4be640a0dc6f77a5b1aa128a1f72c848.ppt
- Количество слайдов: 124
 FLU/WORKSITE WELLNESS PROGRAM 2016 OVERVIEW compassion | ingenuity | optimism
FLU/WORKSITE WELLNESS PROGRAM 2016 OVERVIEW compassion | ingenuity | optimism
 • s/dfa; sdklfja; sldfkj; asldfjasdf https: //youtu. be/OOif_HTj. Dh. Q
• s/dfa; sdklfja; sldfkj; asldfjasdf https: //youtu. be/OOif_HTj. Dh. Q
 Flu/Worksite Wellness Program Overview MVNA 1. 2. 3. 4. 5. Introductions/Contact Info MVNA Mission and Wellness Program goals Dress Code Roles & Responsibilities for Clinic Lead, Non-Lead and CSR Wellness Staff Website 1. 2. 6. 7. 8. 9. 10. 11. 12. 13. Scheduling and Reconciling Clinics Training Materials/Forms Payroll/Timesheets/Expense Forms Consent forms Other Forms Introduction to 2016 Flu Vaccines Mock Clinic Video Supplies and Pick-up (RN ONLY) Vaccine Storage and Transportation (RN ONLY) Vaccine Administration & Documentation (RN ONLY)
Flu/Worksite Wellness Program Overview MVNA 1. 2. 3. 4. 5. Introductions/Contact Info MVNA Mission and Wellness Program goals Dress Code Roles & Responsibilities for Clinic Lead, Non-Lead and CSR Wellness Staff Website 1. 2. 6. 7. 8. 9. 10. 11. 12. 13. Scheduling and Reconciling Clinics Training Materials/Forms Payroll/Timesheets/Expense Forms Consent forms Other Forms Introduction to 2016 Flu Vaccines Mock Clinic Video Supplies and Pick-up (RN ONLY) Vaccine Storage and Transportation (RN ONLY) Vaccine Administration & Documentation (RN ONLY)
 Office Team: Who Do you contact? • Melissa Beebe, Program Manager – Any questions • Melissa. Beebe@hcmed. org or 612 -916 -2661 • Danielle Rice, Program Assistant – Questions about scheduling • Danielle. Rice@hcmed. org or 612 -617 -4624 • Angela Thao, Principle Office Specialist – Questions about payroll • Angela. Thao@hcmed. org or 612 -617 -4706 Other Team Members: • Paula Abramson, Sales and Account Manager • Glory Barduson, Clinic Coordinator • Marni Radcliffe, Clinic Coordinator • Jeanne Portoghese, Clinic Coordinator
Office Team: Who Do you contact? • Melissa Beebe, Program Manager – Any questions • Melissa. Beebe@hcmed. org or 612 -916 -2661 • Danielle Rice, Program Assistant – Questions about scheduling • Danielle. Rice@hcmed. org or 612 -617 -4624 • Angela Thao, Principle Office Specialist – Questions about payroll • Angela. Thao@hcmed. org or 612 -617 -4706 Other Team Members: • Paula Abramson, Sales and Account Manager • Glory Barduson, Clinic Coordinator • Marni Radcliffe, Clinic Coordinator • Jeanne Portoghese, Clinic Coordinator
 Save this number! 612 -617 -4620 • Flu Room Phone • After Hours: • If you do call this number after hours, leave a message for the next business day. • “What if I need to talk to someone BEFORE the next business day? ” • When you call the flu room number after hours, the voicemail message will indicate who is “on-call” and provide their cell number. Please only use this for an issue that needs attention before the start of the next business day.
Save this number! 612 -617 -4620 • Flu Room Phone • After Hours: • If you do call this number after hours, leave a message for the next business day. • “What if I need to talk to someone BEFORE the next business day? ” • When you call the flu room number after hours, the voicemail message will indicate who is “on-call” and provide their cell number. Please only use this for an issue that needs attention before the start of the next business day.
