electrochemistry - 1 (3).pptx
- Количество слайдов: 31
 Foundation Year Program Electrochemistry Lecture A: Principles of electrochemistry & batteries 2015 -16
Foundation Year Program Electrochemistry Lecture A: Principles of electrochemistry & batteries 2015 -16
 Foundation Year Program Learning outcomes 1. Define electrochemistry 2. Redox reactions 3. Electrochemical cells a) Galvanic/Voltaic cell b) Electrolytic cell 4. Battery types 2015 -16
Foundation Year Program Learning outcomes 1. Define electrochemistry 2. Redox reactions 3. Electrochemical cells a) Galvanic/Voltaic cell b) Electrolytic cell 4. Battery types 2015 -16
 Foundation Year Program Have you ever wondered? • How do batteries work? • How is a phone charged? • How can we protect metals from corrosion? 2015 -16
Foundation Year Program Have you ever wondered? • How do batteries work? • How is a phone charged? • How can we protect metals from corrosion? 2015 -16
 Foundation Year Program So what is electrochemistry? • Electrochemistry is the study of chemical processes led by movement of electrons from one element to another in a reaction called reduction and oxidation (redox). 2015 -16
Foundation Year Program So what is electrochemistry? • Electrochemistry is the study of chemical processes led by movement of electrons from one element to another in a reaction called reduction and oxidation (redox). 2015 -16
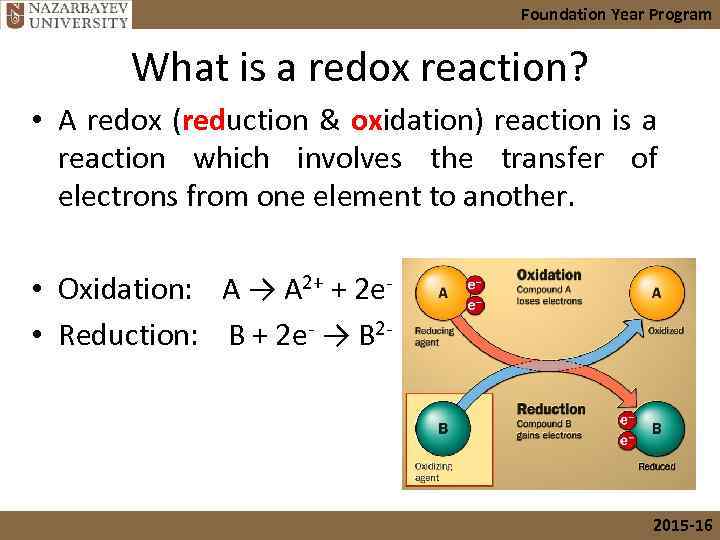 Foundation Year Program What is a redox reaction? • A redox (reduction & oxidation) reaction is a reaction which involves the transfer of electrons from one element to another. • Oxidation: A → A 2+ + 2 e- • Reduction: B + 2 e- → B 2 2015 -16
Foundation Year Program What is a redox reaction? • A redox (reduction & oxidation) reaction is a reaction which involves the transfer of electrons from one element to another. • Oxidation: A → A 2+ + 2 e- • Reduction: B + 2 e- → B 2 2015 -16
 Foundation Year Program Redox reaction 2015 -16
Foundation Year Program Redox reaction 2015 -16
