9a8208b91c8ed0e83f0ee6dbc10cfbb5.ppt
- Количество слайдов: 44
 Forces at work in a molecule
Forces at work in a molecule

 Molecules experience two types of forces: intramolecular (or bonding) and intermolecular (or nonbonding) Intramolecular attraction Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Molecules experience two types of forces: intramolecular (or bonding) and intermolecular (or nonbonding) Intramolecular attraction Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
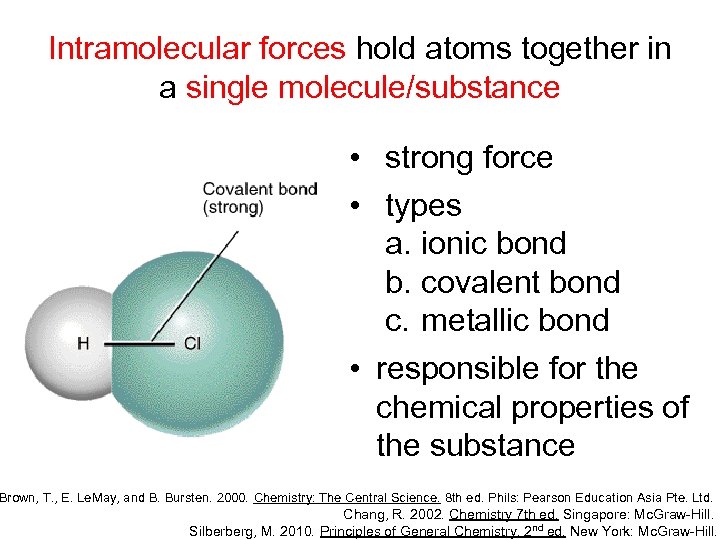 Intramolecular forces hold atoms together in a single molecule/substance • strong force • types a. ionic bond b. covalent bond c. metallic bond • responsible for the chemical properties of the substance Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Intramolecular forces hold atoms together in a single molecule/substance • strong force • types a. ionic bond b. covalent bond c. metallic bond • responsible for the chemical properties of the substance Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
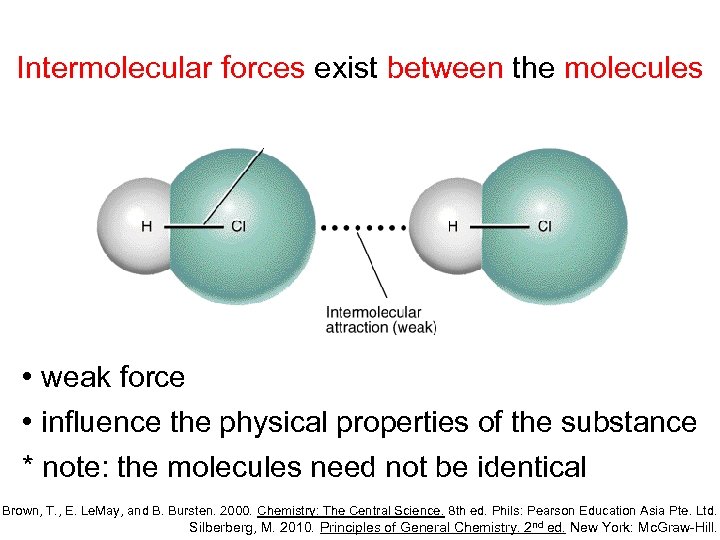 Intermolecular forces exist between the molecules • weak force • influence the physical properties of the substance * note: the molecules need not be identical Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Intermolecular forces exist between the molecules • weak force • influence the physical properties of the substance * note: the molecules need not be identical Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
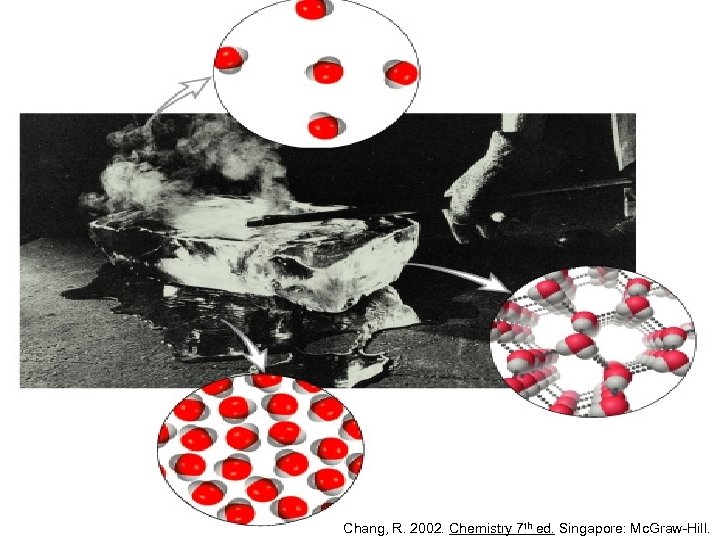 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