 Key Chain
Key Chain
 MVNA Integrated with HCMC January 1 st, 2016: Our Mission We partner with our community, our patients and their families to ensure access to outstanding care for everyone, while improving health and wellness through teaching, patient and community education, and research. Our Values • Patient & Family Centered • Excellence • Teamwork • Respect • Integrity • Compassion
MVNA Integrated with HCMC January 1 st, 2016: Our Mission We partner with our community, our patients and their families to ensure access to outstanding care for everyone, while improving health and wellness through teaching, patient and community education, and research. Our Values • Patient & Family Centered • Excellence • Teamwork • Respect • Integrity • Compassion
 2016 Dress Code v. RNs: Lab coats provided v. CSRs: MVNA logo shirts provided v. Business casual attire at all clinics: -Black or Khaki pants (may wear shorts or capris at State Fair) -Closed toe shoes -Optional: may add plain white long sleeve shirt under polo or plain black cardigan over polo for added warmth
2016 Dress Code v. RNs: Lab coats provided v. CSRs: MVNA logo shirts provided v. Business casual attire at all clinics: -Black or Khaki pants (may wear shorts or capris at State Fair) -Closed toe shoes -Optional: may add plain white long sleeve shirt under polo or plain black cardigan over polo for added warmth
 ROLES & RESPONSBILITIES
ROLES & RESPONSBILITIES
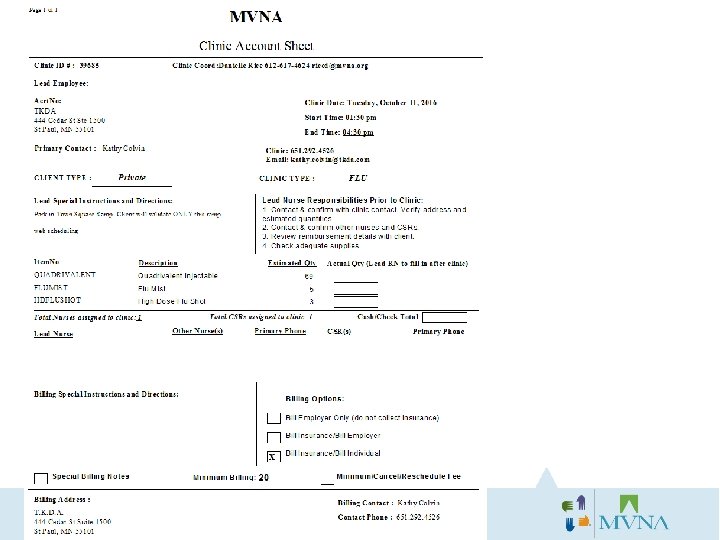
 Clinic Lead RN responsibilities prior to clinic: üThe Account Sheet will be emailed to you 1 week before clinic. Read over the account sheet for any special instructions and clinic schedule to review number of vaccines anticipated. üCalls the client to confirm clinic at least 4 -5 days beforehand. üSchedule supply pick up at MVNA at least 2 full days in advance of pick-up date. üAll supplies should be picked up 1 -7 days before clinic. üReviews the following with the client: • Date and time of event. • Clinic address/location within the building. • Where to park (when applicable). • Billing Option. Remind client that participants should bring their insurance card (if not billing to employer). • Confirm consent forms and VIS documents were received and printed. • If senior residence, asks client if they have any Power of Attorneys (POAs) ready that they need. • Have client remind participants to wear casual/short sleeved shirt. • Review vaccines to be included in clinic and the quantities.
Clinic Lead RN responsibilities prior to clinic: üThe Account Sheet will be emailed to you 1 week before clinic. Read over the account sheet for any special instructions and clinic schedule to review number of vaccines anticipated. üCalls the client to confirm clinic at least 4 -5 days beforehand. üSchedule supply pick up at MVNA at least 2 full days in advance of pick-up date. üAll supplies should be picked up 1 -7 days before clinic. üReviews the following with the client: • Date and time of event. • Clinic address/location within the building. • Where to park (when applicable). • Billing Option. Remind client that participants should bring their insurance card (if not billing to employer). • Confirm consent forms and VIS documents were received and printed. • If senior residence, asks client if they have any Power of Attorneys (POAs) ready that they need. • Have client remind participants to wear casual/short sleeved shirt. • Review vaccines to be included in clinic and the quantities.