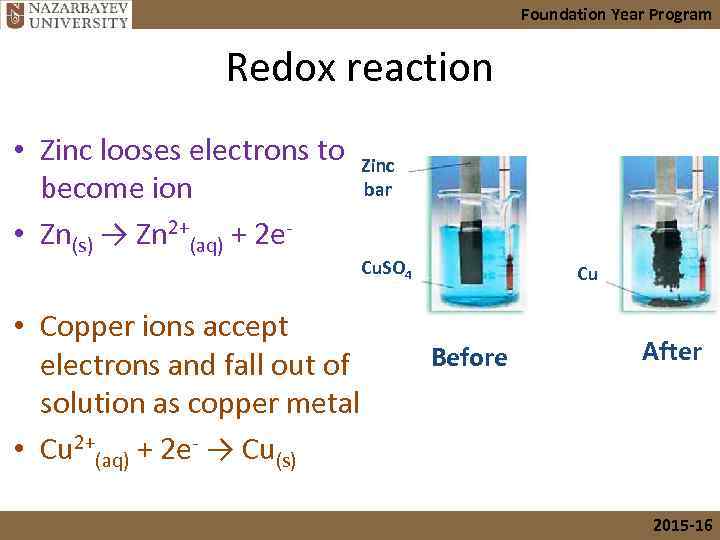 Foundation Year Program Redox reaction • Zinc looses electrons to Zinc bar become ion • Zn(s) → Zn 2+(aq) + 2 e- Cu. SO 4 • Copper ions accept electrons and fall out of solution as copper metal • Cu 2+(aq) + 2 e- → Cu(s) Cu Before After 2015 -16
Foundation Year Program Redox reaction • Zinc looses electrons to Zinc bar become ion • Zn(s) → Zn 2+(aq) + 2 e- Cu. SO 4 • Copper ions accept electrons and fall out of solution as copper metal • Cu 2+(aq) + 2 e- → Cu(s) Cu Before After 2015 -16
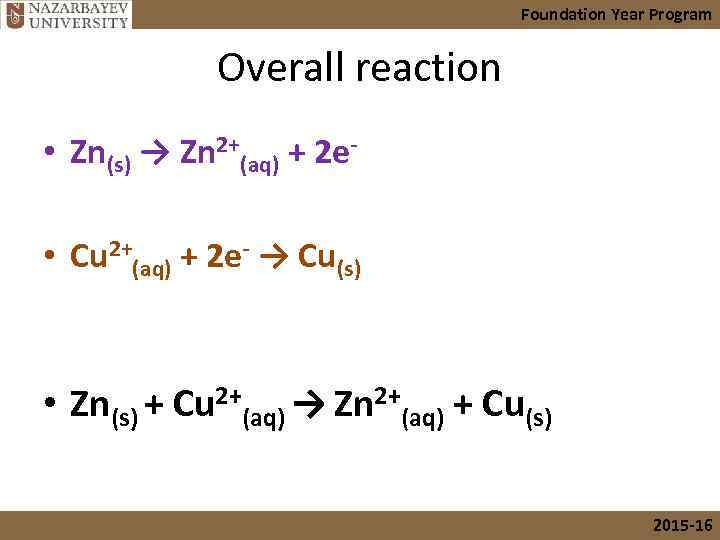 Foundation Year Program Overall reaction • Zn(s) → Zn 2+(aq) + 2 e • Cu 2+(aq) + 2 e- → Cu(s) • Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) 2015 -16
Foundation Year Program Overall reaction • Zn(s) → Zn 2+(aq) + 2 e • Cu 2+(aq) + 2 e- → Cu(s) • Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) 2015 -16
 Foundation Year Program Redox in daily life Batteries Combustion Corrosion Hair colouring Apple darkening 2015 -16
Foundation Year Program Redox in daily life Batteries Combustion Corrosion Hair colouring Apple darkening 2015 -16
 Foundation Year Program Redox in daily life • Ageing of humans is linked with the effects of oxidation. • To delay the effects of oxidation, doctors recommend antioxidants - natural reducing agents such as vitamin C and vitamin E. 2015 -16
Foundation Year Program Redox in daily life • Ageing of humans is linked with the effects of oxidation. • To delay the effects of oxidation, doctors recommend antioxidants - natural reducing agents such as vitamin C and vitamin E. 2015 -16
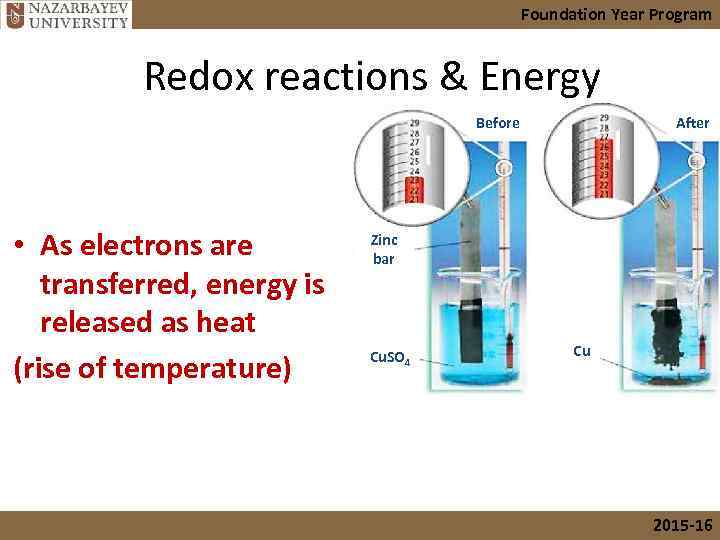 Foundation Year Program Redox reactions & Energy Before • As electrons are transferred, energy is released as heat (rise of temperature) After Zinc bar Cu. SO 4 Cu 2015 -16
Foundation Year Program Redox reactions & Energy Before • As electrons are transferred, energy is released as heat (rise of temperature) After Zinc bar Cu. SO 4 Cu 2015 -16
 Foundation Year Program Redox reactions & Energy • The change of energy can be expressed in the form of electrical energy: the movement of electrons generate electricity. • Electrochemistry looks at the interaction between electrical energy and chemical changes. Chemical changes ↔ Electrical energy 2015 -16
