e26c7cba757c8782fe97dc24e172a570.ppt
- Количество слайдов: 40

FOOD CHEMISTRY BY DR BOOMINATHAN Ph. D. M. Sc. , (Med. Bio, JIPMER), M. Sc. , (FGSWI, Israel), Ph. D (NUS, SINGAPORE) PONDICHERRY UNIVERSITY Fifth lecture 13/August/2012

Goals • • • Structural arrangements of different Gums: Carrageenans, Guar gum, Carbogum, Xanthan, and Gum arabic Composition Physico-chemical properties of Gums Applications of Gums in food industry

Polysaccharides Carrageenans Ionic Gums March 5, 2004. Southern Bio-products Conference
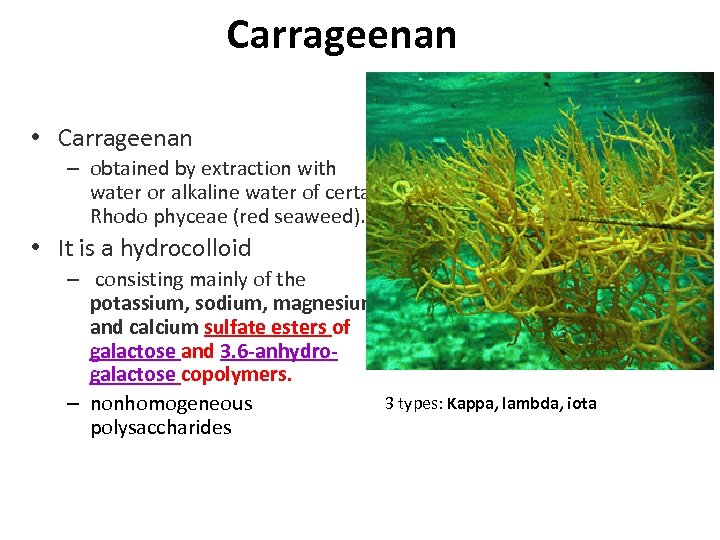
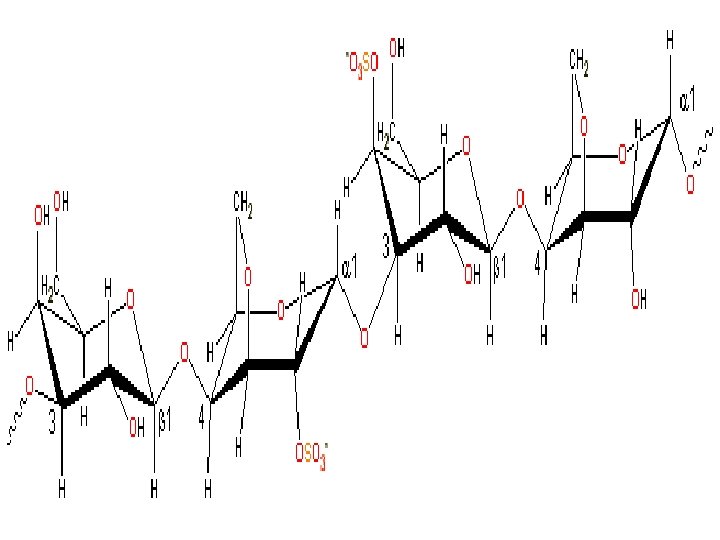
Carrageenan • Carrageenan – obtained by extraction with water or alkaline water of certain Rhodo phyceae (red seaweed). • It is a hydrocolloid – consisting mainly of the potassium, sodium, magnesium, and calcium sulfate esters of galactose and 3. 6 -anhydrogalactose copolymers. 3 types: Kappa, lambda, iota – nonhomogeneous polysaccharides