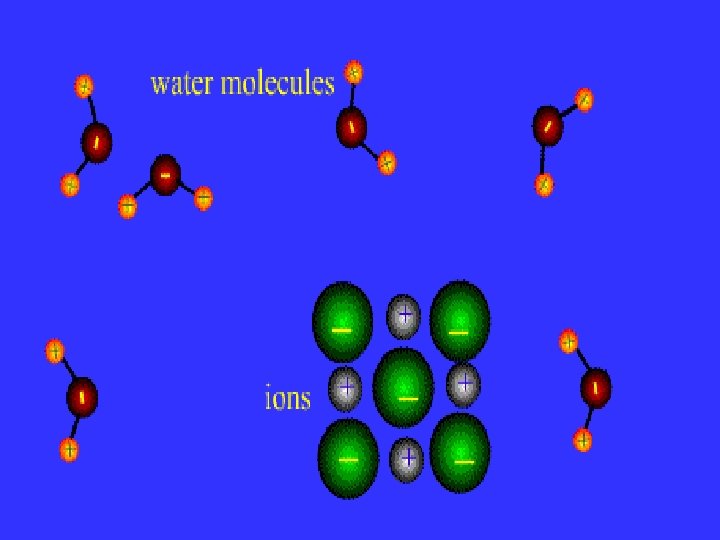
 Intermolecular forces of attraction (IMFA)
Intermolecular forces of attraction (IMFA)
 Opposites Attract
Opposites Attract
 Opposites Attract
Opposites Attract
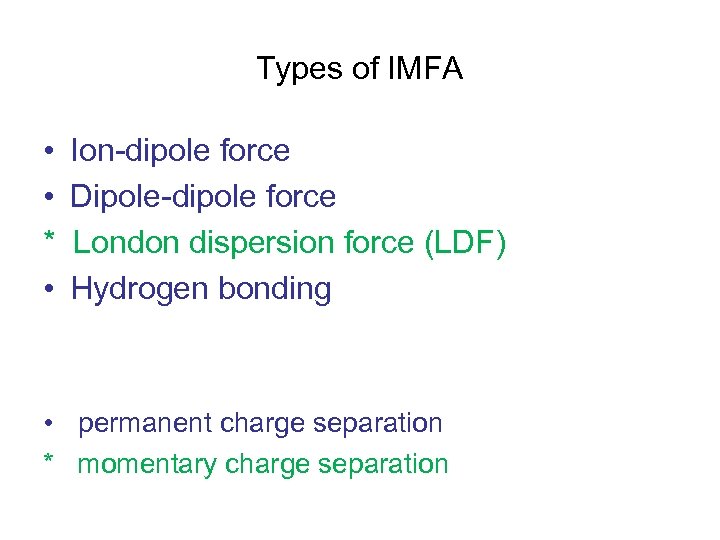
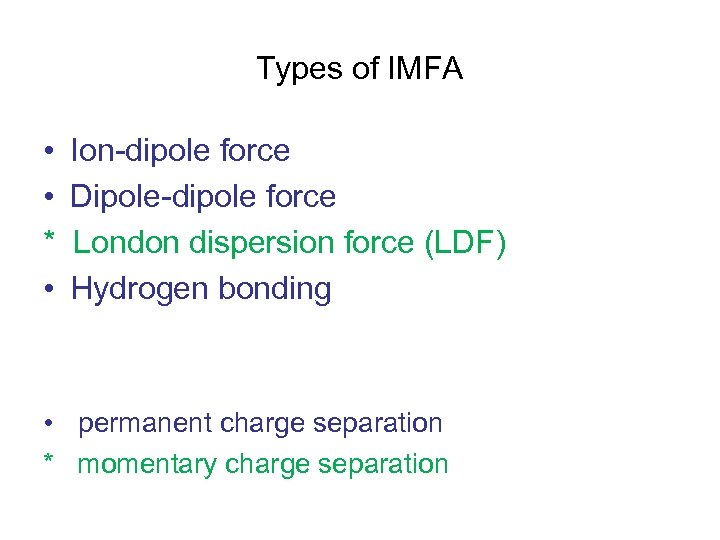 Types of IMFA • • * • Ion-dipole force Dipole-dipole force London dispersion force (LDF) Hydrogen bonding • permanent charge separation * momentary charge separation
Types of IMFA • • * • Ion-dipole force Dipole-dipole force London dispersion force (LDF) Hydrogen bonding • permanent charge separation * momentary charge separation
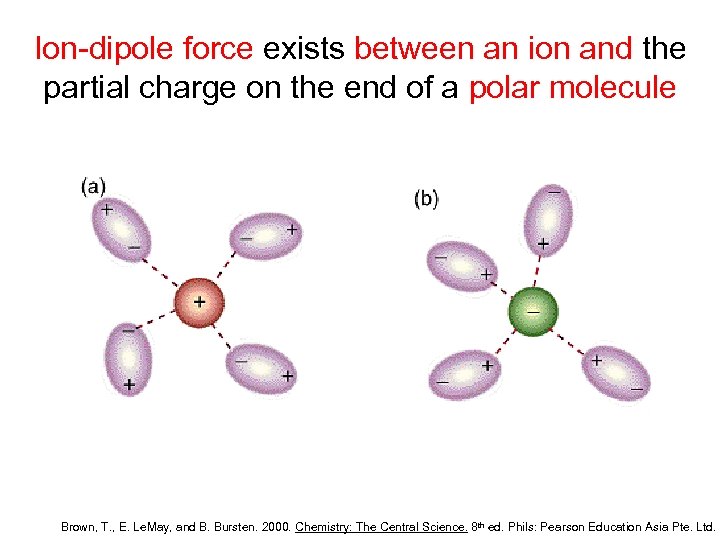 Ion-dipole force exists between an ion and the partial charge on the end of a polar molecule Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
Ion-dipole force exists between an ion and the partial charge on the end of a polar molecule Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
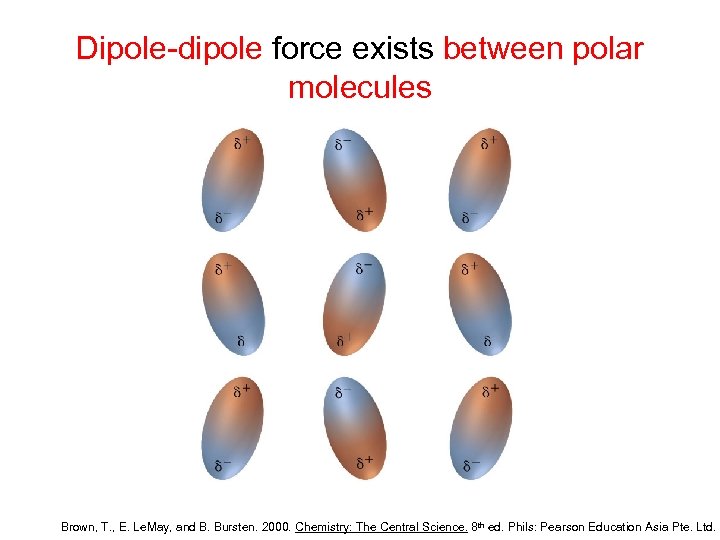 Dipole-dipole force exists between polar molecules Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
Dipole-dipole force exists between polar molecules Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
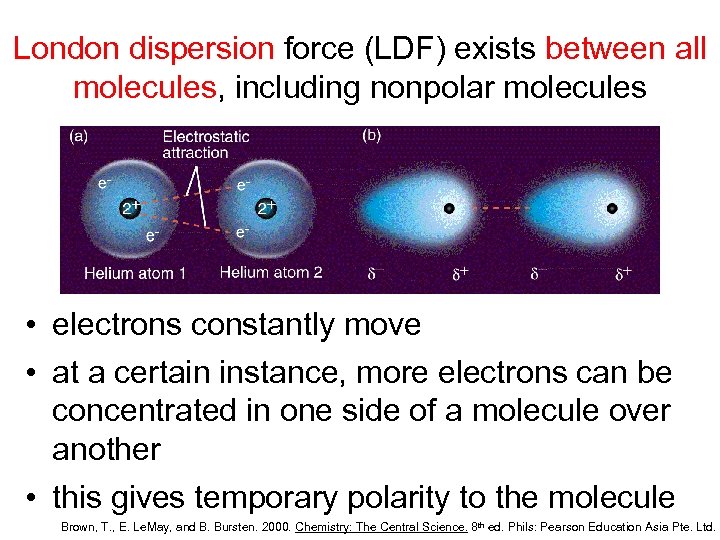 London dispersion force (LDF) exists between all molecules, including nonpolar molecules • electrons constantly move • at a certain instance, more electrons can be concentrated in one side of a molecule over another • this gives temporary polarity to the molecule Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