Foundation Year Program Redox reactions & Energy • The change of energy can be expressed in the form of electrical energy: the movement of electrons generate electricity. • Electrochemistry looks at the interaction between electrical energy and chemical changes. Chemical changes ↔ Electrical energy 2015 -16
 Foundation Year Program Redox reactions & Energy • Processes which produce electrical energy from a redox reaction or promote a chemical reaction by electrical energy occur in devices called electrochemical cells. • E. g. Batteries use a series of electrochemical cells to store chemical energy and transform it into electrical energy. 2015 -16
Foundation Year Program Redox reactions & Energy • Processes which produce electrical energy from a redox reaction or promote a chemical reaction by electrical energy occur in devices called electrochemical cells. • E. g. Batteries use a series of electrochemical cells to store chemical energy and transform it into electrical energy. 2015 -16
 Foundation Year Program Electrochemical cells • Consists of two half-cells containing electrode, electrolyte and salt bridge. • The electrode where oxidation occurs is called the anode. • The electrode where reduction occurs is called the cathode. • Electrons flow from anode to cathode. • A salt bridge is used to keep reaction continuing by maintaining electrical neutrality. 2015 -16
Foundation Year Program Electrochemical cells • Consists of two half-cells containing electrode, electrolyte and salt bridge. • The electrode where oxidation occurs is called the anode. • The electrode where reduction occurs is called the cathode. • Electrons flow from anode to cathode. • A salt bridge is used to keep reaction continuing by maintaining electrical neutrality. 2015 -16
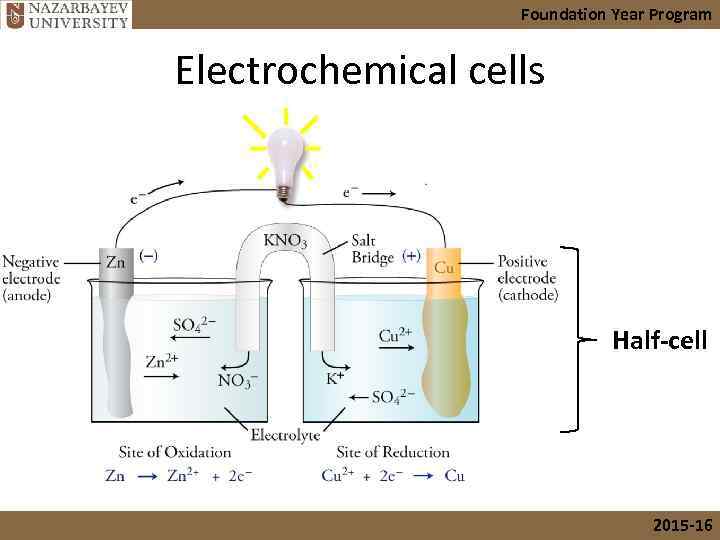 Foundation Year Program Electrochemical cells Half-cell 2015 -16
Foundation Year Program Electrochemical cells Half-cell 2015 -16
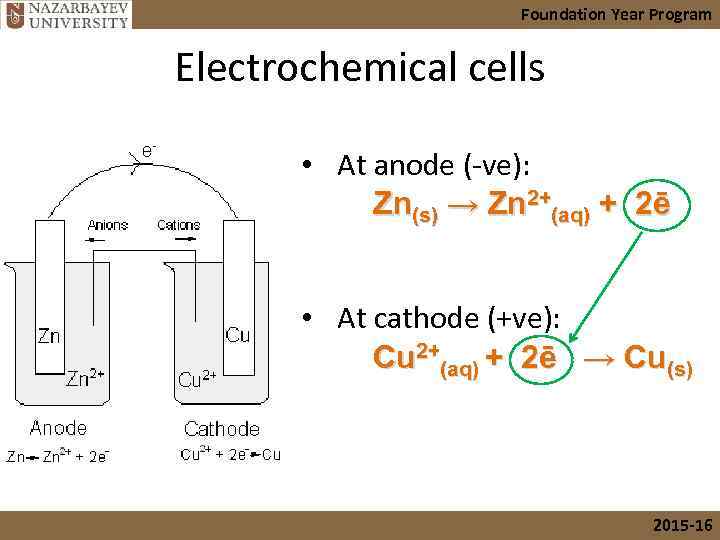 Foundation Year Program Electrochemical cells • At anode (-ve): Zn(s) → Zn 2+(aq) + 2ē • At cathode (+ve): Cu 2+(aq) + 2ē → Cu(s) 2015 -16
