ef6f44b27f658c29e330556f2ad9ef86.ppt
- Количество слайдов: 49

FOOD CHEMISTRY BY DR BOOMINATHAN Ph. D. M. Sc. , (Med. Bio, JIPMER), M. Sc. , (FGS, Israel), Ph. D (NUS, SINGAPORE) PONDICHERRY UNIVERSITY 1/August/2012

Food Science/Chemistry • Food science is an interdisciplinary subject involving primarily bacteriology, chemistry, biology, and engineering. • Food chemistry, a major aspect of food science, deals with the composition and properties of food and the chemical changes it undergoes during handling, processing, and storage.
Molecular Food Biochemistry Carbohydrates Copyright © 1999 -2008 by Joyce J. Diwan. All rights reserved.
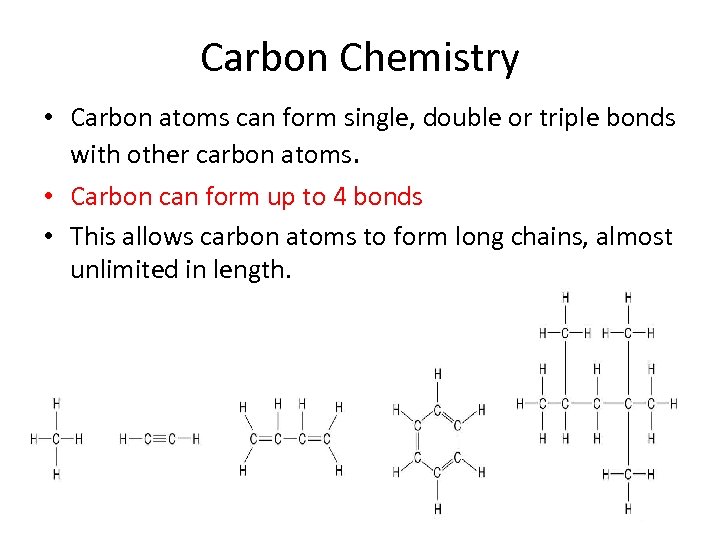
Carbon Chemistry • Carbon atoms can form single, double or triple bonds with other carbon atoms. • Carbon can form up to 4 bonds • This allows carbon atoms to form long chains, almost unlimited in length.
Macromolecules • “GIANT MOLECULES” • Made up of numerous of little molecules. • Formed from a process known as polymerization, in which large molecules are produced by joining small ones together. • The small units (monomers), join together to form large units (polymers)
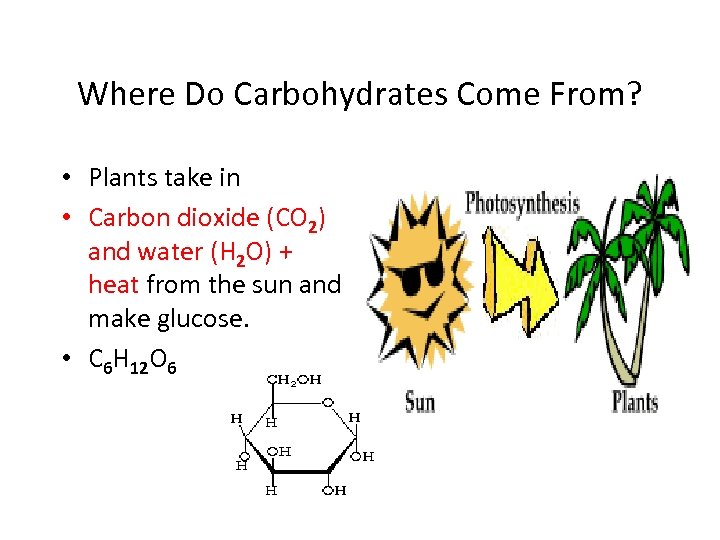
Where Do Carbohydrates Come From? • Plants take in • Carbon dioxide (CO 2) and water (H 2 O) + heat from the sun and make glucose. • C 6 H 12 O 6

Carbohydrates • As the name implies, consist of carbon, hydrogen, and oxygen. • Hydrate=(water) hydrogen and oxygen. • The basic formula for carbohydrates is C-H 2 O, meaning that there is one carbon atom, two hydrogen atoms, and one oxygen atom as the ratio in the structure of carbohydrates • What would be the formula for a carbohydrate that has 3 carbons. • C 3 H 6 O 3

Carbohydrate • Fancy way of saying sugar. • Carbohydrates are energy packed compounds, that can be broken down quickly by organisms to give them energy. • However, the energy supplied by carbohydrates does not last long, and that is why you get hungry every 4 hours. • Carbohydrates are also used for structure.

Saccharides • Scientist use the word saccharides to describe sugars. • If there is only one sugar molecule it is known as a monosaccharide • If there are two it is a disaccharide • When there a whole bunch, it is a polysaccharide.
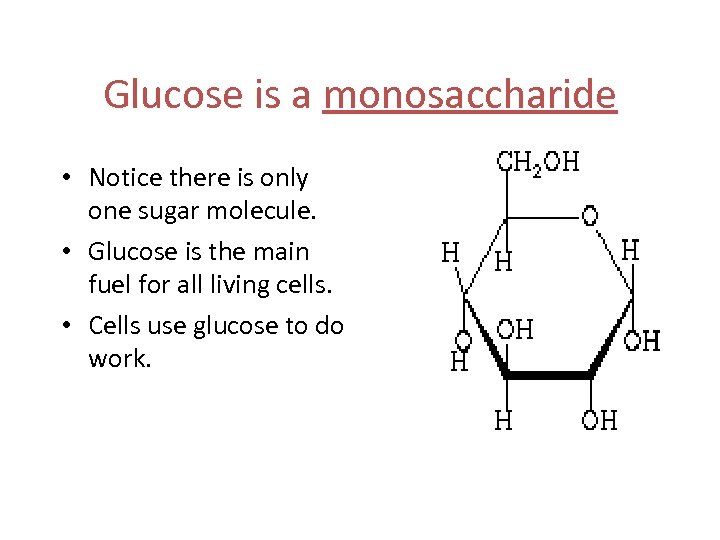
Glucose is a monosaccharide • Notice there is only one sugar molecule. • Glucose is the main fuel for all living cells. • Cells use glucose to do work.