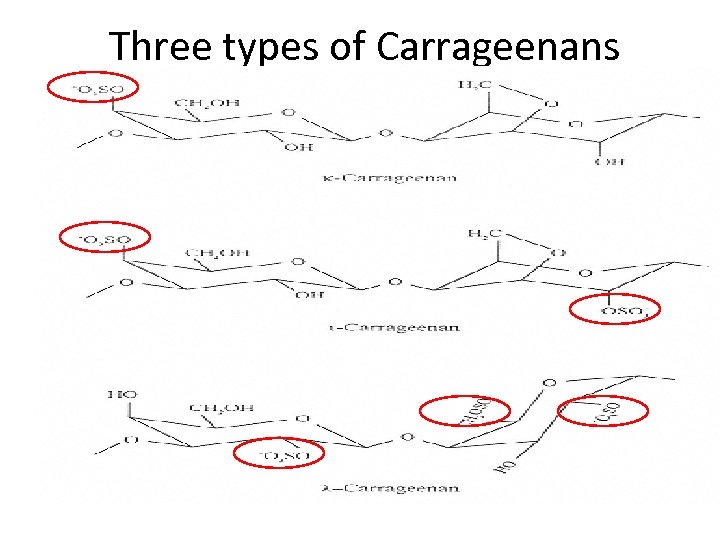
Three types of Carrageenans
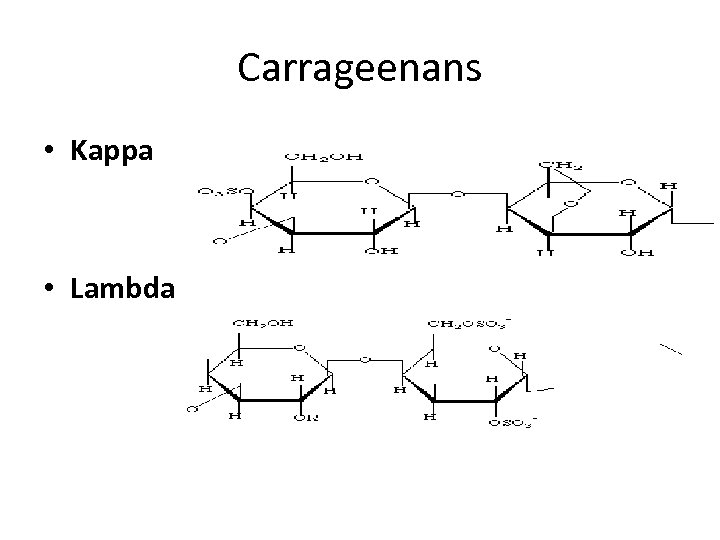
Carrageenans • Kappa • Lambda
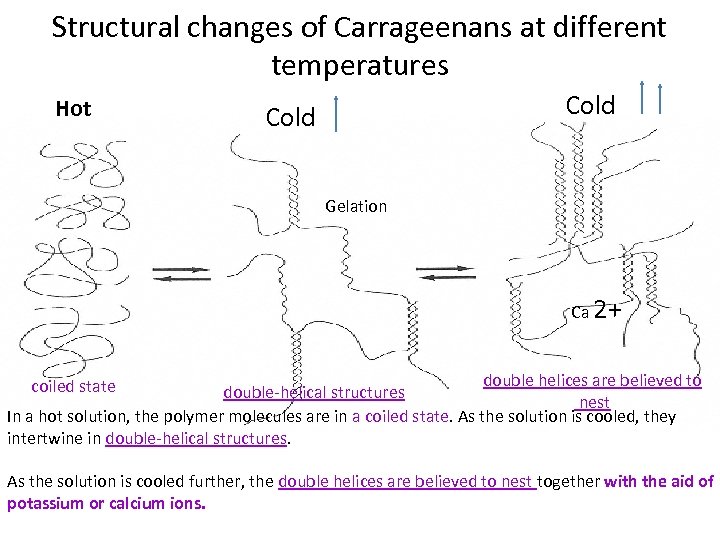
Structural changes of Carrageenans at different temperatures Hot Cold Gelation Ca 2+ double helices are believed to double-helical structures nest In a hot solution, the polymer molecules are in a coiled state. As the solution is cooled, they intertwine in double-helical structures. coiled state As the solution is cooled further, the double helices are believed to nest together with the aid of potassium or calcium ions.
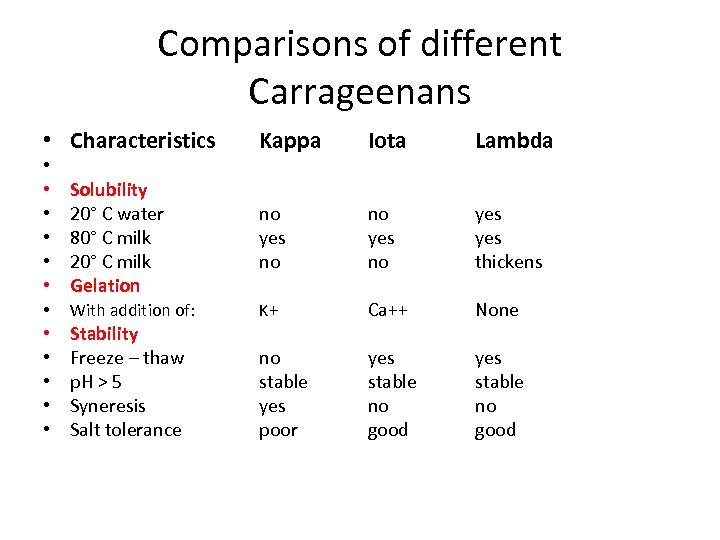
Comparisons of different Carrageenans • Characteristics • • • Solubility 20° C water 80° C milk 20° C milk Gelation • With addition of: • • • Stability Freeze – thaw p. H > 5 Syneresis Salt tolerance Kappa Iota Lambda no yes thickens K+ Ca++ None no stable yes poor yes stable no good