Foundation Year Program Electrochemical cells • At anode (-ve): Zn(s) → Zn 2+(aq) + 2ē • At cathode (+ve): Cu 2+(aq) + 2ē → Cu(s) 2015 -16
 Foundation Year Program 2015 -16
Foundation Year Program 2015 -16
 Foundation Year Program Electrochemical cells: two types 1. Galvanic or Voltaic cells • Chemical reaction causes the production of electric energy; • Chemical E → Electrical E • Oxidation occurs at anode (-ve) • Reduction occurs at cathode is (+ve) • Electrons supplied by species getting oxidised 2. Electrolytic cells • Externally applied electrical energy causes the chemical reaction • Electrical E → Chemical E • Oxidation occurs at anode (+ve) • Reduction occurs at cathode (-ve) • Electrons suppled by external source 2015 -16
Foundation Year Program Electrochemical cells: two types 1. Galvanic or Voltaic cells • Chemical reaction causes the production of electric energy; • Chemical E → Electrical E • Oxidation occurs at anode (-ve) • Reduction occurs at cathode is (+ve) • Electrons supplied by species getting oxidised 2. Electrolytic cells • Externally applied electrical energy causes the chemical reaction • Electrical E → Chemical E • Oxidation occurs at anode (+ve) • Reduction occurs at cathode (-ve) • Electrons suppled by external source 2015 -16
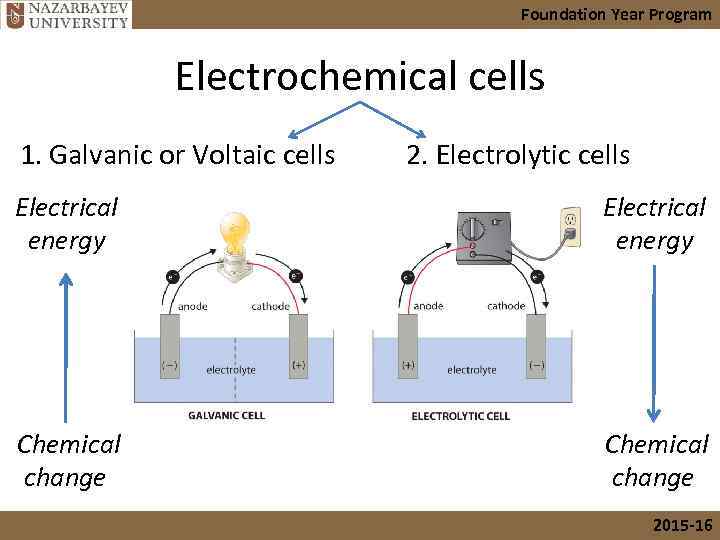 Foundation Year Program Electrochemical cells 1. Galvanic or Voltaic cells 2. Electrolytic cells Electrical energy Chemical change 2015 -16
Foundation Year Program Electrochemical cells 1. Galvanic or Voltaic cells 2. Electrolytic cells Electrical energy Chemical change 2015 -16
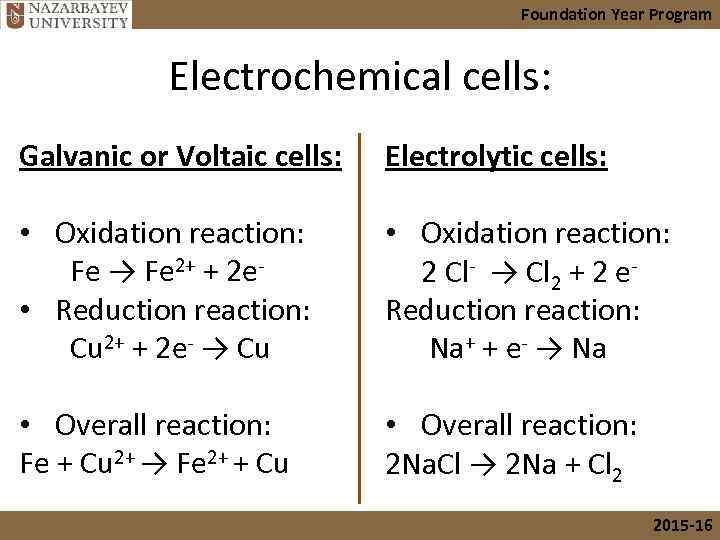 Foundation Year Program Electrochemical cells: Galvanic or Voltaic cells: Electrolytic cells: • Oxidation reaction: Fe → Fe 2+ + 2 e- • Reduction reaction: Cu 2+ + 2 e- → Cu • Oxidation reaction: 2 Cl- → Cl 2 + 2 e. Reduction reaction: Na+ + e- → Na • Overall reaction: Fe + Cu 2+ → Fe 2+ + Cu • Overall reaction: 2 Na. Cl → 2 Na + Cl 2 2015 -16