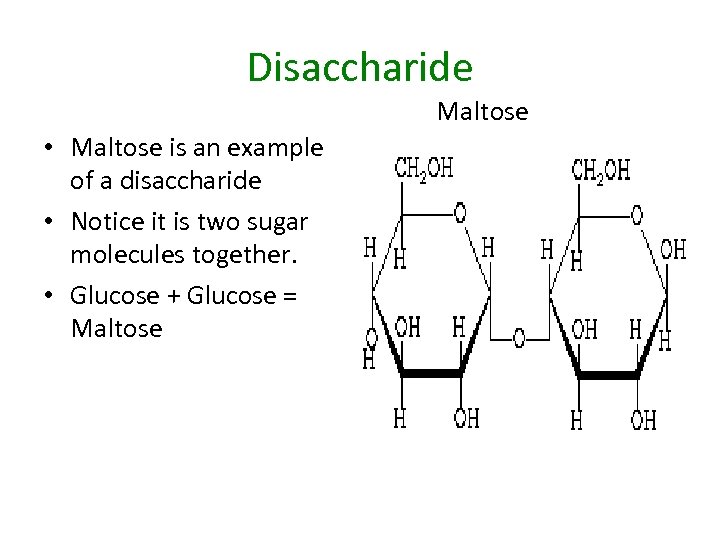
Disaccharide Maltose • Maltose is an example of a disaccharide • Notice it is two sugar molecules together. • Glucose + Glucose = Maltose
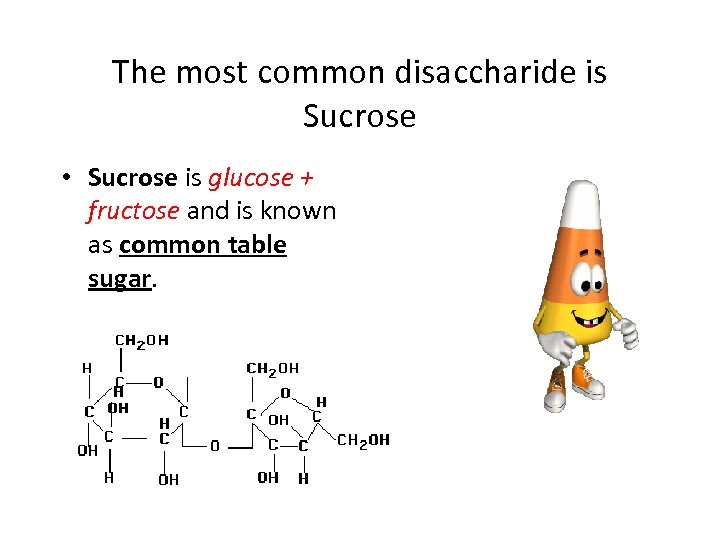
The most common disaccharide is Sucrose • Sucrose is glucose + fructose and is known as common table sugar.
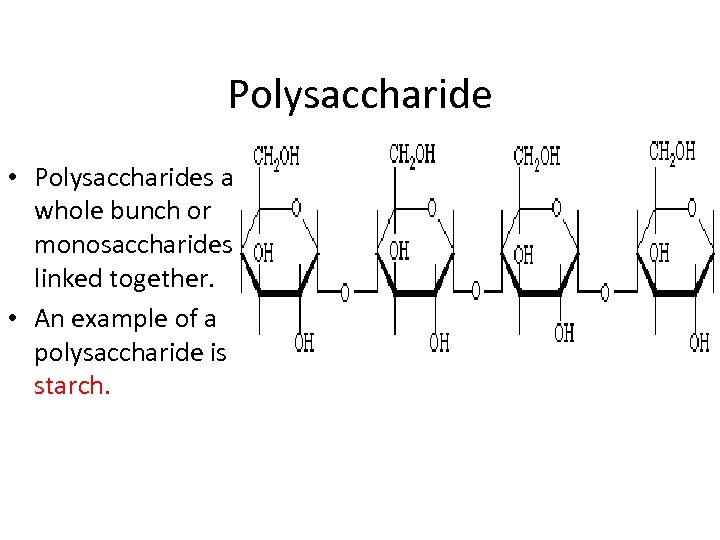
Polysaccharide • Polysaccharides are a whole bunch or monosaccharides linked together. • An example of a polysaccharide is starch.
Polysaccharide • Polysaccharides are a whole bunch or monosaccharides linked together. • An example of a polysaccharide is starch.
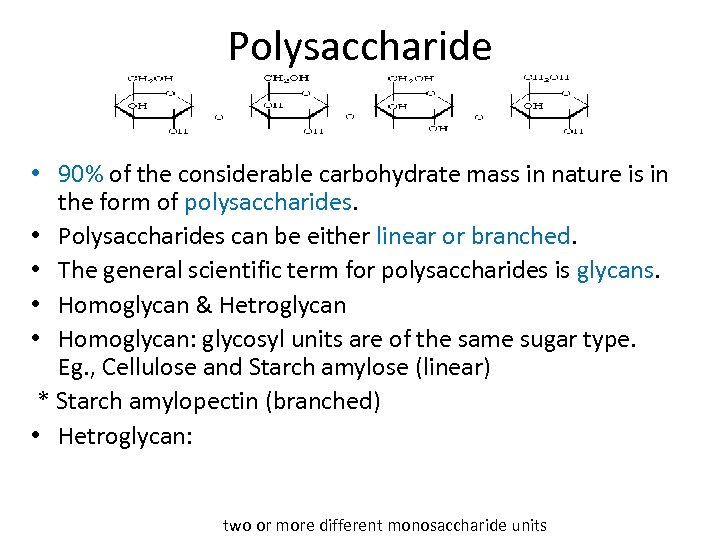
Polysaccharide • 90% of the considerable carbohydrate mass in nature is in the form of polysaccharides. • Polysaccharides can be either linear or branched. • The general scientific term for polysaccharides is glycans. • Homoglycan & Hetroglycan • Homoglycan: glycosyl units are of the same sugar type. Eg. , Cellulose and Starch amylose (linear) * Starch amylopectin (branched) • Hetroglycan: two or more different monosaccharide units

* Diheteroglycans:

Most of the names of carbohydrates end in -ose • • • Glucose-What plants make Maltose- used in making beer (disaccharide) Fructose – found in fruit (monosaccharide) Sucrose- Table sugar (disaccharide) Lactose – In milk (disaccharide)
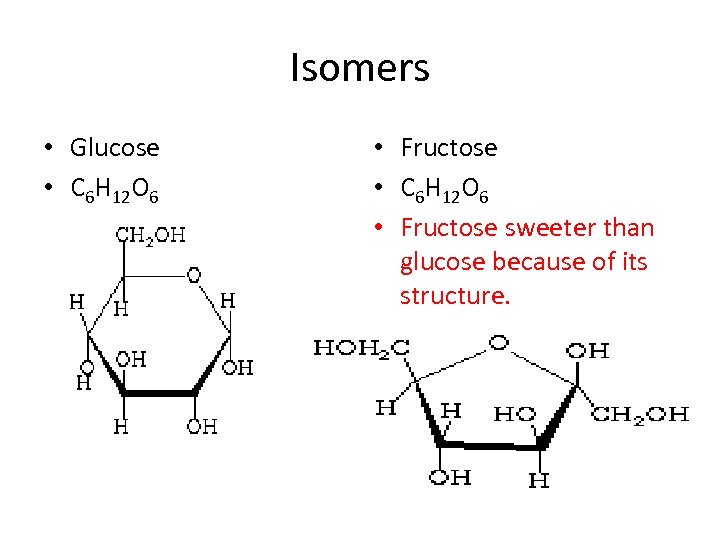
Isomers • Glucose • C 6 H 12 O 6 • Fructose • C 6 H 12 O 6 • Fructose sweeter than glucose because of its structure.