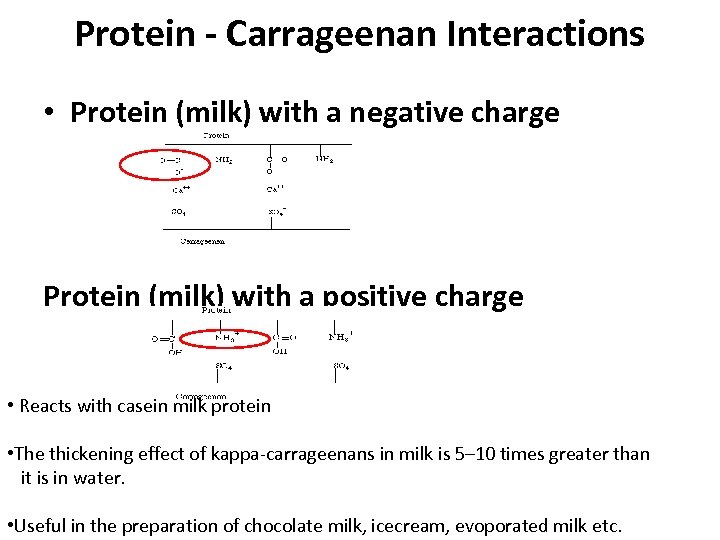
Protein - Carrageenan Interactions • Protein (milk) with a negative charge Protein (milk) with a positive charge • Reacts with casein milk protein • The thickening effect of kappa-carrageenans in milk is 5– 10 times greater than it is in water. • Useful in the preparation of chocolate milk, icecream, evoporated milk etc.
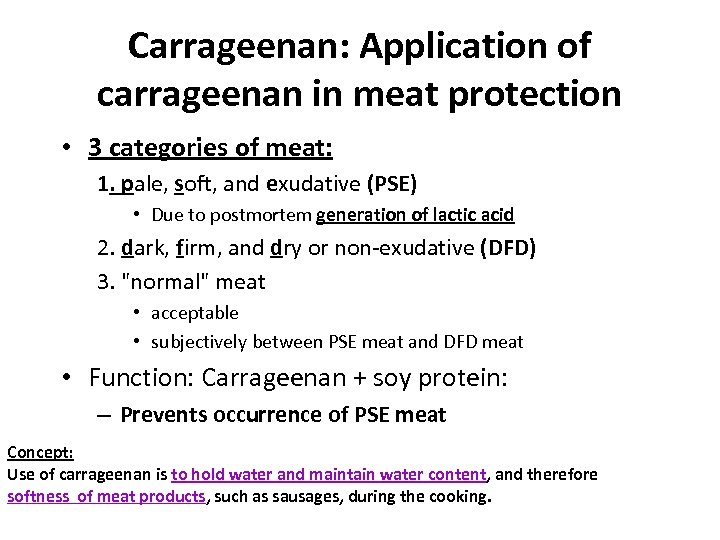
Carrageenan: Application of carrageenan in meat protection • 3 categories of meat: 1. pale, soft, and exudative (PSE) • Due to postmortem generation of lactic acid 2. dark, firm, and dry or non-exudative (DFD) 3. "normal" meat • acceptable • subjectively between PSE meat and DFD meat • Function: Carrageenan + soy protein: – Prevents occurrence of PSE meat Concept: Use of carrageenan is to hold water and maintain water content, and therefore softness of meat products, such as sausages, during the cooking.