Foundation Year Program Electrochemical cells: Galvanic or Voltaic cells: Electrolytic cells: • Oxidation reaction: Fe → Fe 2+ + 2 e- • Reduction reaction: Cu 2+ + 2 e- → Cu • Oxidation reaction: 2 Cl- → Cl 2 + 2 e. Reduction reaction: Na+ + e- → Na • Overall reaction: Fe + Cu 2+ → Fe 2+ + Cu • Overall reaction: 2 Na. Cl → 2 Na + Cl 2 2015 -16
 Foundation Year Program Battery types 1. • • PRIMARY BATTERY Uses Galvanic cell and reactions proceed one way; Chemical E → Electrical E Not rechargeable e. g. used in flashlight, toys, clock, TV remote controller 2. SECONDARY BATTERY • Uses both Galvanic and electrolytic cells • Reactions proceed either way • Electrical E ↔ Chemical E • Rechargeable • e. g. used in mobile phone, laptop, camera 2015 -16
Foundation Year Program Battery types 1. • • PRIMARY BATTERY Uses Galvanic cell and reactions proceed one way; Chemical E → Electrical E Not rechargeable e. g. used in flashlight, toys, clock, TV remote controller 2. SECONDARY BATTERY • Uses both Galvanic and electrolytic cells • Reactions proceed either way • Electrical E ↔ Chemical E • Rechargeable • e. g. used in mobile phone, laptop, camera 2015 -16
 Foundation Year Program Oranges fruit batteries power i. Phone https: //www. youtube. com/watch? v=9_LLj 4_3 ZRA 2015 -16
Foundation Year Program Oranges fruit batteries power i. Phone https: //www. youtube. com/watch? v=9_LLj 4_3 ZRA 2015 -16
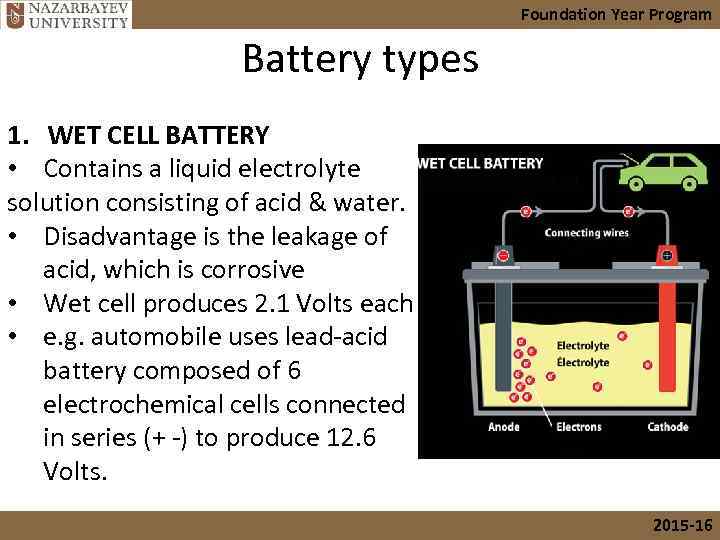 Foundation Year Program Battery types 1. WET CELL BATTERY • Contains a liquid electrolyte solution consisting of acid & water. • Disadvantage is the leakage of acid, which is corrosive • Wet cell produces 2. 1 Volts each • e. g. automobile uses lead-acid battery composed of 6 electrochemical cells connected in series (+ -) to produce 12. 6 Volts. 2015 -16
Foundation Year Program Battery types 1. WET CELL BATTERY • Contains a liquid electrolyte solution consisting of acid & water. • Disadvantage is the leakage of acid, which is corrosive • Wet cell produces 2. 1 Volts each • e. g. automobile uses lead-acid battery composed of 6 electrochemical cells connected in series (+ -) to produce 12. 6 Volts. 2015 -16