London dispersion force (LDF) exists between all molecules, including nonpolar molecules • electrons constantly move • at a certain instance, more electrons can be concentrated in one side of a molecule over another • this gives temporary polarity to the molecule Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
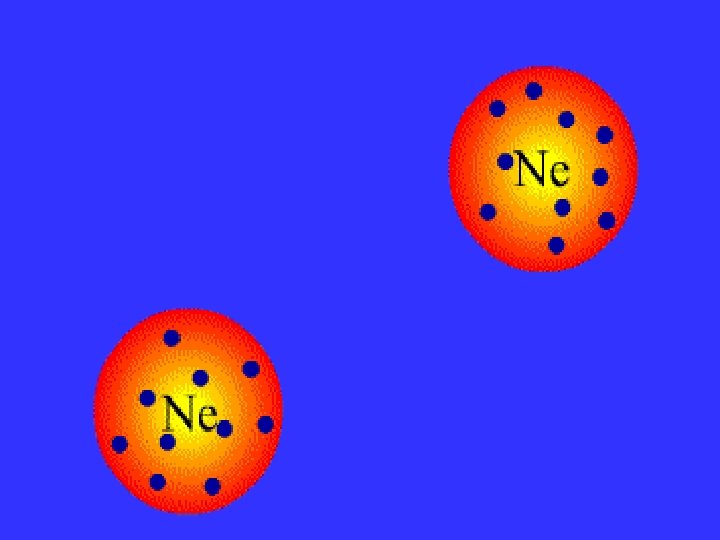
 Types of IMFA • • * • Ion-dipole force Dipole-dipole force London dispersion force (LDF) Hydrogen bonding • permanent charge separation * momentary charge separation
Types of IMFA • • * • Ion-dipole force Dipole-dipole force London dispersion force (LDF) Hydrogen bonding • permanent charge separation * momentary charge separation
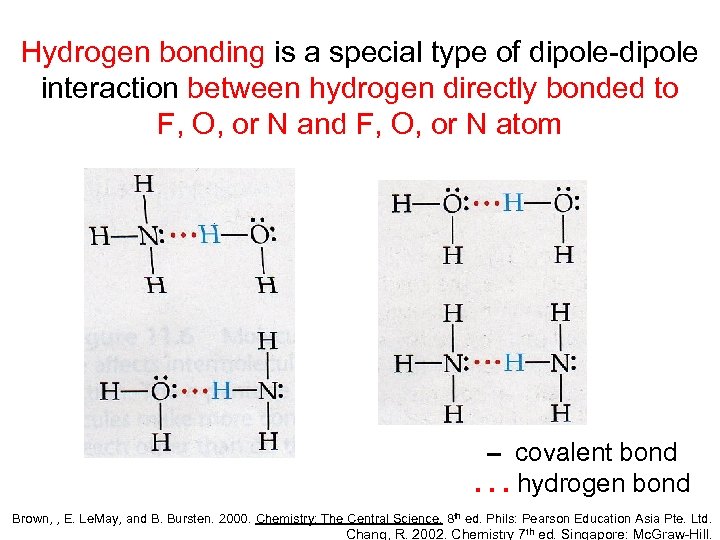 Hydrogen bonding is a special type of dipole-dipole interaction between hydrogen directly bonded to F, O, or N and F, O, or N atom – covalent bond. . . hydrogen bond Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Hydrogen bonding is a special type of dipole-dipole interaction between hydrogen directly bonded to F, O, or N and F, O, or N atom – covalent bond. . . hydrogen bond Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
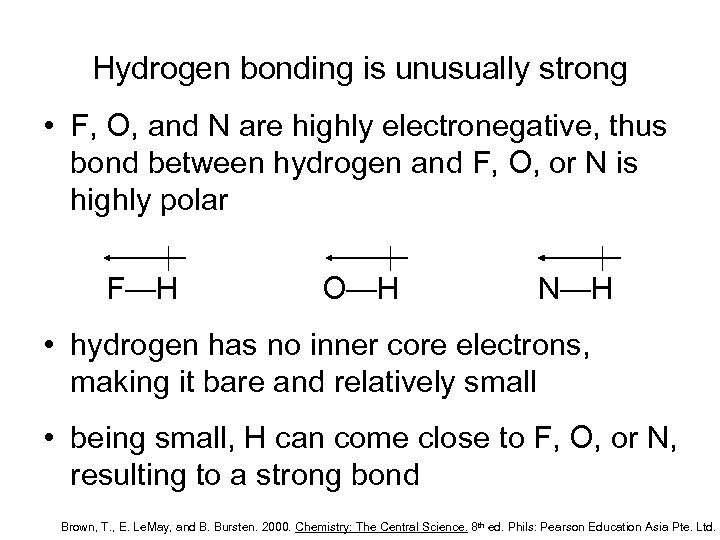 Hydrogen bonding is unusually strong • F, O, and N are highly electronegative, thus bond between hydrogen and F, O, or N is highly polar F—H O—H N—H • hydrogen has no inner core electrons, making it bare and relatively small • being small, H can come close to F, O, or N, resulting to a strong bond Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