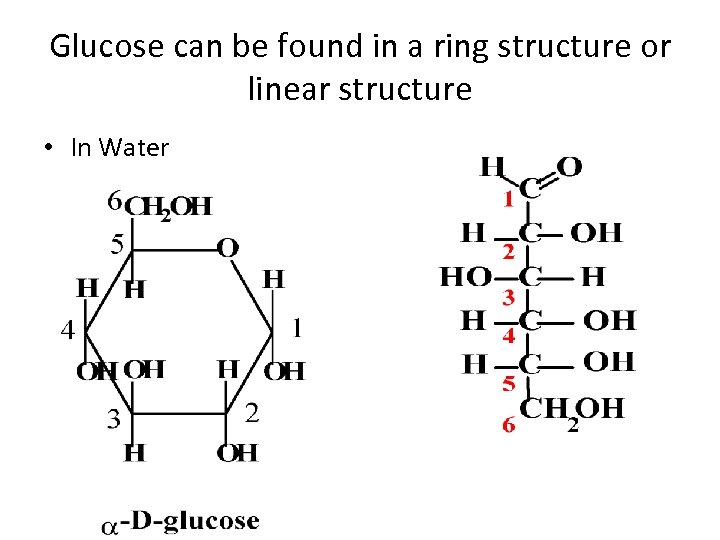
Glucose can be found in a ring structure or linear structure • In Water
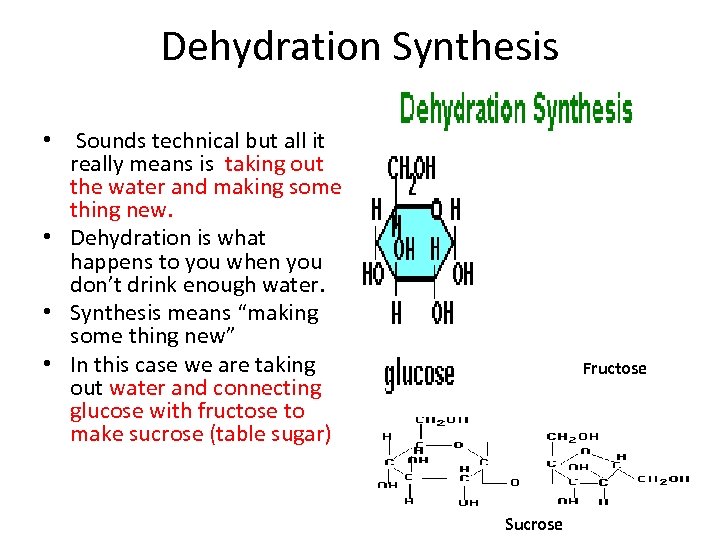
Dehydration Synthesis • Sounds technical but all it really means is taking out the water and making some thing new. • Dehydration is what happens to you when you don’t drink enough water. • Synthesis means “making some thing new” • In this case we are taking out water and connecting glucose with fructose to make sucrose (table sugar) Fructose Sucrose
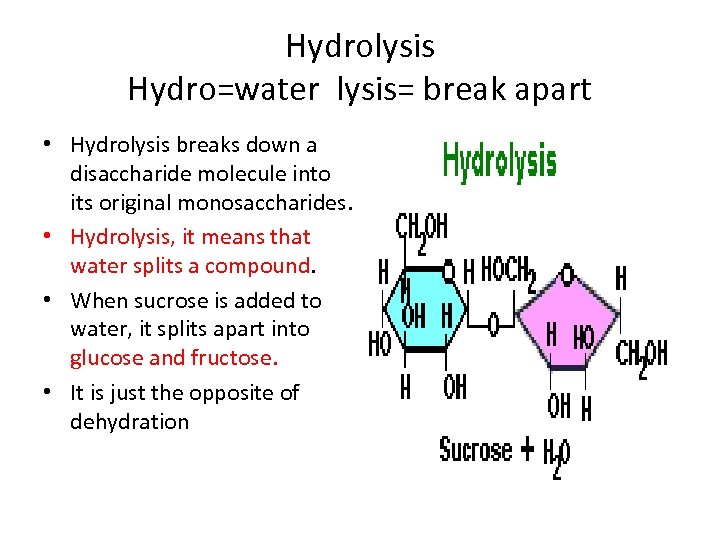
Hydrolysis Hydro=water lysis= break apart • Hydrolysis breaks down a disaccharide molecule into its original monosaccharides. • Hydrolysis, it means that water splits a compound. • When sucrose is added to water, it splits apart into glucose and fructose. • It is just the opposite of dehydration
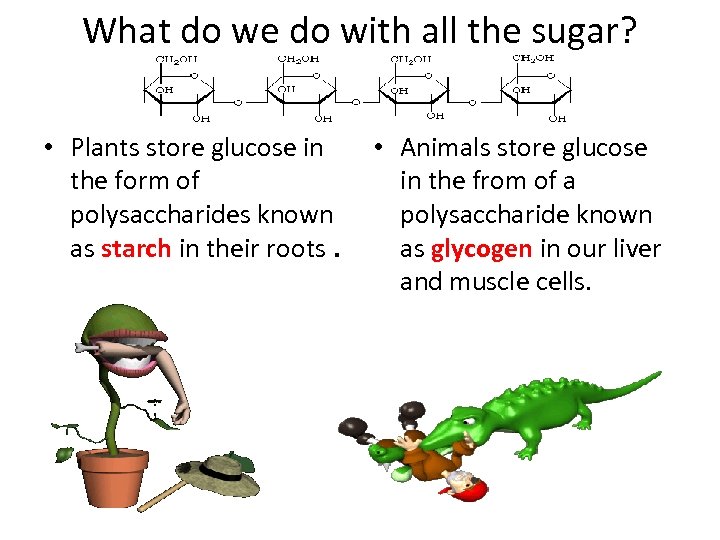
What do we do with all the sugar? • Plants store glucose in the form of polysaccharides known as starch in their roots. • Animals store glucose in the from of a polysaccharide known as glycogen in our liver and muscle cells.