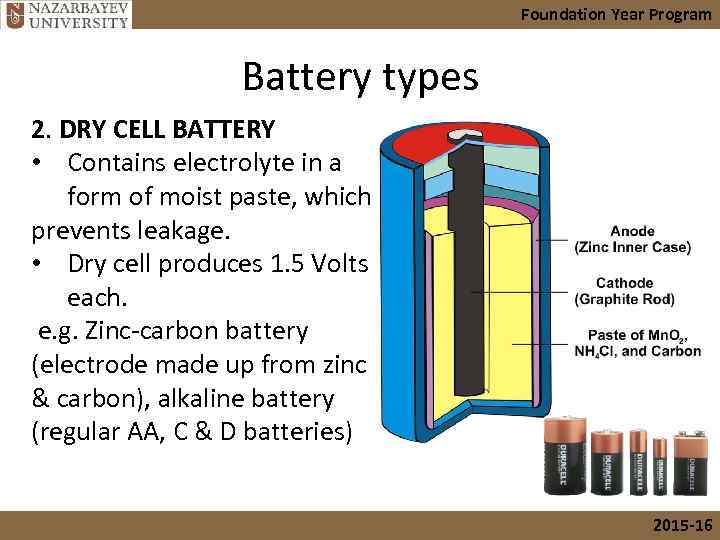 Foundation Year Program Battery types 2. DRY CELL BATTERY • Contains electrolyte in a form of moist paste, which prevents leakage. • Dry cell produces 1. 5 Volts each. e. g. Zinc-carbon battery (electrode made up from zinc & carbon), alkaline battery (regular AA, C & D batteries) 2015 -16
Foundation Year Program Battery types 2. DRY CELL BATTERY • Contains electrolyte in a form of moist paste, which prevents leakage. • Dry cell produces 1. 5 Volts each. e. g. Zinc-carbon battery (electrode made up from zinc & carbon), alkaline battery (regular AA, C & D batteries) 2015 -16
 Foundation Year Program Lithium-ion battery • Variety substances used, but common combination is a lithium cobalt oxide cathode & a carbon anode. • Used in high-performance devices, e. g. cell phone, digital camera, electric car 2015 -16
Foundation Year Program Lithium-ion battery • Variety substances used, but common combination is a lithium cobalt oxide cathode & a carbon anode. • Used in high-performance devices, e. g. cell phone, digital camera, electric car 2015 -16
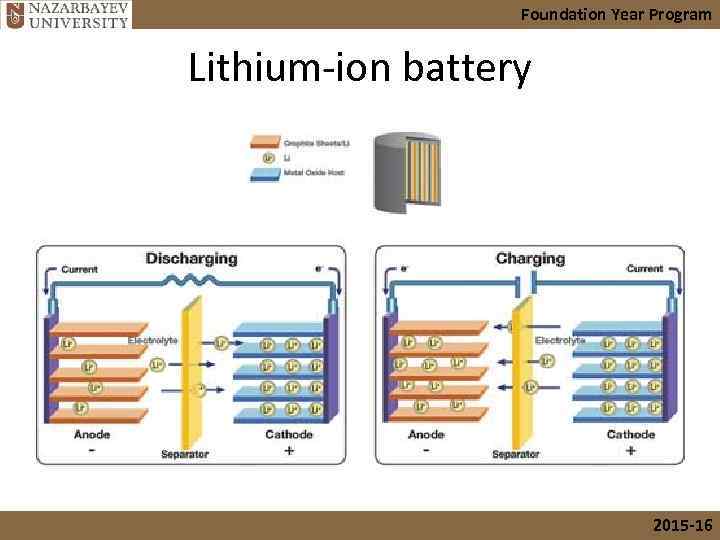 Foundation Year Program Lithium-ion battery 2015 -16
Foundation Year Program Lithium-ion battery 2015 -16
 Foundation Year Program Biological batteries The fish generates a current by pumping charged potassium and sodium ions out of its special muscle cells. The negatively charged ions inside the cells increase until it opens just the right channels to cause electrons to flood out of the cell. Natural electric eel cells generate and release electric pulses of more than 500 volts with eight different channels and pumps. 2015 -16
Foundation Year Program Biological batteries The fish generates a current by pumping charged potassium and sodium ions out of its special muscle cells. The negatively charged ions inside the cells increase until it opens just the right channels to cause electrons to flood out of the cell. Natural electric eel cells generate and release electric pulses of more than 500 volts with eight different channels and pumps. 2015 -16