Carrageenan softens meats • Carrageenan is a water-soluble polymer, also known as a gum, that is used as a fat substitute in processed meats and can be found in condensed milk and some soy milk products. • Normally, fat serves the purpose of maintaining softness, but because of the binding power of carrageenan for protein and its high affinity for water, • Carrageenans can be used to replace in part this function of natural animal fat in lean products.
… However • Carrageenan may cause stomach lesions, cancer • Strawberry cheesecake like this may be stabilized and thickened with carrageenan. • Containers of pudding, ice cream, yogurt, or cottage cheese may include the ingredient carrageenan, a thickener derived from red seaweed. • For decades, it has been presumed to be safe to eat, but new research from a medical doctor on the faculty of the University of Iowa shows that presumption may be wrong. March 5, 2004.
Typical Dairy Applications of Carrageenan • Milk Gels • Cooked flans (open pastry filled with fruit or custard) or custards (Sweetened mixture of milk and eggs baked, boiled or frozen) – Gelation K, K + I • Cooked prepared custards – Thickening – Gelation K, I, L • Pudding & Pie Fillings • Dry mix cooked with milk – Level starch gelatinization • Ready-to-eat – Syneresis control, bodying

Typical Dairy Applications of Carrageenan • Whipped products – Whipped cream Stabilize overrun – Aerosol whipped cream • Stabilize overrun & emulsion • Cold prepared Milks – Instant Breakfast • Suspension, bodying agent – Shakes • Suspension, bodying
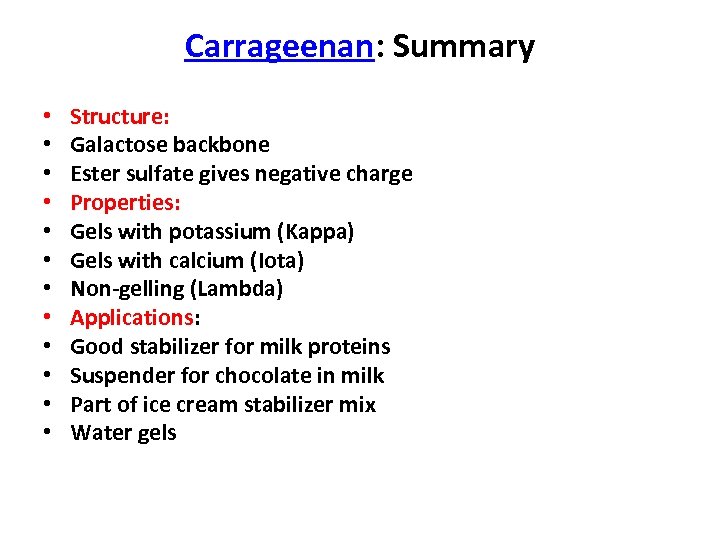
Carrageenan: Summary • • • Structure: Galactose backbone Ester sulfate gives negative charge Properties: Gels with potassium (Kappa) Gels with calcium (Iota) Non-gelling (Lambda) Applications: Good stabilizer for milk proteins Suspender for chocolate in milk Part of ice cream stabilizer mix Water gels

Questions • Commercial carragennan contains: 1. Kappa 2. Iota 3. Lamda 4. a mixture of above • The viscosity of carrageen is quite stable over a wide range of p. H values because • 1. sulfate half-ester groups are always ionized • 2. Phosphate groups are never ionized • 3. Phosphate groups are always ionized • 4. Nitro groups are always ionized

Questions • Lambda carragennan contains: 1. the maximum no. of sulfates 2. the lowest no. of sulfates 3. only one sulfate 4. none • Carrageen softens meat due to its • 1. binding power for protein • 2. high affinity for water • 3. both