Hydrogen bonding is unusually strong • F, O, and N are highly electronegative, thus bond between hydrogen and F, O, or N is highly polar F—H O—H N—H • hydrogen has no inner core electrons, making it bare and relatively small • being small, H can come close to F, O, or N, resulting to a strong bond Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
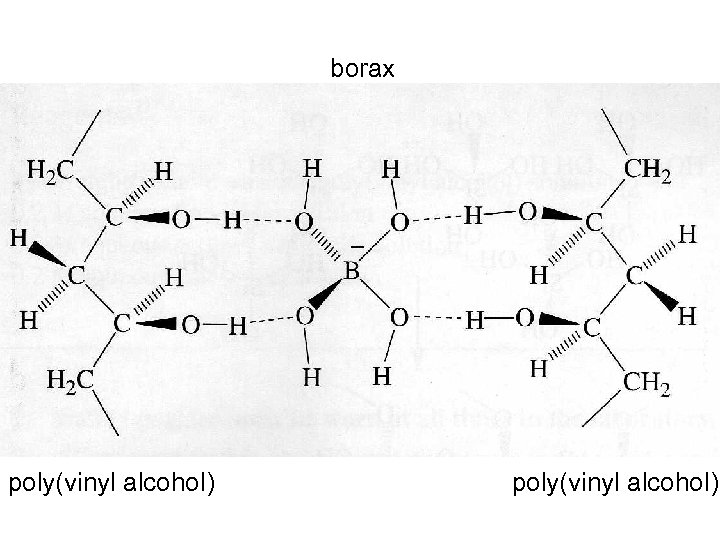 borax poly(vinyl alcohol)
borax poly(vinyl alcohol)
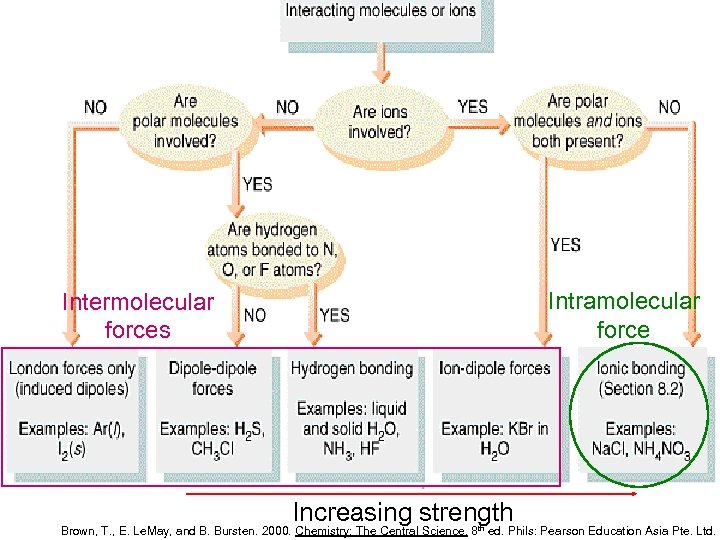 Intramolecular force Intermolecular forces Increasing strength Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
Intramolecular force Intermolecular forces Increasing strength Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
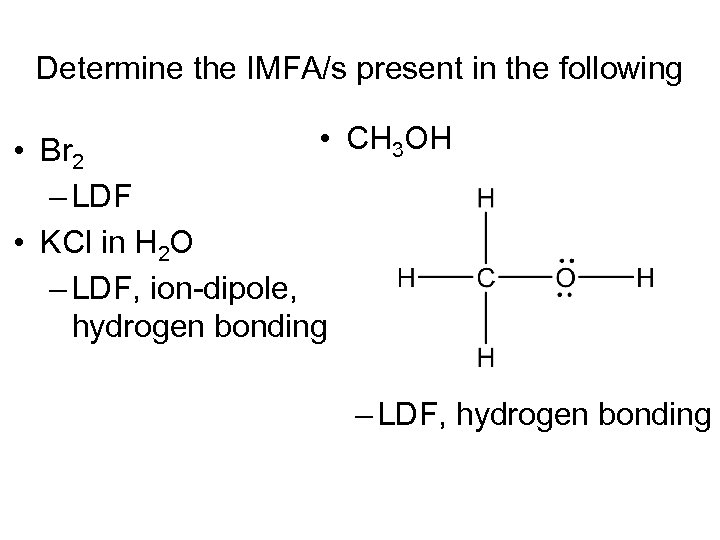 Determine the IMFA/s present in the following • CH 3 OH • Br 2 – LDF • KCl in H 2 O – LDF, ion-dipole, hydrogen bonding – LDF, hydrogen bonding
Determine the IMFA/s present in the following • CH 3 OH • Br 2 – LDF • KCl in H 2 O – LDF, ion-dipole, hydrogen bonding – LDF, hydrogen bonding
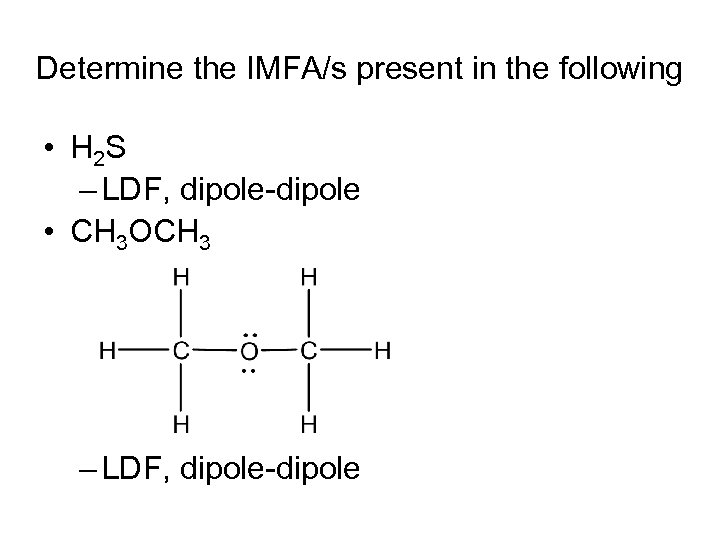 Determine the IMFA/s present in the following • H 2 S – LDF, dipole-dipole • CH 3 OCH 3 – LDF, dipole-dipole
Determine the IMFA/s present in the following • H 2 S – LDF, dipole-dipole • CH 3 OCH 3 – LDF, dipole-dipole
 IMFA and the states of matter
IMFA and the states of matter
 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
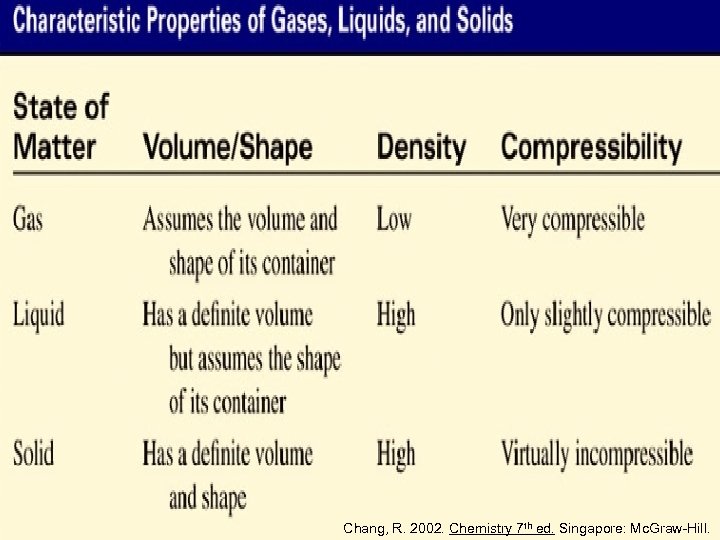
