085379ca15835ca8fa8289bbd26255df.ppt
- Количество слайдов: 35

FP 7 Health theme: Opportunities for Collaborative Research Sarajevo, 23 rd April 2009 Dr. Joana Namorado April 2009 Horizontal Aspects Coordination- Ethics, Gender Issues Directorate Health. DG RTD – European Commission

Main points The 7 th Framework programme (FP 7) Ø Rationale and approach Ø Basic principles, including prioritisation Ø Structure and content Ø Next calls for proposals in Health Ø Outcome of first calls in Health Ø Factors for success
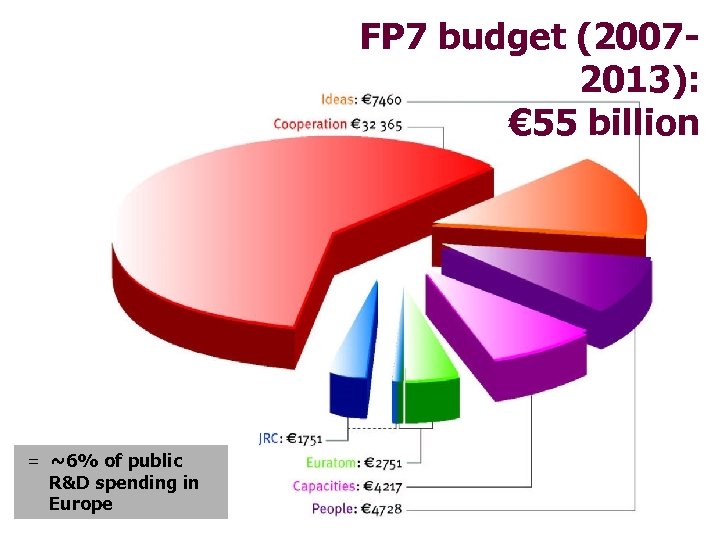
FP 7 budget (20072013): € 55 billion = ~6% of public R&D spending in Europe
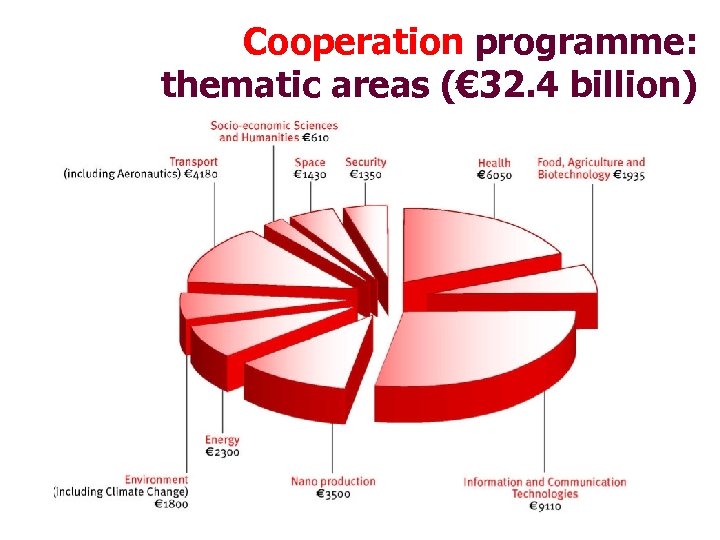
Cooperation programme: thematic areas (€ 32. 4 billion)

Collaborative research in FP 7: The Cooperation programme Thematic Priorities 1. 2. 3. 4. 5. 6. 7. 8. 9. Health Food, agriculture, fisheries and biotechnology Information and communication technologies Nanosciences, nanotechnologies, materials and new production technologies Energy Environment (including climate change) Transport (including aeronautics) Socio-economic sciences and the humanities Security & 10. Space Total for collaborative research 6. 1 1. 9 9. 1 3. 5 2. 3 1. 9 4. 2 0. 6 2. 8 € 32. 4 billion

