
Fluorine made by : Zinaidova D. , Kuznetsova D. , Shamina O.

Fluorine SYMBOL F CHEMICAL FORMULA F 2 ATOMIC NUMBER 9 ATOMIC MASS 18. 998404 FAMILY Group 17 (VIIA) Halogen

Discovery and naming In the early 1500 s, German scholar Georgius Agricola (1494 - 1555) described a mineral he called fluorspar(calcium fluoride - Ca. F 2 ). The name fluorspar comes from the Latin word fluere, meaning "to flow. " In 1670, German glass cutter Heinrich Schwanhard discovered that a mixture of fluorspar and acid formed a substance that could be used to etch glass. Etching is a process by which a pattern is drawn into glass. Etching is used to produce artistic shapes on glass as well as precise scientific measuring instruments. The new etching material was identified in 1771 by Swedish chemist Carl Wilhelm Scheele (1742 -86). Scheele described, in detail, the properties of this material, hydrofluoric acid (HF). His work set off an intense study of the acid and its composition. Finally, in 1888, the problem was solved. Moissan made a solution of hydrofluoric (HF) acid in potassium hydrogen fluoride (KHF 2 ). He then cooled the solution to -23°C and passed an electric current through it. A gas appeared at one end of the apparatus. He gave the name fluorine to the new element. The name comes from the mineral fluorspar.

Physical properties Fluorine is a pale yellow gas with a density of 2 grams per liter. That makes fluorine about 1. 3 times as dense as air. Fluorine changes from a gas to a liquid at a temperature of -188°C and from a liquid to a solid at -219°C. Fluorine has a strong and characteristic odor that can be detected in very small amounts, as low as 20 parts per billion. This property is very helpful to those who work with fluorine. It means that the gas can be detected and avoided in case it leaks into a room.
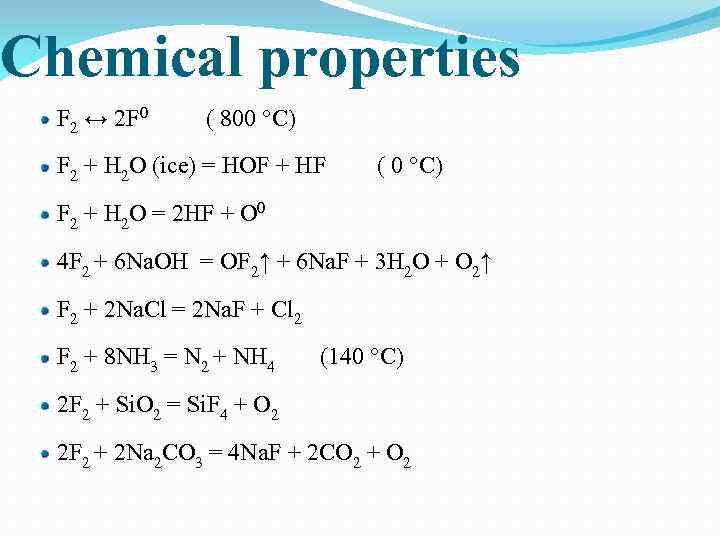
Chemical properties F 2 ↔ 2 F 0 ( 800 °С) F 2 + H 2 O (ice) = HOF + HF ( 0 °С) F 2 + H 2 O = 2 HF + O 0 4 F 2 + 6 Na. OH = OF 2↑ + 6 Na. F + 3 H 2 О + O 2↑ F 2 + 2 Na. Cl = 2 Na. F + Cl 2 F 2 + 8 NH 3 = N 2 + NH 4 (140 °С) 2 F 2 + Si. O 2 = Si. F 4 + O 2 2 F 2 + 2 Na 2 CO 3 = 4 Na. F + 2 CO 2 + O 2
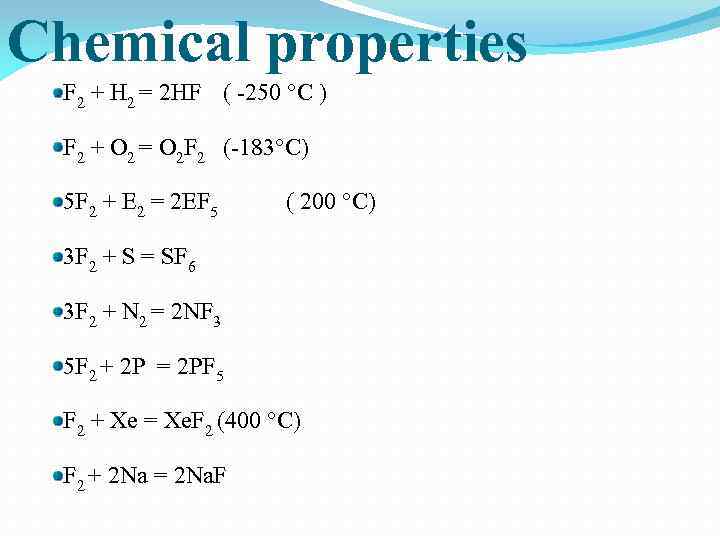
Chemical properties F 2 + H 2 = 2 HF ( -250 °С ) F 2 + O 2 = O 2 F 2 (-183°С) 5 F 2 + E 2 = 2 EF 5 ( 200 °С) 3 F 2 + S = SF 6 3 F 2 + N 2 = 2 NF 3 5 F 2 + 2 P = 2 PF 5 F 2 + Xe = Xe. F 2 (400 °С) F 2 + 2 Na = 2 Na. F

