2. 2: Human Development and Ageing Integrative systems biology and comparative 2. 2. 2 -1 genomics for studying human ageing and most common age-related conditions Investigator-driven clinical trials for optimisation 2. 2. 2 -2 of treatment for elderly patients with multiple diseases EIP 6, 000 Driving Innovation > 30% EIP 6, 000 • Focus on use and application of –omics knowledge and tools to gain a clear understanding of the fundamental mechanisms of human ageing • Clinical trials should compare outcomes of various treatment regimens Technology Strategy Board >1 >1 Clin >15%
2. 2. 2: Human Development and Ageing Integrative systems biology and comparative 2. 2. 2 -1 genomics for studying human ageing and most common age-related conditions Investigator-driven clinical trials for optimisation 2. 2. 2 -2 of treatment for elderly patients with multiple diseases EIP 6, 000 Driving Innovation > 30% EIP 6, 000 • Focus on use and application of –omics knowledge and tools to gain a clear understanding of the fundamental mechanisms of human ageing • Clinical trials should compare outcomes of various treatment regimens Technology Strategy Board >1 >1 Clin >15%
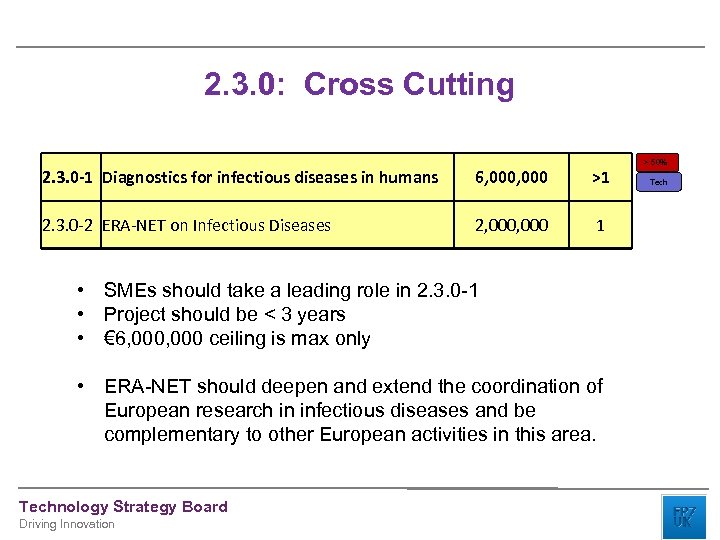 2. 3. 0: Cross Cutting 2. 3. 0 -1 Diagnostics for infectious diseases in humans 6, 000 >1 2. 3. 0 -2 ERA-NET on Infectious Diseases 2, 000 1 • SMEs should take a leading role in 2. 3. 0 -1 • Project should be < 3 years • € 6, 000 ceiling is max only • ERA-NET should deepen and extend the coordination of European research in infectious diseases and be complementary to other European activities in this area. Technology Strategy Board Driving Innovation > 50% Tech
2. 3. 0: Cross Cutting 2. 3. 0 -1 Diagnostics for infectious diseases in humans 6, 000 >1 2. 3. 0 -2 ERA-NET on Infectious Diseases 2, 000 1 • SMEs should take a leading role in 2. 3. 0 -1 • Project should be < 3 years • € 6, 000 ceiling is max only • ERA-NET should deepen and extend the coordination of European research in infectious diseases and be complementary to other European activities in this area. Technology Strategy Board Driving Innovation > 50% Tech