Collaborative research across borders and other barriers • between countries: – multinational consortia, with at least 3 partners from 27 EU Member States (MS) + Associated Countries (AC) eg: Albania, Bosnia, Croatia, FYROM, Iceland, Israel, Liechtenstein, Montenegro, Norway, Serbia, Switzerland, Turkey. – researchers from any country in the world can participate, from industrialised countries and from developing countries. eg: Argentina, Australia, Brazil, Canada, China, India, Japan, Korea, New-Zealand, Russia, South-Africa, USA, … … … • between different types of organizations Public & private sector: universities, research centres, large companies, Small and medium-sized enterprises (SMEs), etc. • between disciplines: multidisciplinary, translational research
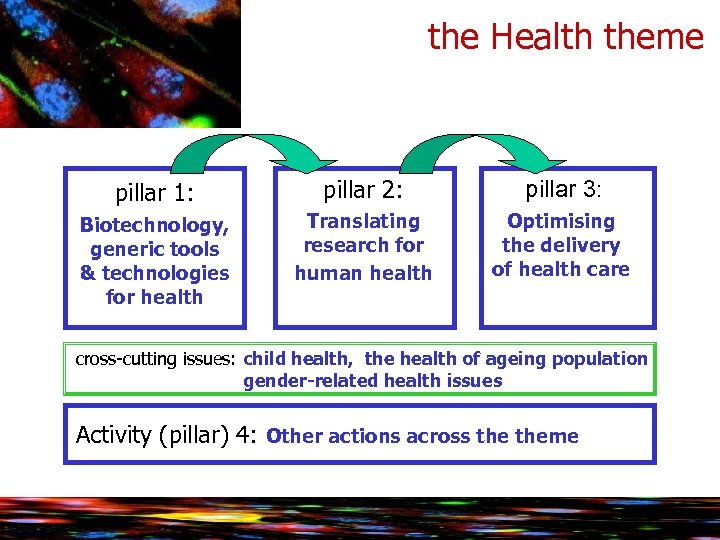
the Health theme pillar 1: pillar 2: pillar 3: Biotechnology, generic tools & technologies for health Translating research for human health Optimising the delivery of health care cross-cutting issues: child health, the health of ageing population gender-related health issues Activity (pillar) 4: Other actions across theme

the Health theme 1: Biotechnology, generic tools and technologies Ø High-throughput research enhancing data generation, standardisation, acquisition & analysis Ø Detection, diagnosis and monitoring with emphasis on non-invasive or minimally invasive approaches Ø Predicting suitability, safety and efficacy of therapies develop and validate parameters, tools, methods and standards and alternatives to animal testing (mainly through IMI) Ø Innovative therapeutic approaches and interventions gene and cell therapy, regenerative medicine, immunotherapy and vaccines.

the Health theme 2: Translating research for human health Ø Integrating biological data and processes: large-scale data gathering, systems biology Ø Research on the brain and related diseases, human development and ageing Ø Translational research in major infectious diseases to confront major threats to public health antimicrobial drug resistance, HIV/AIDS, malaria and TB, emerging epidemics, neglected infectious diseases Ø Translational research in other major diseases: cancer, cardiovascular disease, diabetes and obesity, rare diseases, and other chronic diseases

Cooperation programme Health theme 3: Optimising the delivery of healthcare Ø Translating clinical research into clinical practice Ø Quality, efficiency and solidarity of healthcare systems Ø Enhanced health promotion and disease prevention

Collaborative research on the Health theme 4. Actions across the Theme Ø Coordination & Support Actions across theme Ø Responding to EU policy needs Ø Specific International Cooperation Actions (SICAs)

Scope of research in the Health Theme • In the Health theme, the Framework programme can support both basic and applied research • This includes discovery activities, translational research and early clinical trials (normally only phase I and II).