Cellulose • The most abundant organic molecule on earth. • Gives trees and plants structure and strength. • Most animals can not break the glucose linkage by normal means of hydrolysis. Need special enzymes. • We need cellulose (fiber) to keep our digestive tracts clean and healthy.
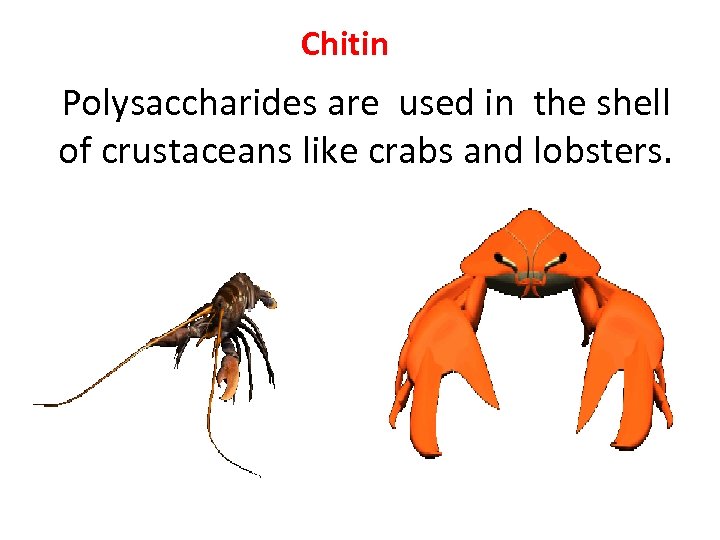
Chitin Polysaccharides are used in the shell of crustaceans like crabs and lobsters.
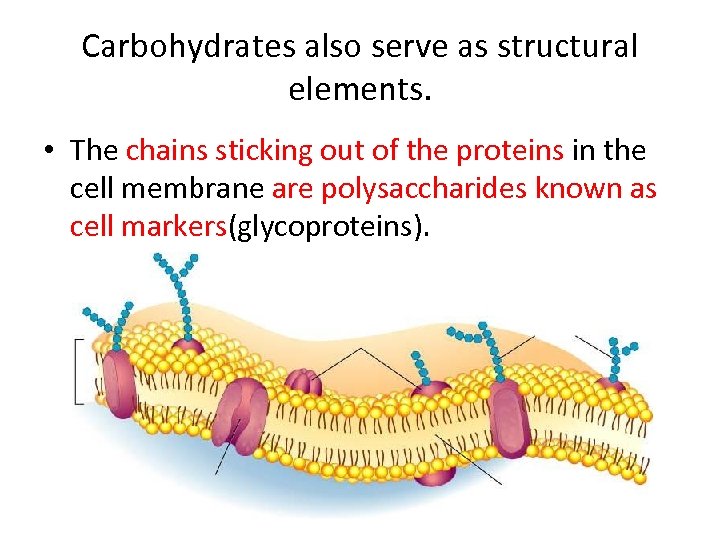
Carbohydrates also serve as structural elements. • The chains sticking out of the proteins in the cell membrane are polysaccharides known as cell markers(glycoproteins).

How Sweet It Is • The human tongue has four basic taste qualities. • Bitter • Salty • Sour • Sweet • We perceive taste qualities when receptors on our tongue send a message to our brain.

Its all about how tightly the molecules fit into the receptors on the tongue. • The chemical structure of a compound determines its shape, which in turn will determine how well it will fit into a receptor. • Compounds that bind more tightly to “sweet” taste receptors send stronger “sweet” messages to the brain.
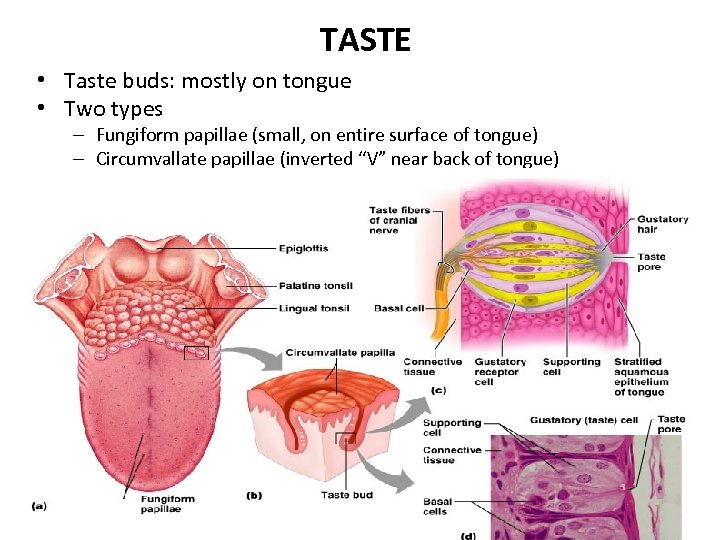
TASTE • Taste buds: mostly on tongue • Two types – Fungiform papillae (small, on entire surface of tongue) – Circumvallate papillae (inverted “V” near back of tongue) 28
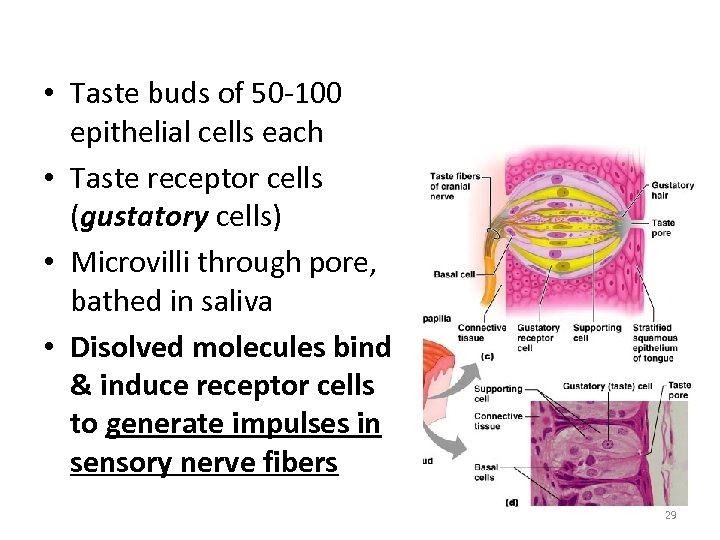
• Taste buds of 50 -100 epithelial cells each • Taste receptor cells (gustatory cells) • Microvilli through pore, bathed in saliva • Disolved molecules bind & induce receptor cells to generate impulses in sensory nerve fibers 29
Carbohydrate Structure
Carbohydrates • • • Cx(H 2 O)y 70 -80% human energy needs >90% dry matter of plants Monomers and polymers Functional properties – Sweetness – Chemical reactivity – Polymer functionality