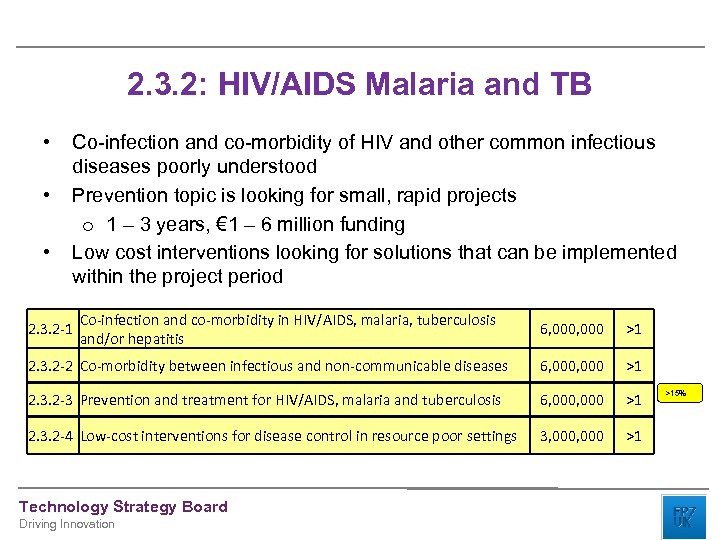 2. 3. 2: HIV/AIDS Malaria and TB • • • Co-infection and co-morbidity of HIV and other common infectious diseases poorly understood Prevention topic is looking for small, rapid projects o 1 – 3 years, € 1 – 6 million funding Low cost interventions looking for solutions that can be implemented within the project period 2. 3. 2 -1 Co-infection and co-morbidity in HIV/AIDS, malaria, tuberculosis and/or hepatitis 6, 000 >1 2. 3. 2 -2 Co-morbidity between infectious and non-communicable diseases 6, 000 >1 2. 3. 2 -3 Prevention and treatment for HIV/AIDS, malaria and tuberculosis 6, 000 >1 2. 3. 2 -4 Low-cost interventions for disease control in resource poor settings 3, 000 >1 Technology Strategy Board Driving Innovation >15%
2. 3. 2: HIV/AIDS Malaria and TB • • • Co-infection and co-morbidity of HIV and other common infectious diseases poorly understood Prevention topic is looking for small, rapid projects o 1 – 3 years, € 1 – 6 million funding Low cost interventions looking for solutions that can be implemented within the project period 2. 3. 2 -1 Co-infection and co-morbidity in HIV/AIDS, malaria, tuberculosis and/or hepatitis 6, 000 >1 2. 3. 2 -2 Co-morbidity between infectious and non-communicable diseases 6, 000 >1 2. 3. 2 -3 Prevention and treatment for HIV/AIDS, malaria and tuberculosis 6, 000 >1 2. 3. 2 -4 Low-cost interventions for disease control in resource poor settings 3, 000 >1 Technology Strategy Board Driving Innovation >15%
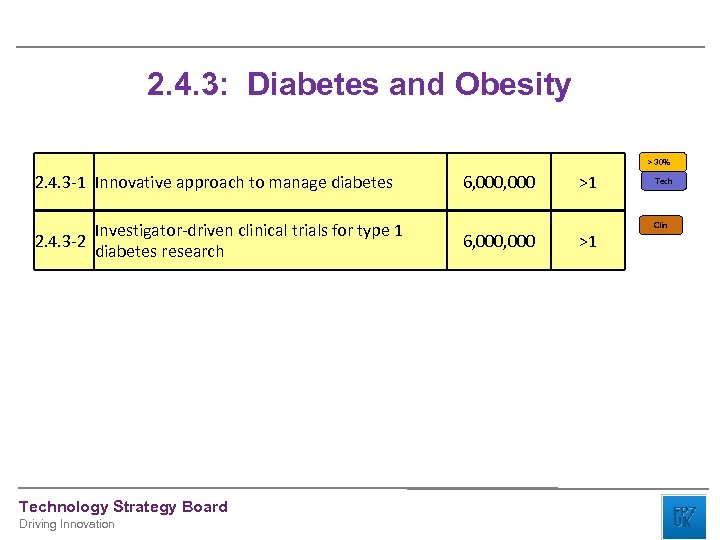 2. 4. 3: Diabetes and Obesity > 30% 2. 4. 3 -1 Innovative approach to manage diabetes Investigator-driven clinical trials for type 1 2. 4. 3 -2 diabetes research Technology Strategy Board Driving Innovation 6, 000, 000 >1 >1 Tech Clin
2. 4. 3: Diabetes and Obesity > 30% 2. 4. 3 -1 Innovative approach to manage diabetes Investigator-driven clinical trials for type 1 2. 4. 3 -2 diabetes research Technology Strategy Board Driving Innovation 6, 000, 000 >1 >1 Tech Clin
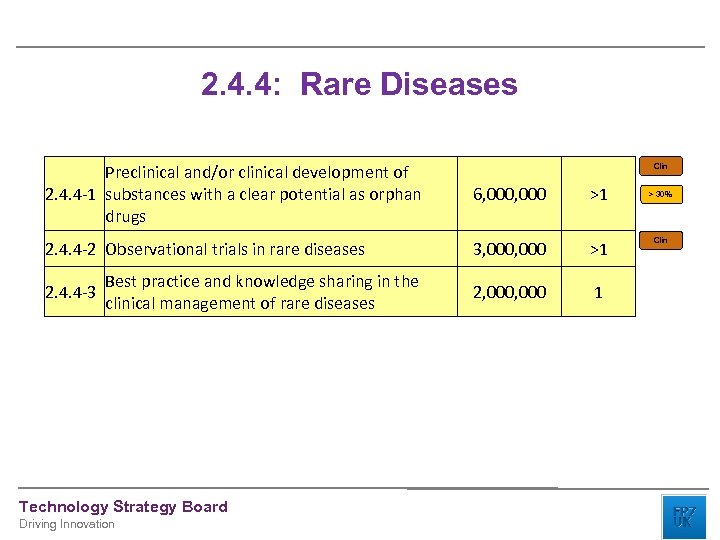 2. 4. 4: Rare Diseases Preclinical and/or clinical development of 2. 4. 4 -1 substances with a clear potential as orphan drugs 6, 000 >1 2. 4. 4 -2 Observational trials in rare diseases 3, 000 >1 2, 000 1 2. 4. 4 -3 Best practice and knowledge sharing in the clinical management of rare diseases Technology Strategy Board Driving Innovation Clin > 30% Clin