Trends: SME participation International Cooperation Renewed emphasis and new measures for: Ø SMEs Ø Ø Participation encouraged in all areas Special topics for SMEs Support actions more favourable conditions (75% of costs covered) Ø International Cooperation Ø Ø Participation possible in all areas Special International Cooperation Actions (SICA) Coordination or Support actions new mechanisms: coordinated programmes and topics

International participation in FP 7 - Health research 3 different avenues: 1. All activities open for International Cooperation Partner Countries (ICPCs) can participate in projects and receive EC funding as in FP 6 § Industrialised countries may be funded if their participation is seen as essential for the project § Minimum number of participants: 3 from MS/AS § 2. Specific International Cooperation Actions § Address specific issues that partner countries face or have a global character, on the basis of mutual interest and benefit § Minimum n° of participants: 2+2 (2 from MS/AS + 2 from ICPCs) 3. Bilateral agreements for targeted co-funding § Individual agreements with specific countries (CN, RU…)

Attractive conditions for research intensive SMEs • Medium/long term vision: 7 -year programme • Significant commitment for SMEs (15%) (>€ 900 m for Health) • Improved conditions in FP 7 • Strong support: up to 75% of total R&D costs for research and up to 100% for management and training • Better protection: IPR rules more attentive to SMEs needs • Administrative simplification: reduced need for financial checks and bank guarantees

Scientific excellence at European level From policy to funding the best research projects: • The policy for Health research is described in the FP 7 specific programme (available on CORDIS) • Each year, a work programme is prepared by the EC, based on consultation of scientific community and consideration of funded actions. Discussed with the Advisory Group and with the Programme Committee representing the MS and AC. • Through calls for proposals the EC invites researchers from all Europe and beyond to submit their proposals • The very best projects are selected on the basis of evaluation by a panel of independent experts (peer review) • After negotiation of a grant agreement, a project is funded for 2 -5 y

Scientific excellence at European level Topics selection: • FP 6 and FP 7 actions are taken into consideration • Workshops and conferences with scientific communitiy and others • Early input from MS & AC (strategic priorities) • Topics are drafted by the Commission services, to implement FP 7. • Not all topics / areas can be opened at any one time: some areas are closed in some call, emphasis placed on others • Trying to have a structuring effect on European research

Submission & evaluation Basic principles: Ø annual calls – single-stage or two-stage Ø eligibility check (partners, limits, scope, deadline) Ø evaluation by panels of independent experts overseen by Independent Observers Ø 3 criteria: Ø Science & Technology excellence Ø Implementation & Management Ø Potential Impact Thresholds: 3/5 3/5 overall 10/15 Ø feedback: Evaluation Summary Reports (ESRs)
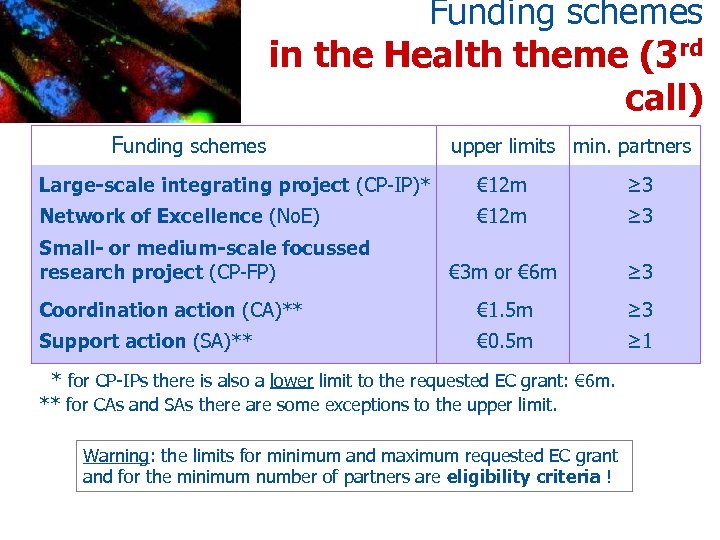
Funding schemes in the Health theme (3 rd call) Funding schemes upper limits min. partners Large-scale integrating project (CP-IP)* € 12 m ≥ 3 Network of Excellence (No. E) € 12 m ≥ 3 € 3 m or € 6 m ≥ 3 Coordination action (CA)** € 1. 5 m ≥ 3 Support action (SA)** € 0. 5 m ≥ 1 Small- or medium-scale focussed research project (CP-FP) * for CP-IPs there is also a lower limit to the requested EC grant: € 6 m. ** for CAs and SAs there are some exceptions to the upper limit. Warning: the limits for minimum and maximum requested EC grant and for the minimum number of partners are eligibility criteria !