Non-ionic Gums • Guar gum & Carobgum

• Guar gum
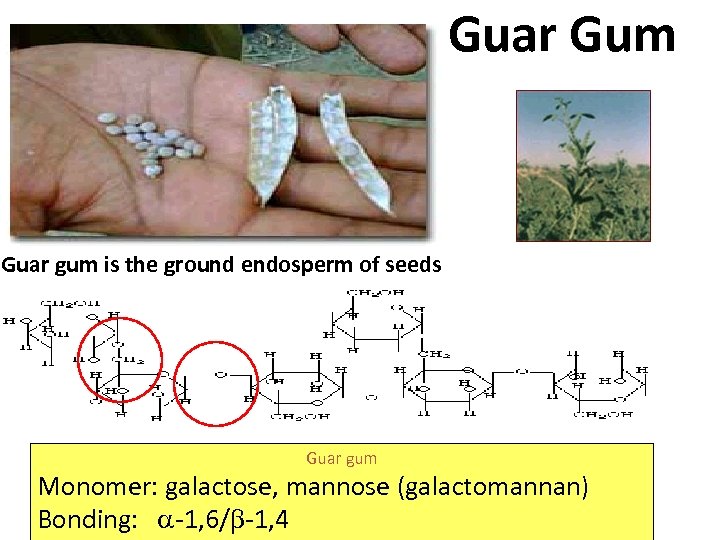
Guar Gum Guar gum is the ground endosperm of seeds Guar gum Monomer: galactose, mannose (galactomannan) Bonding: -1, 6/ -1, 4
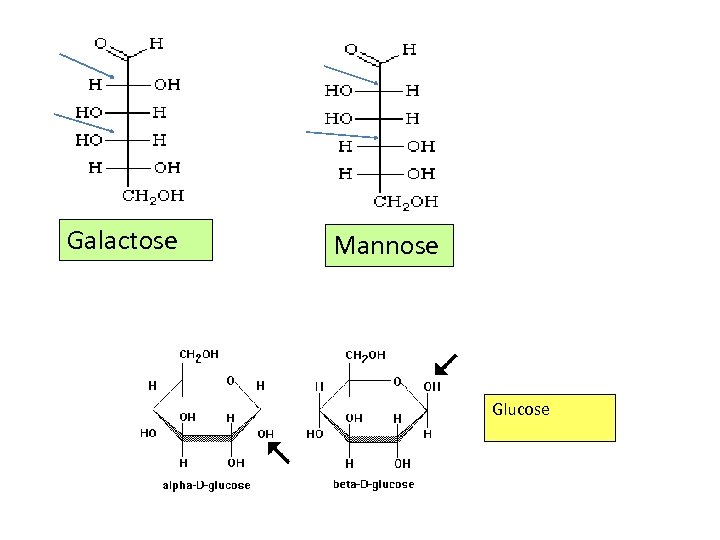
Galactose Mannose Glucose
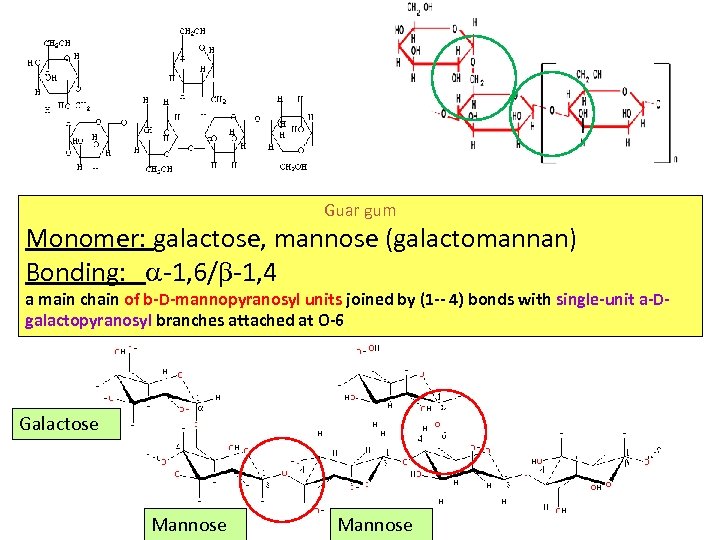
Guar gum Monomer: galactose, mannose (galactomannan) Bonding: -1, 6/ -1, 4 a main chain of b-D-mannopyranosyl units joined by (1 -- 4) bonds with single-unit a-Dgalactopyranosyl branches attached at O-6 Galactose Mannose