 Gas molecules have weak IMFAs • interaction between the molecules is minimal, and so gas molecules fill a container • distances between gas molecules are so great, and so gases are highly compressible and have low densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Gas molecules have weak IMFAs • interaction between the molecules is minimal, and so gas molecules fill a container • distances between gas molecules are so great, and so gases are highly compressible and have low densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Liquid molecules have stronger IMFAs • molecules are held together by one or more types of attractive forces, and so liquid molecules have definite volume but indefinite shape • distances between liquid molecules are small, and so liquids are slightly compressible and have high densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Liquid molecules have stronger IMFAs • molecules are held together by one or more types of attractive forces, and so liquid molecules have definite volume but indefinite shape • distances between liquid molecules are small, and so liquids are slightly compressible and have high densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Solid molecules have very strong IMFAs • molecules are held rigidly in position, and so solid molecules have definite volume and definite shape • distances between solid molecules are even smaller than in liquids, and so solids are almost incompressible and have high densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Solid molecules have very strong IMFAs • molecules are held rigidly in position, and so solid molecules have definite volume and definite shape • distances between solid molecules are even smaller than in liquids, and so solids are almost incompressible and have high densities Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
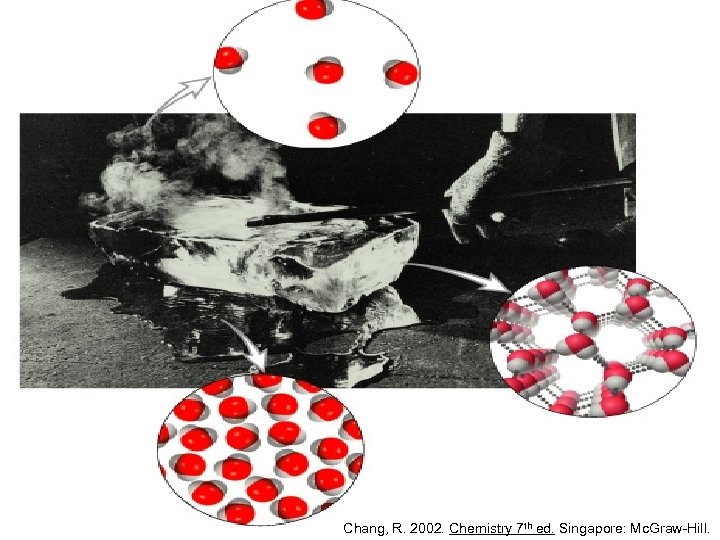
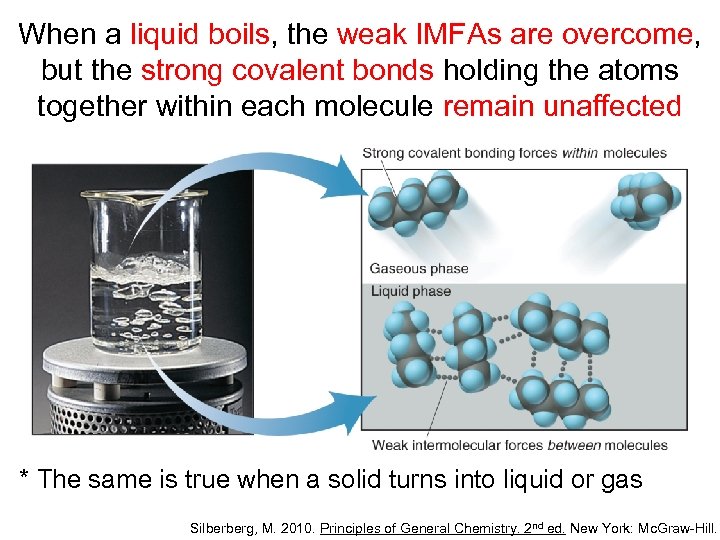 When a liquid boils, the weak IMFAs are overcome, but the strong covalent bonds holding the atoms together within each molecule remain unaffected * The same is true when a solid turns into liquid or gas Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
When a liquid boils, the weak IMFAs are overcome, but the strong covalent bonds holding the atoms together within each molecule remain unaffected * The same is true when a solid turns into liquid or gas Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
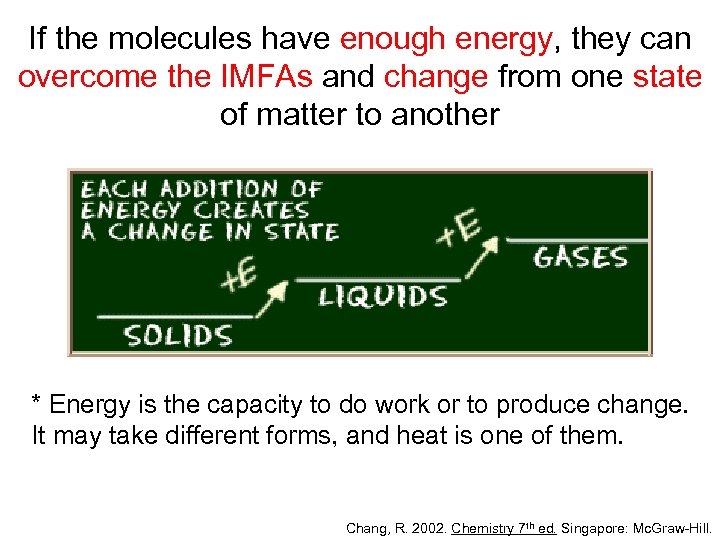 If the molecules have enough energy, they can overcome the IMFAs and change from one state of matter to another * Energy is the capacity to do work or to produce change. It may take different forms, and heat is one of them. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
If the molecules have enough energy, they can overcome the IMFAs and change from one state of matter to another * Energy is the capacity to do work or to produce change. It may take different forms, and heat is one of them. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 QUESTION: Why is boiling point of alcohol lower than the boiling point of water?
QUESTION: Why is boiling point of alcohol lower than the boiling point of water?