2. 4. 4: Rare Diseases Preclinical and/or clinical development of 2. 4. 4 -1 substances with a clear potential as orphan drugs 6, 000 >1 2. 4. 4 -2 Observational trials in rare diseases 3, 000 >1 2, 000 1 2. 4. 4 -3 Best practice and knowledge sharing in the clinical management of rare diseases Technology Strategy Board Driving Innovation Clin > 30% Clin
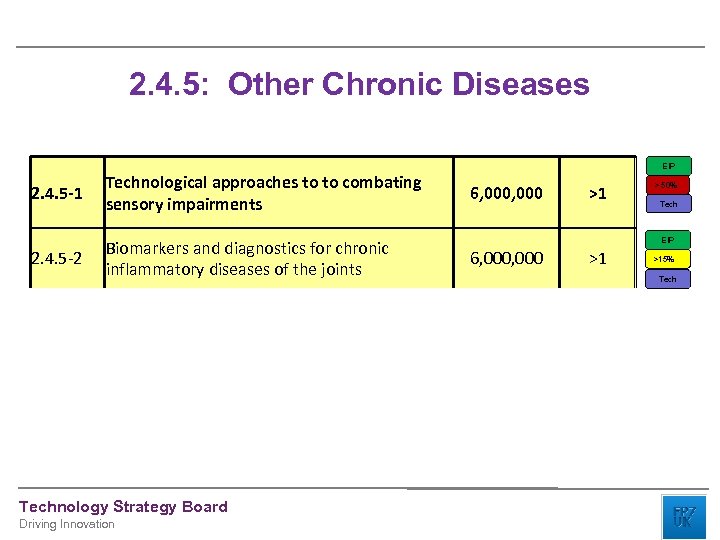 2. 4. 5: Other Chronic Diseases EIP 2. 4. 5 -1 Technological approaches to to combating sensory impairments 2. 4. 5 -2 Biomarkers and diagnostics for chronic inflammatory diseases of the joints Technology Strategy Board Driving Innovation 6, 000 >1 > 50% Tech EIP 6, 000 >1 >15% Tech
2. 4. 5: Other Chronic Diseases EIP 2. 4. 5 -1 Technological approaches to to combating sensory impairments 2. 4. 5 -2 Biomarkers and diagnostics for chronic inflammatory diseases of the joints Technology Strategy Board Driving Innovation 6, 000 >1 > 50% Tech EIP 6, 000 >1 >15% Tech
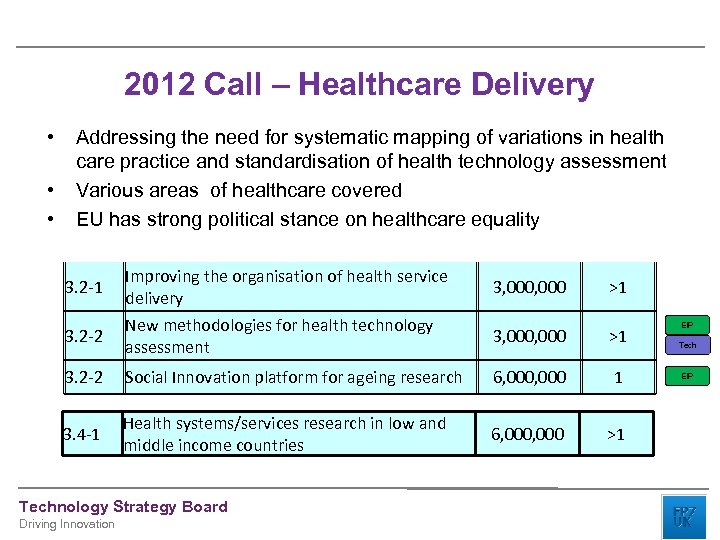 2012 Call – Healthcare Delivery • • • Addressing the need for systematic mapping of variations in health care practice and standardisation of health technology assessment Various areas of healthcare covered EU has strong political stance on healthcare equality 3. 2 -1 Improving the organisation of health service delivery 3. 2 -2 New methodologies for health technology assessment 3, 000 >1 3. 2 -2 Social Innovation platform for ageing research 6, 000 1 3. 4 -1 Health systems/services research in low and middle income countries 6, 000 >1 Technology Strategy Board Driving Innovation 3, 000 >1 EIP Tech EIP
2012 Call – Healthcare Delivery • • • Addressing the need for systematic mapping of variations in health care practice and standardisation of health technology assessment Various areas of healthcare covered EU has strong political stance on healthcare equality 3. 2 -1 Improving the organisation of health service delivery 3. 2 -2 New methodologies for health technology assessment 3, 000 >1 3. 2 -2 Social Innovation platform for ageing research 6, 000 1 3. 4 -1 Health systems/services research in low and middle income countries 6, 000 >1 Technology Strategy Board Driving Innovation 3, 000 >1 EIP Tech EIP