 Clinic Lead RN responsibilities prior to clinic Cont’d: üThe Clinic Lead calls RN and CSR staff assigned 2 -4 days prior confirming their participation, time, location and special instructions. üIf Clinic Lead finds s/he will require assistance in securing additional staff (due to illness, cancellation or if staff is unreachable), call Danielle Rice 612 -6174624 M-F during business hours to obtain additional staff needed or Melissa Beebe at 612 -916 -2661. üClinic Lead and staff arrive 15 minutes prior to start of clinic and introduces self to client contact, arranges room and supplies, finds location of land line phone, sets up and posts any information (HIPAA, VIS) and re-confirms number of participants to pass through clinic. üClinic Lead reviews with staff the various vaccinations offered and allows for brief Q & A prior to the start of the clinic. üEnsures proper storage of vaccine & supplies.
Clinic Lead RN responsibilities prior to clinic Cont’d: üThe Clinic Lead calls RN and CSR staff assigned 2 -4 days prior confirming their participation, time, location and special instructions. üIf Clinic Lead finds s/he will require assistance in securing additional staff (due to illness, cancellation or if staff is unreachable), call Danielle Rice 612 -6174624 M-F during business hours to obtain additional staff needed or Melissa Beebe at 612 -916 -2661. üClinic Lead and staff arrive 15 minutes prior to start of clinic and introduces self to client contact, arranges room and supplies, finds location of land line phone, sets up and posts any information (HIPAA, VIS) and re-confirms number of participants to pass through clinic. üClinic Lead reviews with staff the various vaccinations offered and allows for brief Q & A prior to the start of the clinic. üEnsures proper storage of vaccine & supplies.
 Clinic Lead RN responsibilities during clinic: üEnsures Client Service Representative (CSR) and participant complete the consent form in its entirety. The participant may not receive his/her vaccine until the form is completed. üMonitor staff ensuring compliance to policy and procedures. üTake lead role in management of: ümedical emergencies üReportable Occurrence üon-site client relationship and üIf the clinic is slow please discharge additional staff early. Also talk to the on-site coordinator and ask for their help to announce there is no waiting, etc.
Clinic Lead RN responsibilities during clinic: üEnsures Client Service Representative (CSR) and participant complete the consent form in its entirety. The participant may not receive his/her vaccine until the form is completed. üMonitor staff ensuring compliance to policy and procedures. üTake lead role in management of: ümedical emergencies üReportable Occurrence üon-site client relationship and üIf the clinic is slow please discharge additional staff early. Also talk to the on-site coordinator and ask for their help to announce there is no waiting, etc.
 Clinic Lead RN responsibilities after clinic: üEnsure all staff participate in the clean up, packing and proper disposal of materials used in the clinic. üEnsure all consent forms are signed and reconciled before departure and return them to the MVNA within 2 -3 business days. If outside of metro, mail them back to MVNA (you will be reimbursed). üCommunicate with on-site client contact those participants that did not show and obtain any missing information on forms. üAccess MVNA website to enter number of immunizations provided at each clinic site. üLeave the clinic incomplete if there any clinic time or staff changes. Type a short explanation. Return supplies and forms or pick up additional supplies at MVNA. üEnsure proper storage of vaccines and supplies. üReport any changes to clinic set-up, parking, etc noted so MVNA office staff can ensure the changes get added to clinic notes on account sheet. üProvide clinic and staff feedback to MVNA office staff.