Simple Sugars • Cannot be broken down by mild acid hydrolysis • C 3 -9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles
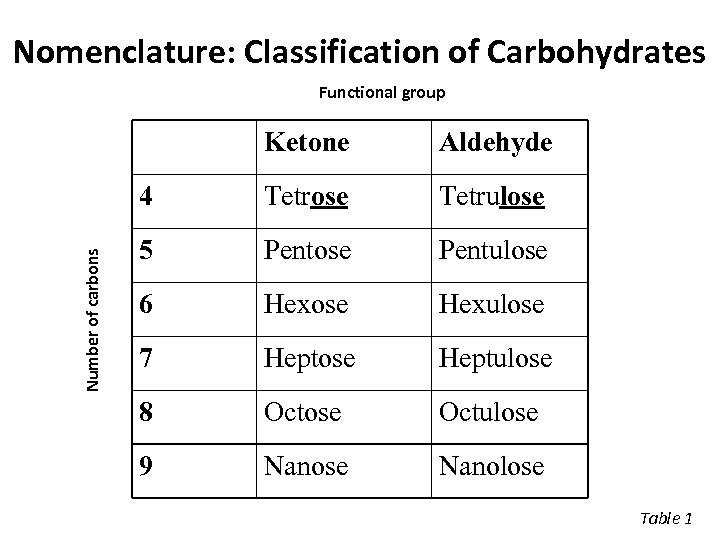
Nomenclature: Classification of Carbohydrates Functional group Aldehyde 4 Number of carbons Ketone Tetrose Tetrulose 5 Pentose Pentulose 6 Hexose Hexulose 7 Heptose Heptulose 8 Octose Octulose 9 Nanose Nanolose Table 1
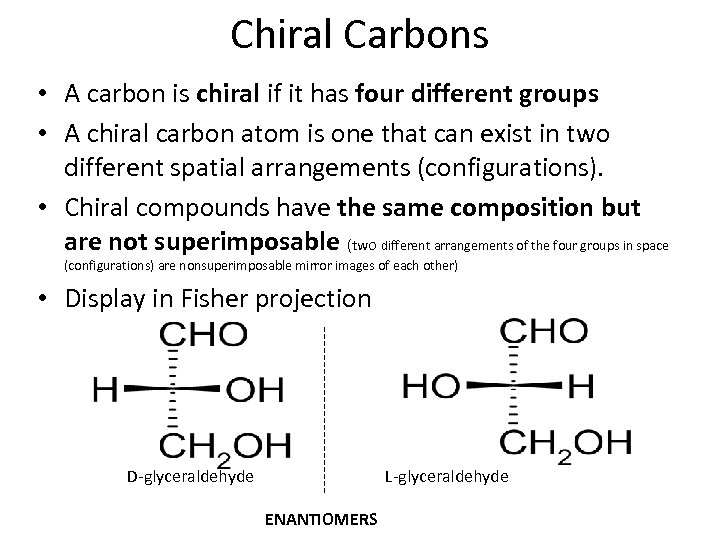
Chiral Carbons • A carbon is chiral if it has four different groups • A chiral carbon atom is one that can exist in two different spatial arrangements (configurations). • Chiral compounds have the same composition but are not superimposable (two different arrangements of the four groups in space (configurations) are nonsuperimposable mirror images of each other) • Display in Fisher projection D-glyceraldehyde L-glyceraldehyde ENANTIOMERS
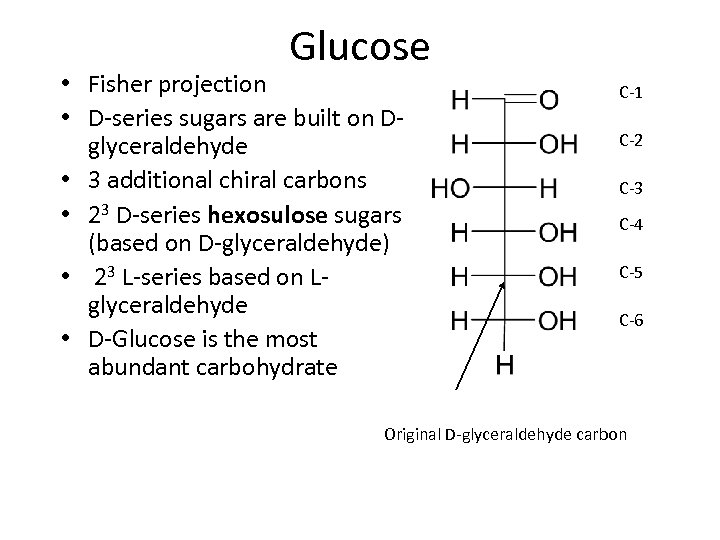
Glucose • Fisher projection • D-series sugars are built on Dglyceraldehyde • 3 additional chiral carbons • 23 D-series hexosulose sugars (based on D-glyceraldehyde) • 23 L-series based on Lglyceraldehyde • D-Glucose is the most abundant carbohydrate C-1 C-2 C-3 C-4 C-5 C-6 Original D-glyceraldehyde carbon
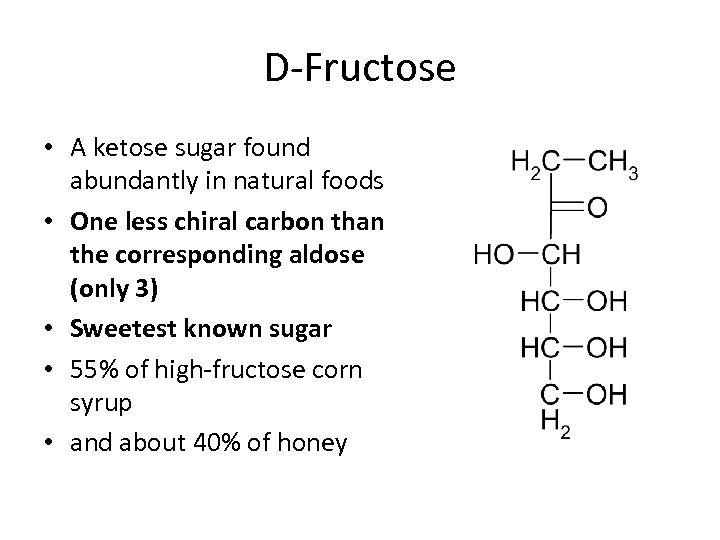
D-Fructose • A ketose sugar found abundantly in natural foods • One less chiral carbon than the corresponding aldose (only 3) • Sweetest known sugar • 55% of high-fructose corn syrup • and about 40% of honey
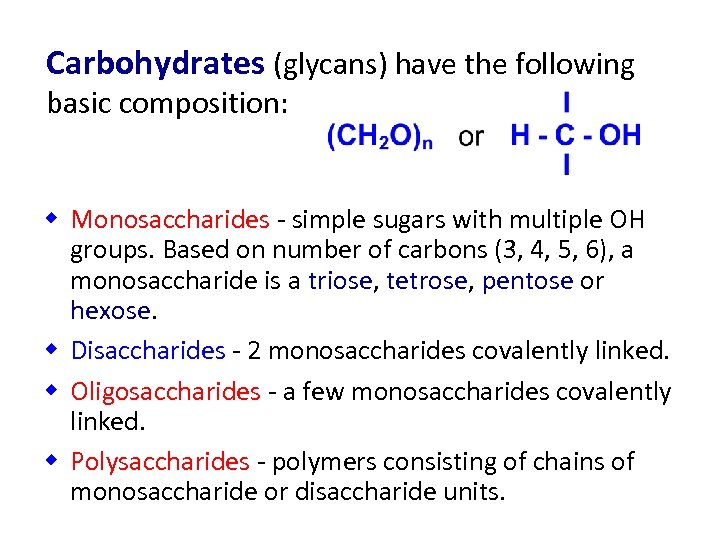
Carbohydrates (glycans) have the following basic composition: w Monosaccharides - simple sugars with multiple OH groups. Based on number of carbons (3, 4, 5, 6), a monosaccharide is a triose, tetrose, pentose or hexose. w Disaccharides - 2 monosaccharides covalently linked. w Oligosaccharides - a few monosaccharides covalently linked. w Polysaccharides - polymers consisting of chains of monosaccharide or disaccharide units.