Guar Gum • It produces the highest viscosity of any natural, commercial gum • Galactomannan (Mannose (1 -4) + Galactose (1 -6) every other Mannose • Galactomannans consist of MW 220, 000 ± 20, 000 • Particle size affects viscosity and hydration • Cold water swelling - Turbid solutions

Guar Gum • Hydration increased by heating • High water binding • High viscosity form - up to 100, 000 CP • Low viscosity from - up to 10, 000 CP • Modifies properties when used with: – Carrageenan – Xanthan
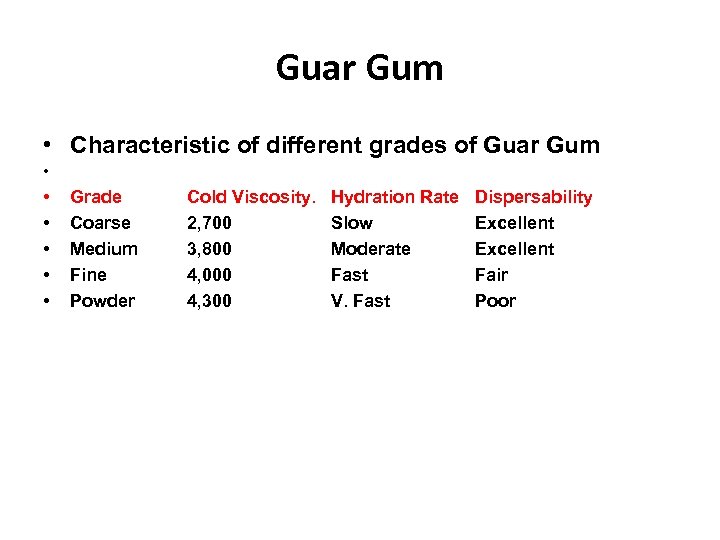
Guar Gum • Characteristic of different grades of Guar Gum • • • Grade Coarse Medium Fine Powder Cold Viscosity. 2, 700 3, 800 4, 000 4, 300 Hydration Rate Slow Moderate Fast V. Fast Dispersability Excellent Fair Poor

Food Uses Ice cream prevents ice crystal formation, slows meltdown, improves heat shock resistance Salad dressing viscosity Cheese improves spreading

Guar gum uses in food industry • Ice creams: Smooth creamy texture • Bakery products: Texture, moisture retention • Noodles: Moisture retention, machine runnability • Beverages: mouth feel • Meat: Binder, absorb water • Dressings: Thickener, emulsion stabilizer

Carobgum
Carobgum is the ground endosperm of seeds 30

Carobgum • Carobgum – obtained from the endosperm of the Carob or locust seed (Ceratonia siliqua). March 5, 2004. Southern Bio-products Conference
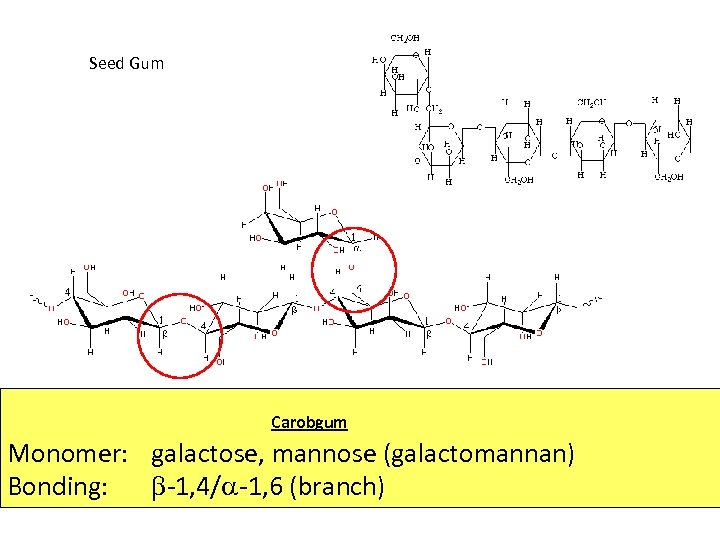
Seed Gum Carobgum Monomer: galactose, mannose (galactomannan) Bonding: -1, 4/ -1, 6 (branch)
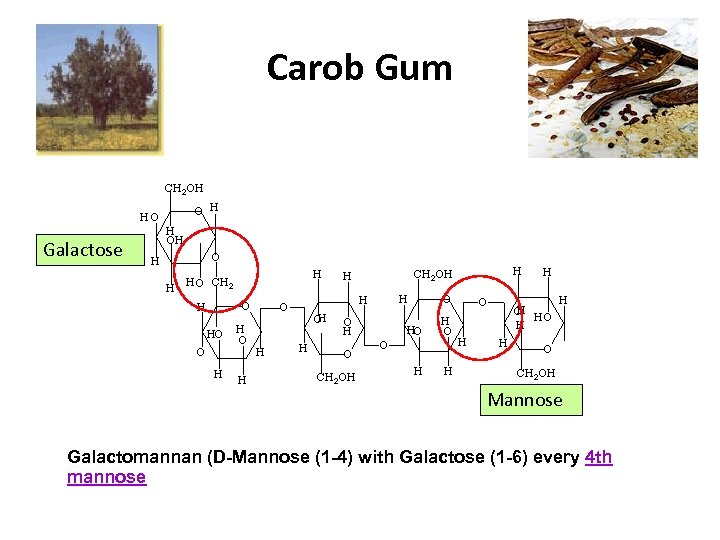
Carob Gum Galactose Mannose Galactomannan (D-Mannose (1 -4) with Galactose (1 -6) every 4 th mannose