Clinic Lead RN responsibilities after clinic: üEnsure all staff participate in the clean up, packing and proper disposal of materials used in the clinic. üEnsure all consent forms are signed and reconciled before departure and return them to the MVNA within 2 -3 business days. If outside of metro, mail them back to MVNA (you will be reimbursed). üCommunicate with on-site client contact those participants that did not show and obtain any missing information on forms. üAccess MVNA website to enter number of immunizations provided at each clinic site. üLeave the clinic incomplete if there any clinic time or staff changes. Type a short explanation. Return supplies and forms or pick up additional supplies at MVNA. üEnsure proper storage of vaccines and supplies. üReport any changes to clinic set-up, parking, etc noted so MVNA office staff can ensure the changes get added to clinic notes on account sheet. üProvide clinic and staff feedback to MVNA office staff.
 Responsibilities of Client Service Rep (CSR) ü The CSR reports to Melissa Beebe 612 -617 -4723 (office) 612 -916 -2661(after hours) ü Responsibilities prior to clinic: • Call Melissa Beebe at the above number if the lead nurse has not contacted you 2 days before the clinic. • Introduce self to clinic lead and the client contact. • Set up the registration table with pens, consent forms, VIS and HIPAA sheets. ü Responsibilities during clinic: • Greet each participant and assist them with completion of the consent form as needed. • Review and confirm consent form is fully completed before directing participant to the vaccine table. • If there is a wait please ask participants to have a seat and convey wait time. • Help manage the flow of the line/participants, keeping track of who is next in line and keeping the pace moving. • Have fun, be friendly and helpful. Help make it a great experience for each person. • Encourage participation ü Responsibilities after clinic: • You are not expected to stay and help clean up after the clinic end time, because we can not pay for this extra time.
Responsibilities of Client Service Rep (CSR) ü The CSR reports to Melissa Beebe 612 -617 -4723 (office) 612 -916 -2661(after hours) ü Responsibilities prior to clinic: • Call Melissa Beebe at the above number if the lead nurse has not contacted you 2 days before the clinic. • Introduce self to clinic lead and the client contact. • Set up the registration table with pens, consent forms, VIS and HIPAA sheets. ü Responsibilities during clinic: • Greet each participant and assist them with completion of the consent form as needed. • Review and confirm consent form is fully completed before directing participant to the vaccine table. • If there is a wait please ask participants to have a seat and convey wait time. • Help manage the flow of the line/participants, keeping track of who is next in line and keeping the pace moving. • Have fun, be friendly and helpful. Help make it a great experience for each person. • Encourage participation ü Responsibilities after clinic: • You are not expected to stay and help clean up after the clinic end time, because we can not pay for this extra time.
 Computer Responsibilities Check your email daily for Updates – check your ü junk/spam mail. ü Check your schedule board regularly ü Save Clinic Confirmations and Account Sheets ü Clinic Lead reconciles immunizations and staffing daily ü HCMC Email – Check monthly to keep active ü Online Trainings ü (Computers available after training if you would like to get started on your online trainings)
Computer Responsibilities Check your email daily for Updates – check your ü junk/spam mail. ü Check your schedule board regularly ü Save Clinic Confirmations and Account Sheets ü Clinic Lead reconciles immunizations and staffing daily ü HCMC Email – Check monthly to keep active ü Online Trainings ü (Computers available after training if you would like to get started on your online trainings)
 Online Training • You received a packet with information on accessing and completing your online training. • Your login info is on your new key chains. • There will be time available after training if you would like to practice accessing this site OR the Wellness Staff site.
Online Training • You received a packet with information on accessing and completing your online training. • Your login info is on your new key chains. • There will be time available after training if you would like to practice accessing this site OR the Wellness Staff site.