Annual calls for proposals in the Health theme Ø 1 st call (2007 budget): € 641 million Ø 2 nd call (2008 budget): € 577 million Ø 3 rd call (2009 budget): ~€ 620 million Ø calls closed Ø 4 th call (2010 budget): ~€ 650 million (tbc) Ø to be published end-July 2009

4 th call for proposals for the Health theme Publication expected 30 July 2008 Ø drawing on the budget for 2010: ~€ 650 million Ø there will be two main calls: Ø ‘FP 7 -HEALTH-2010 -single-stage’ for some areas and topics of the work programme expected deadline: Nov. 2009 (tbc) Ø ‘FP 7 -HEALTH-2010 -two-stage’ for some areas and topics of the work programme. expected deadline for 1 st stage: Oct. 2009 (tbc) Ø plus a coordinated call with the Food and Environment themes: Ø ‘FP 7 -AFRICA-2010 for six topics of the work programme. expected deadline: Jan. 2010 (tbc)

Conditions for two-stage submission/evaluation Ø First stage: Ø proposal size limited to 6 pages (5 pages max. on proposed research and expected impact + 1 page max. to describe consortium & financial resources) Ø only the coordinator needs to complete the ‘A forms’ Ø Evaluation of 2 criteria only (S/T quality and Impact) Ø Higher thresholds Ø Second stage: Ø only coordinators of proposals passing Stage 1 will be invited to submit full proposals for Stage 2 Ø evaluation on all 3 criteria, independently from Stage 1.
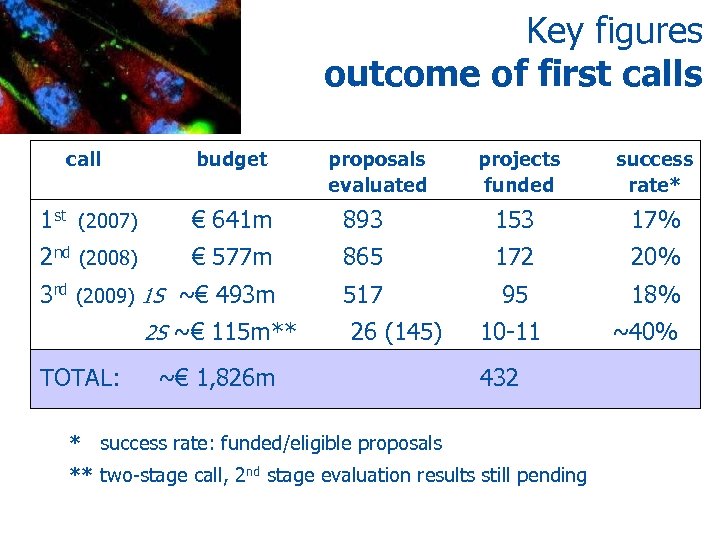