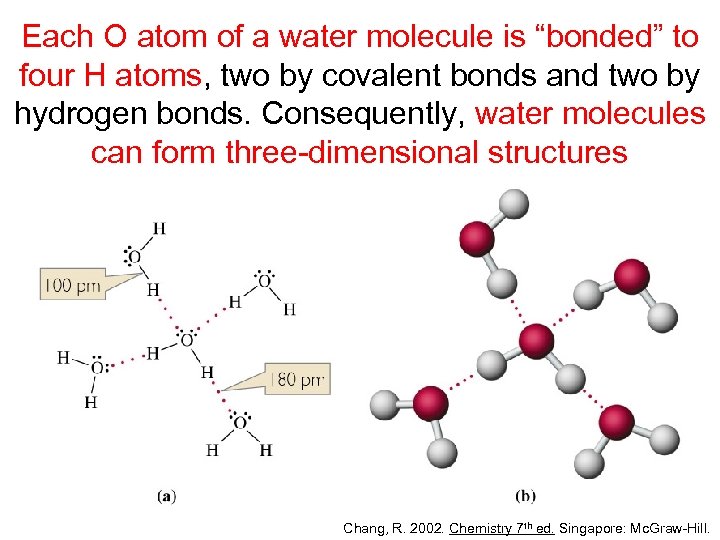 Each O atom of a water molecule is “bonded” to four H atoms, two by covalent bonds and two by hydrogen bonds. Consequently, water molecules can form three-dimensional structures Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Each O atom of a water molecule is “bonded” to four H atoms, two by covalent bonds and two by hydrogen bonds. Consequently, water molecules can form three-dimensional structures Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
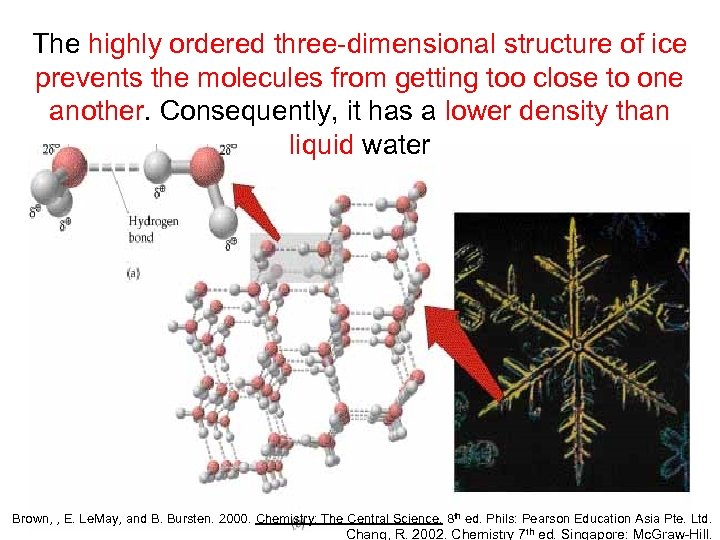 The highly ordered three-dimensional structure of ice prevents the molecules from getting too close to one another. Consequently, it has a lower density than liquid water Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
The highly ordered three-dimensional structure of ice prevents the molecules from getting too close to one another. Consequently, it has a lower density than liquid water Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 The ice layer that forms on the surface (and not at the bottom) of a lake insulates the water beneath and maintains a high enough temperature to sustain aquatic life Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
The ice layer that forms on the surface (and not at the bottom) of a lake insulates the water beneath and maintains a high enough temperature to sustain aquatic life Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Soap
Soap
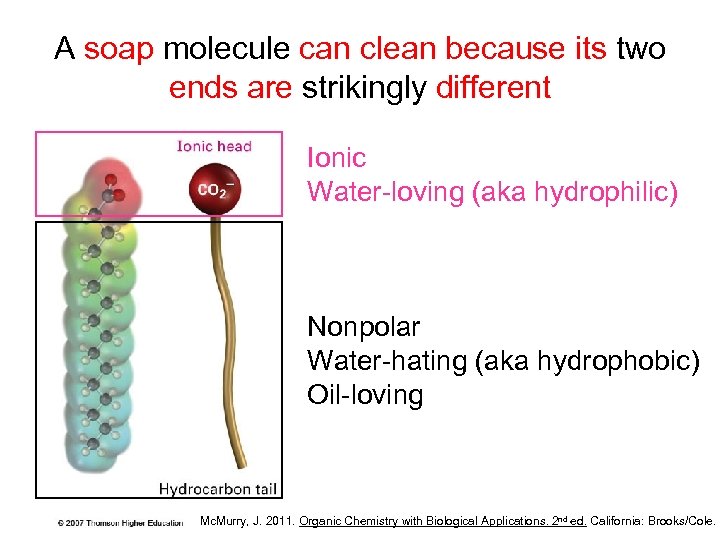 A soap molecule can clean because its two ends are strikingly different Ionic Water-loving (aka hydrophilic) Nonpolar Water-hating (aka hydrophobic) Oil-loving Mc. Murry, J. 2011. Organic Chemistry with Biological Applications. 2 nd ed. California: Brooks/Cole.
A soap molecule can clean because its two ends are strikingly different Ionic Water-loving (aka hydrophilic) Nonpolar Water-hating (aka hydrophobic) Oil-loving Mc. Murry, J. 2011. Organic Chemistry with Biological Applications. 2 nd ed. California: Brooks/Cole.
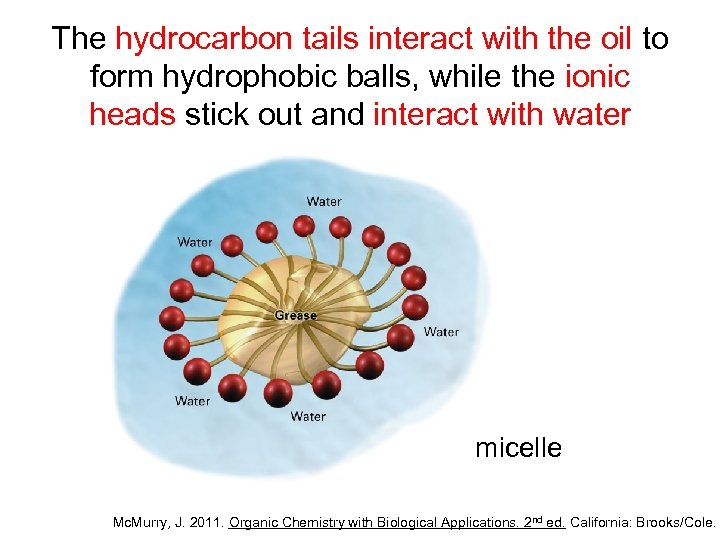 The hydrocarbon tails interact with the oil to form hydrophobic balls, while the ionic heads stick out and interact with water micelle Mc. Murry, J. 2011. Organic Chemistry with Biological Applications. 2 nd ed. California: Brooks/Cole.