 Foundation Year Program Electric Eel Powers Christmas Tree • https: //www. youtube. com/watch? t=69&v=GO 00 t. PIYSUQ 2015 -16
Foundation Year Program Electric Eel Powers Christmas Tree • https: //www. youtube. com/watch? t=69&v=GO 00 t. PIYSUQ 2015 -16
 Foundation Year Program Battery performance is affected by several factors: • Self-discharge: disposable batteries lose 8 -20% of their original charge per year because of “side” chemical reactions. • Corrosion: internal parts may corrode and fail. • Environmental conditions: should withstand stress, vibration (e. g. sensitivity of automobile batteries); should be stored at low temperature since high temperature fastens “side” reactions; 2015 -16
Foundation Year Program Battery performance is affected by several factors: • Self-discharge: disposable batteries lose 8 -20% of their original charge per year because of “side” chemical reactions. • Corrosion: internal parts may corrode and fail. • Environmental conditions: should withstand stress, vibration (e. g. sensitivity of automobile batteries); should be stored at low temperature since high temperature fastens “side” reactions; 2015 -16
 Foundation Year Program The origin of a battery • The Baghdad Battery (Parthian Battery), about 2000 years old, is a clay pot which encapsulates a copper cylinder with an iron rod suspended in the center. 2015 -16
Foundation Year Program The origin of a battery • The Baghdad Battery (Parthian Battery), about 2000 years old, is a clay pot which encapsulates a copper cylinder with an iron rod suspended in the center. 2015 -16
 Foundation Year Program The origin of a battery • After the Second World War, Willard Gray built replicas and, filling them with an electrolyte, found that the devices could produce 2 volts of electricity. • The question remains, if it really was a battery, what was it used to power? 2015 -16
Foundation Year Program The origin of a battery • After the Second World War, Willard Gray built replicas and, filling them with an electrolyte, found that the devices could produce 2 volts of electricity. • The question remains, if it really was a battery, what was it used to power? 2015 -16