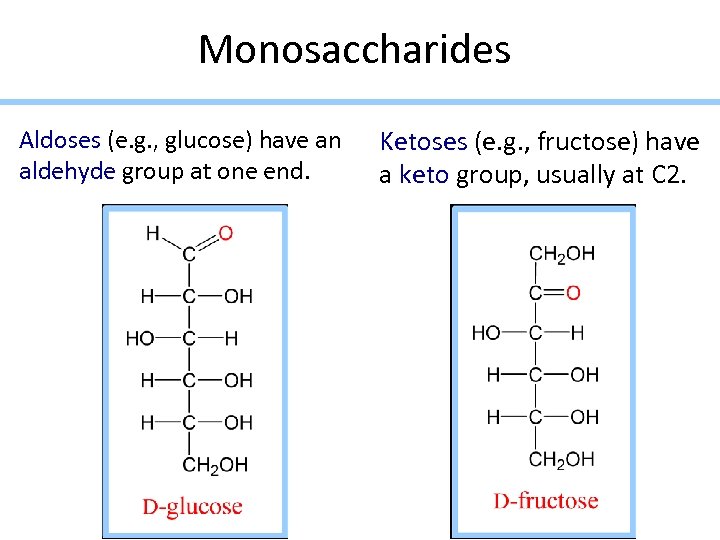
Monosaccharides Aldoses (e. g. , glucose) have an aldehyde group at one end. Ketoses (e. g. , fructose) have a keto group, usually at C 2.
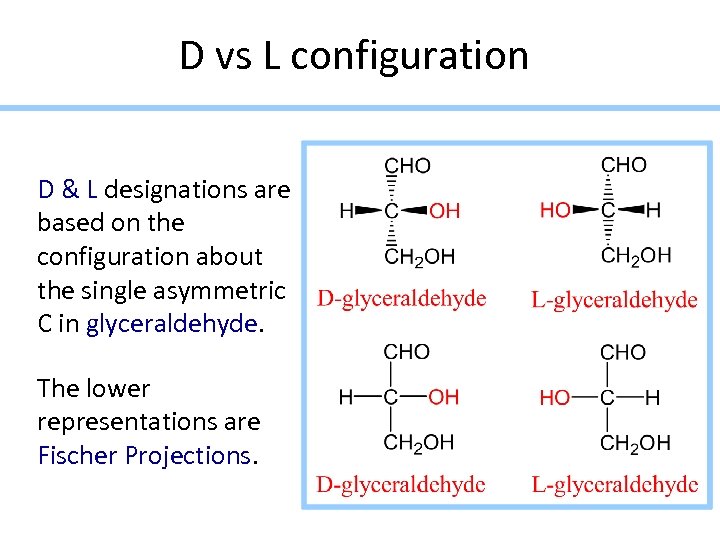
D vs L configuration D & L designations are based on the configuration about the single asymmetric C in glyceraldehyde. The lower representations are Fischer Projections.
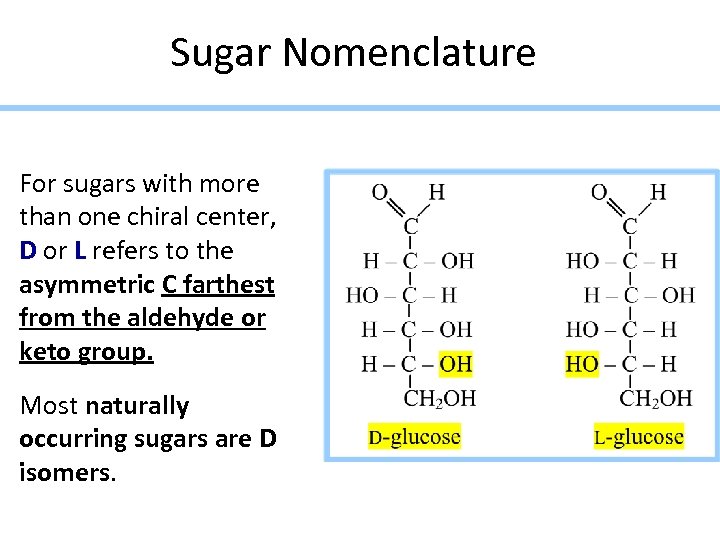
Sugar Nomenclature For sugars with more than one chiral center, D or L refers to the asymmetric C farthest from the aldehyde or keto group. Most naturally occurring sugars are D isomers.