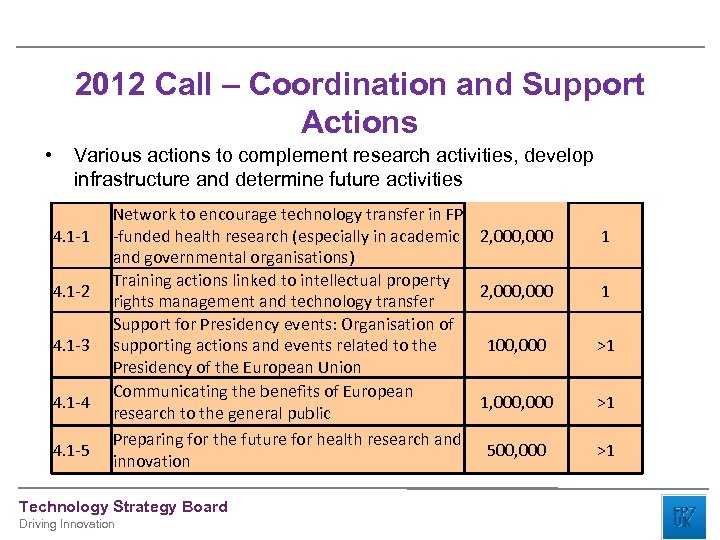 2012 Call – Coordination and Support Actions • Various actions to complement research activities, develop infrastructure and determine future activities 4. 1 -1 4. 1 -2 4. 1 -3 4. 1 -4 4. 1 -5 Network to encourage technology transfer in FP -funded health research (especially in academic 2, 000 and governmental organisations) Training actions linked to intellectual property 2, 000 rights management and technology transfer Support for Presidency events: Organisation of supporting actions and events related to the 100, 000 Presidency of the European Union Communicating the benefits of European 1, 000 research to the general public Preparing for the future for health research and 500, 000 innovation Technology Strategy Board Driving Innovation 1 1 >1 >1 >1
2012 Call – Coordination and Support Actions • Various actions to complement research activities, develop infrastructure and determine future activities 4. 1 -1 4. 1 -2 4. 1 -3 4. 1 -4 4. 1 -5 Network to encourage technology transfer in FP -funded health research (especially in academic 2, 000 and governmental organisations) Training actions linked to intellectual property 2, 000 rights management and technology transfer Support for Presidency events: Organisation of supporting actions and events related to the 100, 000 Presidency of the European Union Communicating the benefits of European 1, 000 research to the general public Preparing for the future for health research and 500, 000 innovation Technology Strategy Board Driving Innovation 1 1 >1 >1 >1
 Key Dates 09 June 2011: Open Info Day, Brussels 10 June 2011: Brokerage Event, Brussels 20 July 2011: Call Opens – EPSS available 27 Sept 2011: likely Stage 1 deadline (INNOVATION 2) 04 Oct 2011: likely Stage 1 deadline (INNOVATION 1) Dec 2011: Stage 1 notification Feb 2012: Stage 2 deadline Technology Strategy Board Driving Innovation
Key Dates 09 June 2011: Open Info Day, Brussels 10 June 2011: Brokerage Event, Brussels 20 July 2011: Call Opens – EPSS available 27 Sept 2011: likely Stage 1 deadline (INNOVATION 2) 04 Oct 2011: likely Stage 1 deadline (INNOVATION 1) Dec 2011: Stage 1 notification Feb 2012: Stage 2 deadline Technology Strategy Board Driving Innovation