Carobgum • Galactomannan (D-Mannose (1 -4) with Galactose (1 -6) every 4 th mannose • Molecular weight 330, 000 ± 30, 000 • Neutral - relatively unaffected by ions, p. H. • Not soluble in cold water • Fully hydrated if heated 10 minutes at 80° C • Solutions are turbid, off-white • Modify properties of – Carrageenan – Xanthan Gum
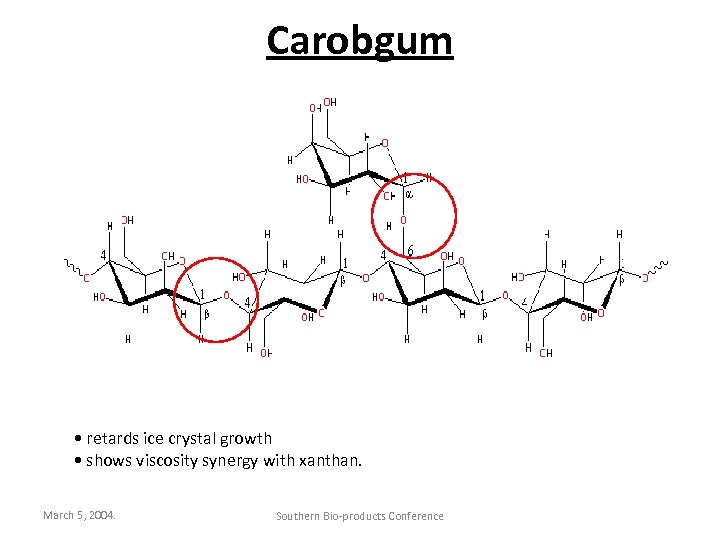
Carobgum • retards ice crystal growth • shows viscosity synergy with xanthan. March 5, 2004. Southern Bio-products Conference