Occurrence in nature Fluorine never occurs as a free element in nature. The most common fluorine minerals are fluorspar, fluorapatite, and cryolite. Apatite is a complex mineral containing primarily calcium, phosphorus, and oxygen, usually with fluorine. Cryolite is also known as Greenland spar. (The island of Greenland is the only commercial source of this mineral. ) It consists primarily of sodium aluminum fluoride (Na 3 Al. F 6 ). The major sources of these minerals are China, Mexico, Mongolia, and South Africa. The United States once produced small amounts of fluorspar, but its last remaining mine closed in 1995. The United States now imports the fluorine minerals it needs. Fluorine is an abundant element in the Earth's crust, estimated at about 1 percent in the earth. That makes it about the 13 th most common element in the crust.
Isotopes There is only one naturally occurring isotope of fluorine, fluroine-19. Only one radioactive isotopes of fluorine, fluorine-18, has been prepared. A radioactive isotope is one that breaks apart and gives off some form of radiation. Fluroine-18 is sometimes used for medical studies. It is injected into the body where it travels primarily to bones. Its presence in bones can be detected by the radiation it gives off. The radiation pattern discloses how normal bones are. Fluorine-18 is sometimes used in a similar way to study brain function.

Extraction Fluorine is commercially made by Moissan's method. An electric current is passed through a mixture of hydrogen fluoride and potassium hydrogen fluoride.
Installing of Moissan. The London Science Museum Installing Moissan by electrolysis to produce fluorine HF

Uses and compounds A familiar use of some fluoride compounds is in toothpastes. Studies show that small amounts of fluorides can help reduce tooth decay. Fluorides are deposited as new tooth material is formed, making it strong and resistant to decay. One use of elemental fluorine is in rocket fuels. It helps other materials burn, like oxygen does. The greatest majority of fluorine is used to make compounds of fluorine. Some cities add fluorides to their water supply. By doing so, they hope to improve the dental health of everyone living in the city. The process of adding fluorides to public water supplies is called fluoridation.

Teflon One of the most profitable discoveries made this way is of the material known as Teflon is the trade name of a kind of plastic made by the Du. Pont Chemical Company. Teflon pans need no oil or butter. Teflon was discovered by accident in 1938 by a Du. Pont chemist named Roy Plunkett (1911 -94). Plunkett was working on the development of chlorofluorocarbons (CFCs) for Du. Pont.

Health effects Fluorine can be quite dangerous. If inhaled in small amounts, it causes severe irritation to the respiratory system (nose, throat, and lungs). In larger amounts, it can cause death. The highest recommended exposure to fluorine is one part per million of air over an eighthour period.
Thank you for your attention!