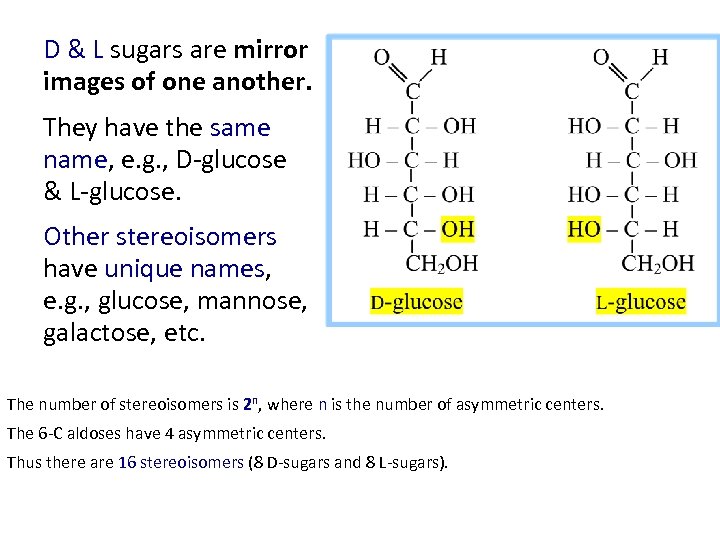
D & L sugars are mirror images of one another. They have the same name, e. g. , D-glucose & L-glucose. Other stereoisomers have unique names, e. g. , glucose, mannose, galactose, etc. The number of stereoisomers is 2 n, where n is the number of asymmetric centers. The 6 -C aldoses have 4 asymmetric centers. Thus there are 16 stereoisomers (8 D-sugars and 8 L-sugars).
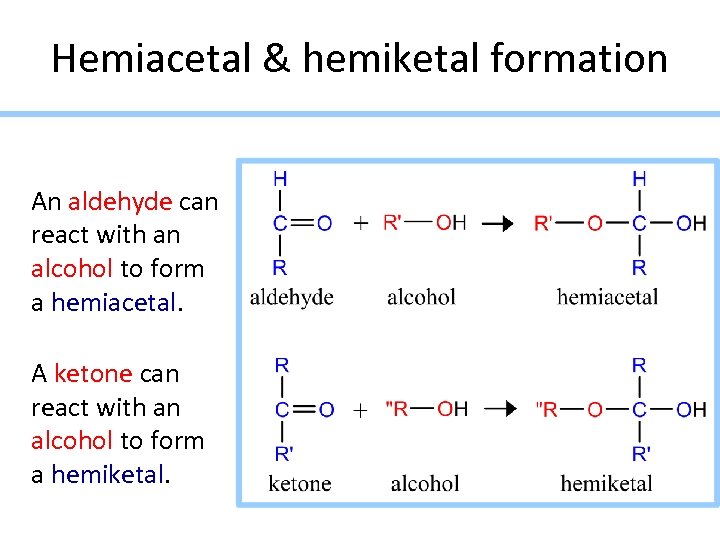
Hemiacetal & hemiketal formation An aldehyde can react with an alcohol to form a hemiacetal. A ketone can react with an alcohol to form a hemiketal.
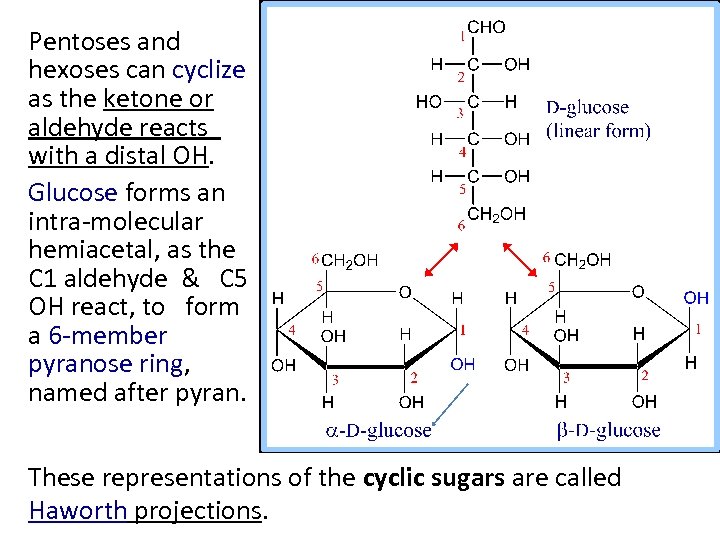
Pentoses and hexoses can cyclize as the ketone or aldehyde reacts with a distal OH. Glucose forms an intra-molecular hemiacetal, as the C 1 aldehyde & C 5 OH react, to form a 6 -member pyranose ring, named after pyran. These representations of the cyclic sugars are called Haworth projections.
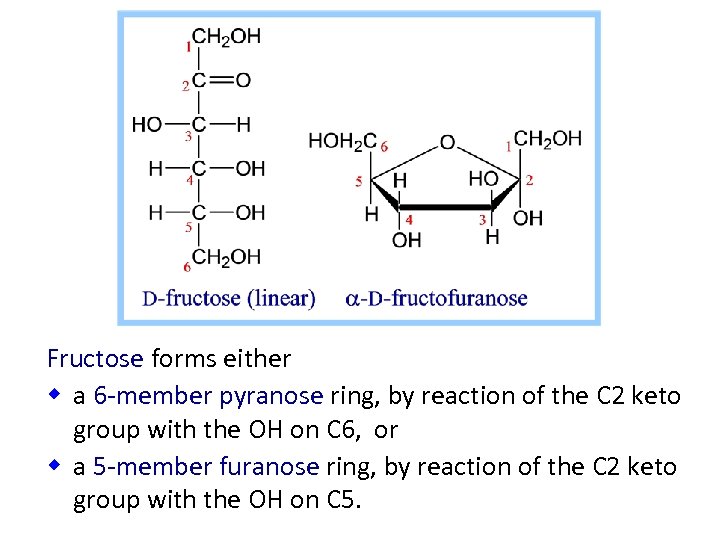
Fructose forms either w a 6 -member pyranose ring, by reaction of the C 2 keto group with the OH on C 6, or w a 5 -member furanose ring, by reaction of the C 2 keto group with the OH on C 5.
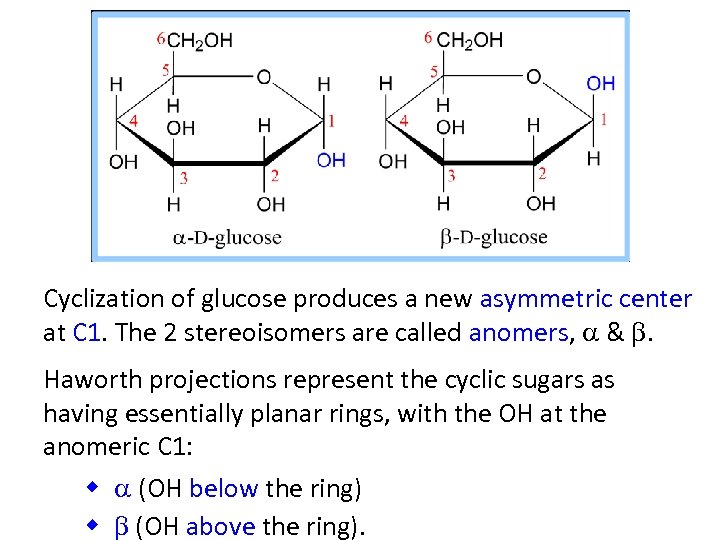
Cyclization of glucose produces a new asymmetric center at C 1. The 2 stereoisomers are called anomers, a & b. Haworth projections represent the cyclic sugars as having essentially planar rings, with the OH at the anomeric C 1: w a (OH below the ring) w b (OH above the ring).