Guar gum and Carobgum
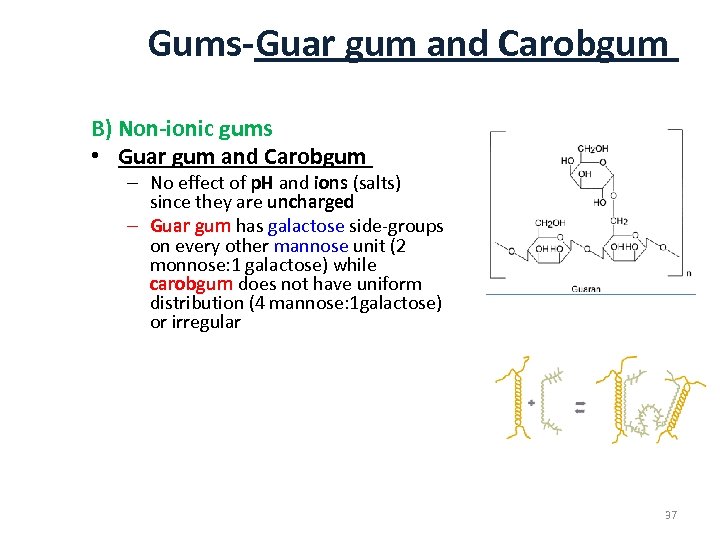
Gums-Guar gum and Carobgum B) Non-ionic gums • Guar gum and Carobgum – No effect of p. H and ions (salts) since they are uncharged – Guar gum has galactose side-groups on every other mannose unit (2 monnose: 1 galactose) while carobgum does not have uniform distribution (4 mannose: 1 galactose) or irregular 37
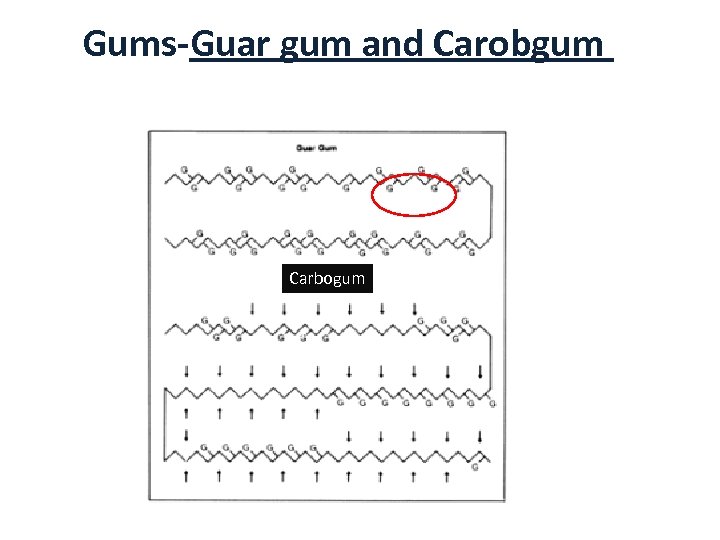
Gums-Guar gum and Carobgum Carbogum
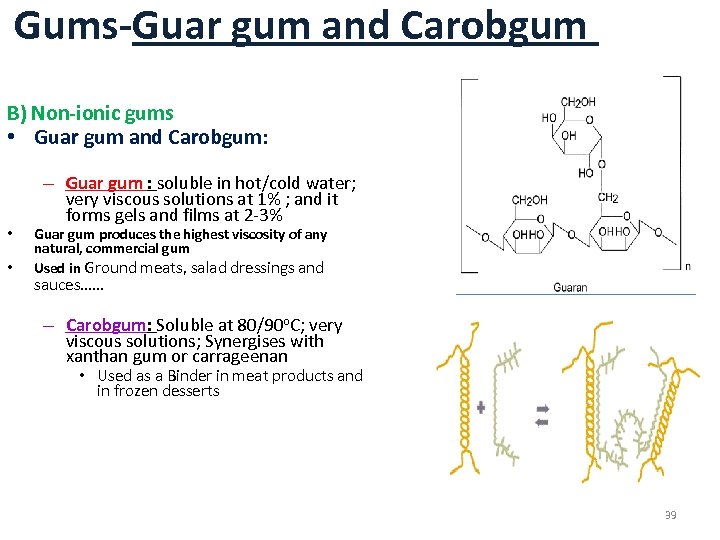
Gums-Guar gum and Carobgum B) Non-ionic gums • Guar gum and Carobgum: • • – Guar gum : soluble in hot/cold water; very viscous solutions at 1% ; and it forms gels and films at 2 -3% Guar gum produces the highest viscosity of any natural, commercial gum Used in Ground meats, salad dressings and sauces…… – Carobgum: Soluble at 80/90 o. C; very viscous solutions; Synergises with xanthan gum or carrageenan • Used as a Binder in meat products and in frozen desserts 39

Questions Guar gum and carbogum are examples for: 1. Ionic gums 2. Non-ionic gums 3. Both 4. None p. H and ions can change the property of Guar gum and carbogum 1. 2. 3. 4. True False Only p. H Only ions
e26c7cba757c8782fe97dc24e172a570.ppt