Key figures outcome of first calls call budget 1 st (2007) € 641 m 2 nd (2008) projects funded success rate* 893 153 17% € 577 m 865 172 20% 3 rd (2009) 1 S ~€ 493 m 517 95 18% 2 S ~€ 115 m** TOTAL: proposals evaluated 26 (145) ~€ 1, 826 m 10 -11 432 * success rate: funded/eligible proposals ** two-stage call, 2 nd stage evaluation results still pending ~40%
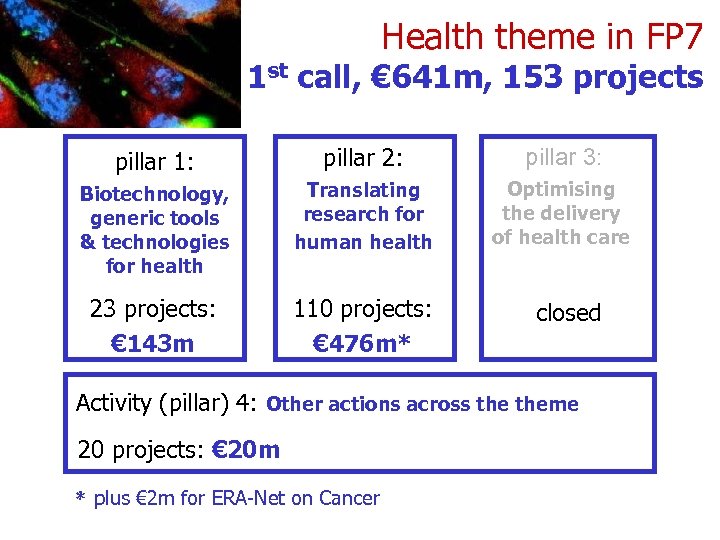
Health theme in FP 7 1 st call, € 641 m, 153 projects pillar 1: pillar 2: pillar 3: Biotechnology, generic tools & technologies for health Translating research for human health Optimising the delivery of health care 23 projects: € 143 m 110 projects: € 476 m* closed Activity (pillar) 4: Other actions across theme 20 projects: € 20 m * plus € 2 m for ERA-Net on Cancer
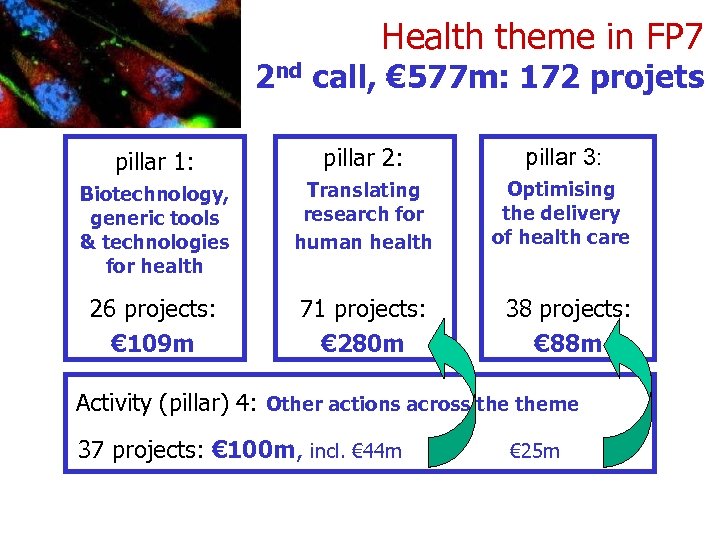
Health theme in FP 7 2 nd call, € 577 m: 172 projets pillar 1: pillar 2: pillar 3: Biotechnology, generic tools & technologies for health Translating research for human health Optimising the delivery of health care 26 projects: € 109 m 71 projects: € 280 m 38 projects: € 88 m Activity (pillar) 4: Other actions across theme 37 projects: € 100 m, incl. € 44 m & € 25 m
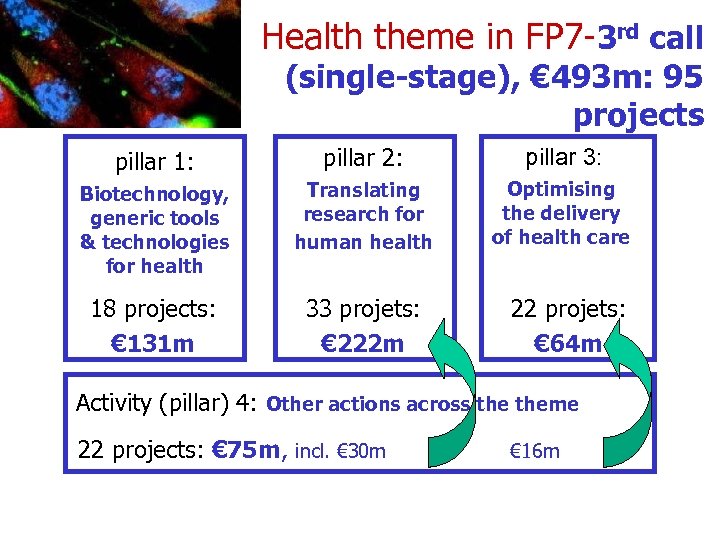
Health theme in FP 7 -3 rd call (single-stage), € 493 m: 95 projects pillar 1: pillar 2: pillar 3: Biotechnology, generic tools & technologies for health Translating research for human health Optimising the delivery of health care 18 projects: € 131 m 33 projets: € 222 m 22 projets: € 64 m Activity (pillar) 4: Other actions across theme 22 projects: € 75 m, incl. € 30 m & € 16 m