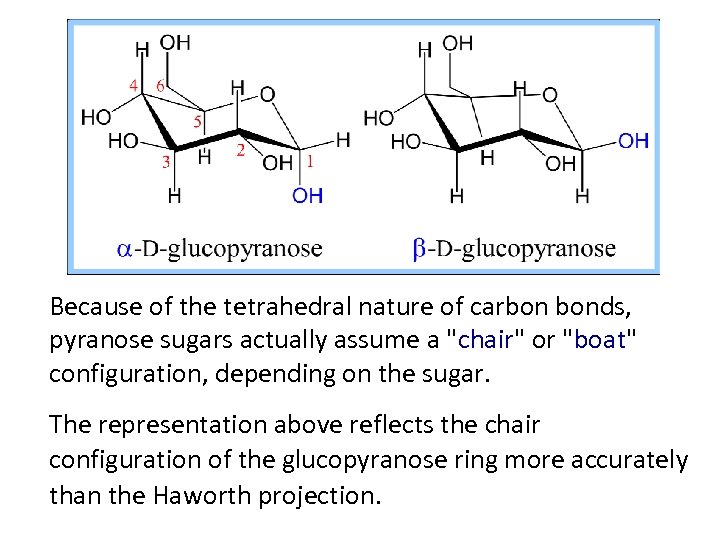
Because of the tetrahedral nature of carbon bonds, pyranose sugars actually assume a "chair" or "boat" configuration, depending on the sugar. The representation above reflects the chair configuration of the glucopyranose ring more accurately than the Haworth projection.
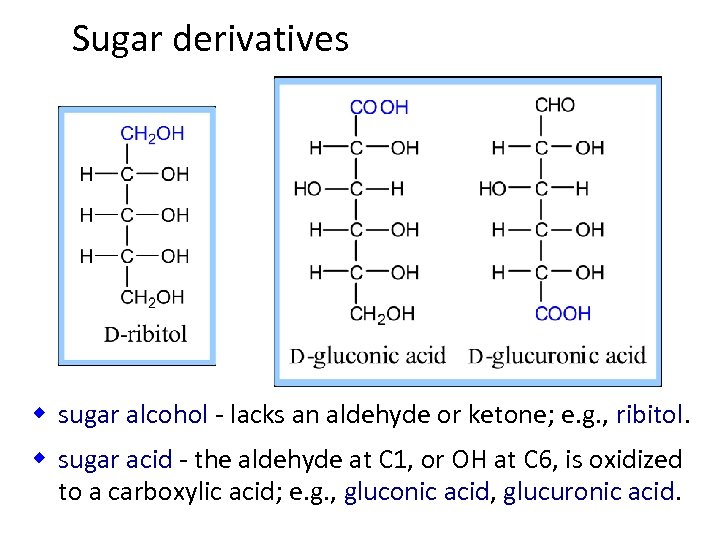
Sugar derivatives w sugar alcohol - lacks an aldehyde or ketone; e. g. , ribitol. w sugar acid - the aldehyde at C 1, or OH at C 6, is oxidized to a carboxylic acid; e. g. , gluconic acid, glucuronic acid.
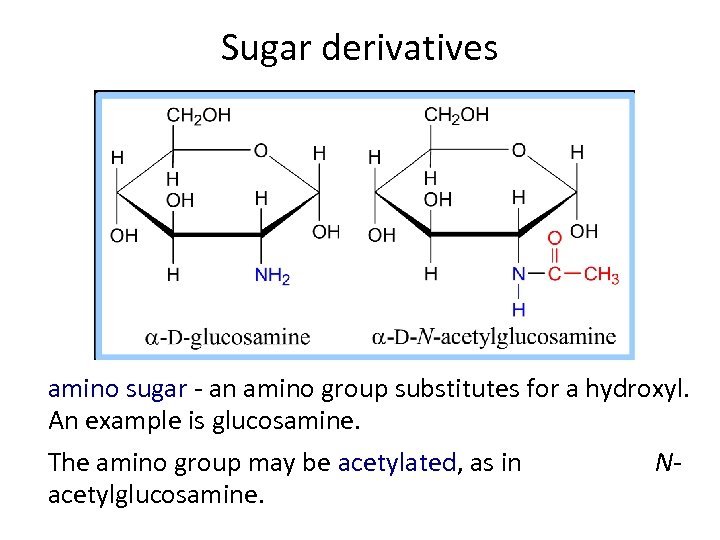
Sugar derivatives amino sugar - an amino group substitutes for a hydroxyl. An example is glucosamine. The amino group may be acetylated, as in acetylglucosamine. N-
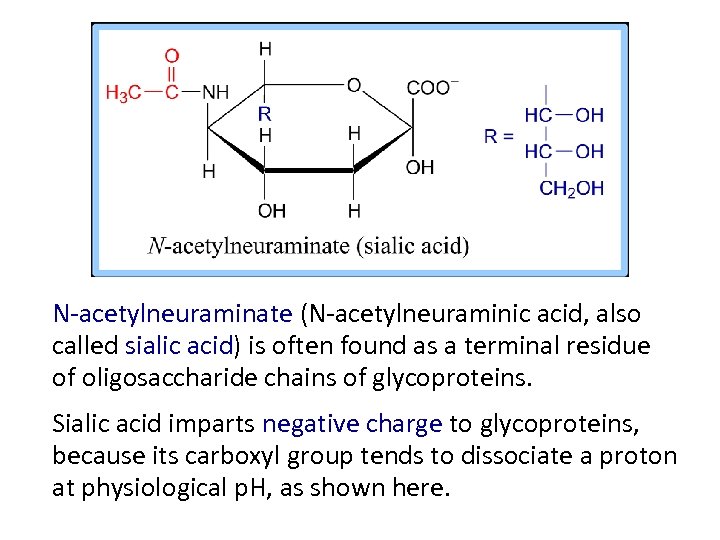
N-acetylneuraminate (N-acetylneuraminic acid, also called sialic acid) is often found as a terminal residue of oligosaccharide chains of glycoproteins. Sialic acid imparts negative charge to glycoproteins, because its carboxyl group tends to dissociate a proton at physiological p. H, as shown here.
ef6f44b27f658c29e330556f2ad9ef86.ppt