The hydrocarbon tails interact with the oil to form hydrophobic balls, while the ionic heads stick out and interact with water micelle Mc. Murry, J. 2011. Organic Chemistry with Biological Applications. 2 nd ed. California: Brooks/Cole.
 And others…
And others…
 Compared to tissue, blotting paper is better at removing oil from the face http: //badkittyexoticwear. com/shop/facecareoilblottingpaper-p-1617. html http: //pics. drugstore. com/prodimg/68807/300. jpg
Compared to tissue, blotting paper is better at removing oil from the face http: //badkittyexoticwear. com/shop/facecareoilblottingpaper-p-1617. html http: //pics. drugstore. com/prodimg/68807/300. jpg
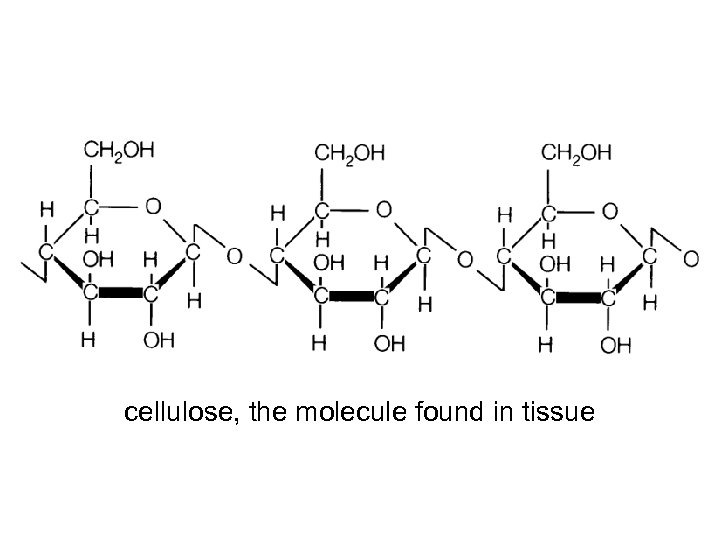 cellulose, the molecule found in tissue
cellulose, the molecule found in tissue
 To cook bulalo, one needs to boil beef in water with salt. During the process, the fat from the meat is seen floating on top of the broth, while the salt is not visible. http: //norecipes. com/blog/2009/06/11/bulalo-recipe/
To cook bulalo, one needs to boil beef in water with salt. During the process, the fat from the meat is seen floating on top of the broth, while the salt is not visible. http: //norecipes. com/blog/2009/06/11/bulalo-recipe/
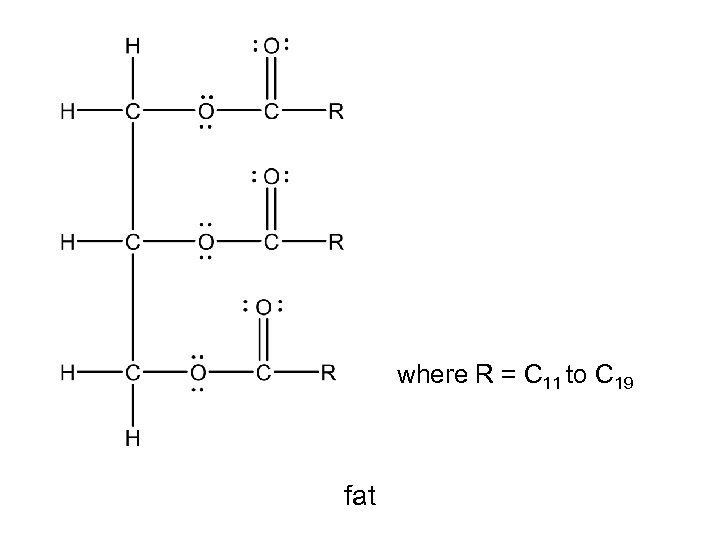 where R = C 11 to C 19 fat
where R = C 11 to C 19 fat
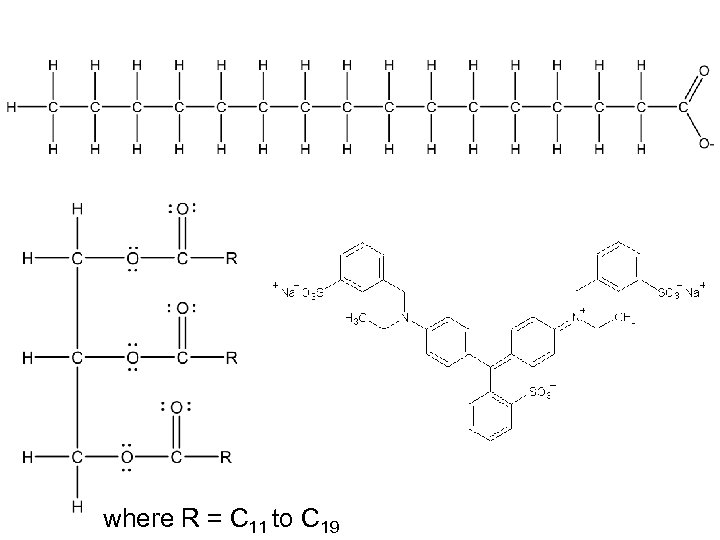 where R = C 11 to C 19
where R = C 11 to C 19