Evaluation process for 3 rd call ‘single-stage’ • 517 proposals evaluated (provisional) – About 684 evaluators – Remote evaluation using RIVET began on 12 December – Consensus meetings in Brussels (19/1 – 27/2) – Ethics screening (2 -13/3) – Independent Observers

Key factors for success in FP 7 Competition is tough: only the best projects get funded Ø The proposal must be in scope with the topic and the work programme (not wishful thinking) Ø The consortium of partners must be excellent and appropriate for the task (select the right partners) Ø The proposal must address all criteria Ø Convince the evaluators (don’t rely on reputation) Ø and, of course, respect the basic rules.

Eligibility & quality issues • Requirements for eligibility: Ø meet the deadline with complete proposal Ø have the minimum number of partners Ø respect ceilings for max. EU contribution per project • Quality is a prerequisite for success: Ø page number limitations for proposals (new in FP 7) Ø added requirements for information on Ethics issues

Causes for ineligibility in first calls • Ineligibility cases in 1 st call: 21/914 Ø ceilings not respected: 11 Ø minimum n° partners: 5 Ø withdrawn, test or incomplete: 5 • Ineligibility cases in the 2 nd call: 37/902 Ø ceilings not respected: 23 Ø minimum n° partners: 11 Ø withdrawn: 1 Ø out of scope: 3

Ethics: Panorama for FP 7 Ethical Review Process Evolving Applicants are first expected to identify key ethical issues raised by their proposals and explain how they address them. They are: • • • Informed Consent Research on Human Embryos/Foetuses Data Protection and Privacy Research on Animals Research involving Developing Countries Dual Use (research that could have a military application)

Ethics: Panorama for FP 7 The Proposal • The Proposal includes a section dedicated to ETHICS. • The applicant fills out a table of ethics issues in the proposal • Scientific evaluators are asked to signal any ethical issues that arise in a proposal including those which have not been highlighted by the applicant.

Contacts & Information Consult the web page for Health: http: //cordis. europa. eu/fp 7/cooperation/health_en. html including NCPs, Ethics, registration as an Expert International Cooperation: Dr. Indridi Benediktsson Tel. +32 2 299 3137 – Email: indridi. benediktsson@ec. europa. eu SME participation: Dr. Ludovica Serafini Tel. +32 2 295 6759 – Email: ludovica. serafini@ec. europa. eu Ethical issues: Dr. Joana Namorado Tel. +32 2 298 5466 – Email: joana. namorado@ec. europa. eu

Contacts in the Health Directorate Director – Dr. Ruxandra Draghia-Akli : Medical and Public Health Research unit – Dr. Manuel Hallen Email: manuel. hallen@ec. europa. eu Cancer – Dr. Maria Vidal Email: maria-jose. vidal-ragout@ec. europa. eu Public Health sector – Dr. Kevin Mc. Carthy Email: kevin. mccarthy@ec. europa. eu Infectious Diseases unit – Dr. Bernard Mulligan Email: bernard. mulligan@ec. europa. eu Emerging Infectious diseases sector – Dr. Anna Lönnroth Email: anna. lonnroth@ec. europa. eu Genomics and Systems Biology unit – Patrik Kolar Email: patrik. kolar@ec. europa. eu Health Biotechnology unit – Dr. Arnd Hoeveler Email: arnd. hoeveler@ec. europa. eu Coordination unit – Stéphane Hogan Email: stephane. hogan@ec. europa. eu

Thank You Dr. Joana Namorado Directorate Health DG RTD – European Commission FP 7 –Health theme
085379ca15835ca8fa8289bbd26255df.ppt