 WELLNESS STAFF WEBSITE
WELLNESS STAFF WEBSITE
 Accessing the Wellness Staff Log-in Site q. Go to www. mvna. org q. Click on Employee Portal tab in the blue banner q. Click on Wellness Staff Login q. Your username and password are on the Keychain
Accessing the Wellness Staff Log-in Site q. Go to www. mvna. org q. Click on Employee Portal tab in the blue banner q. Click on Wellness Staff Login q. Your username and password are on the Keychain
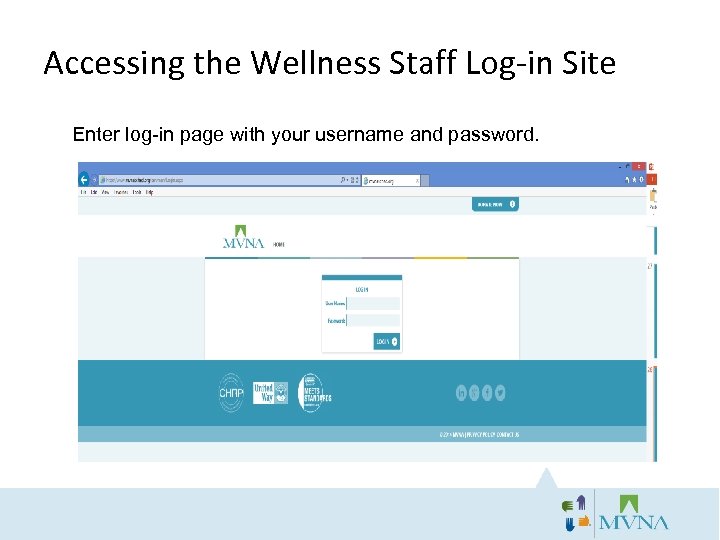 Accessing the Wellness Staff Log-in Site Enter log-in page with your username and password.
Accessing the Wellness Staff Log-in Site Enter log-in page with your username and password.
 Wellness Staff Log-in Site Overview • One-Stop shop for: • • Access Training materials Timesheet/Expense Forms Helpful Checklists Schedule Supply Pickup Submit your Availability Check your Schedule Reconcile Clinics
Wellness Staff Log-in Site Overview • One-Stop shop for: • • Access Training materials Timesheet/Expense Forms Helpful Checklists Schedule Supply Pickup Submit your Availability Check your Schedule Reconcile Clinics
 SCHEDULING AND RECONCILING CLINICS
SCHEDULING AND RECONCILING CLINICS
 Overview of 2016 Scheduling Process v. You will submit your availability from the MVNA staff log-in page. All availability must be updated at least three full weeks in advance. v. You will complete a survey about which geographic areas (zones) you prefer to work. v. You will be scheduled based on your availability & zones selected. v. You can check your weekly schedule from the MVNA staff log-in page: http: //www. mvnasched. org/servman/
Overview of 2016 Scheduling Process v. You will submit your availability from the MVNA staff log-in page. All availability must be updated at least three full weeks in advance. v. You will complete a survey about which geographic areas (zones) you prefer to work. v. You will be scheduled based on your availability & zones selected. v. You can check your weekly schedule from the MVNA staff log-in page: http: //www. mvnasched. org/servman/
 Clinic Assignments ØYou will have all your clinic assignments at least two weeks in advance. ØYou will be contacted before being scheduled into a last minute clinic. ØYou will receive an email notification of each individual clinic you are scheduled at. ØAll scheduled clinics will also appear on your schedule board. Ø You will also receive an email with the Account Sheet attached 1 week before your clinic. ØYou are responsible for checking your schedule board frequently and closely as changes may occur. Contact Danielle Rice at 612 -617 -4624 immediately about conflicts/errors/questions. ØNo reminders will be sent from the office about clinics. The Clinic Lead will contact the client and all staff at least 2 -4 days before the clinic. If you do not hear from your Clinic Lead at least 24 hours before the clinic please contact the office staff immediately.
Clinic Assignments ØYou will have all your clinic assignments at least two weeks in advance. ØYou will be contacted before being scheduled into a last minute clinic. ØYou will receive an email notification of each individual clinic you are scheduled at. ØAll scheduled clinics will also appear on your schedule board. Ø You will also receive an email with the Account Sheet attached 1 week before your clinic. ØYou are responsible for checking your schedule board frequently and closely as changes may occur. Contact Danielle Rice at 612 -617 -4624 immediately about conflicts/errors/questions. ØNo reminders will be sent from the office about clinics. The Clinic Lead will contact the client and all staff at least 2 -4 days before the clinic. If you do not hear from your Clinic Lead at least 24 hours before the clinic please contact the office staff immediately.
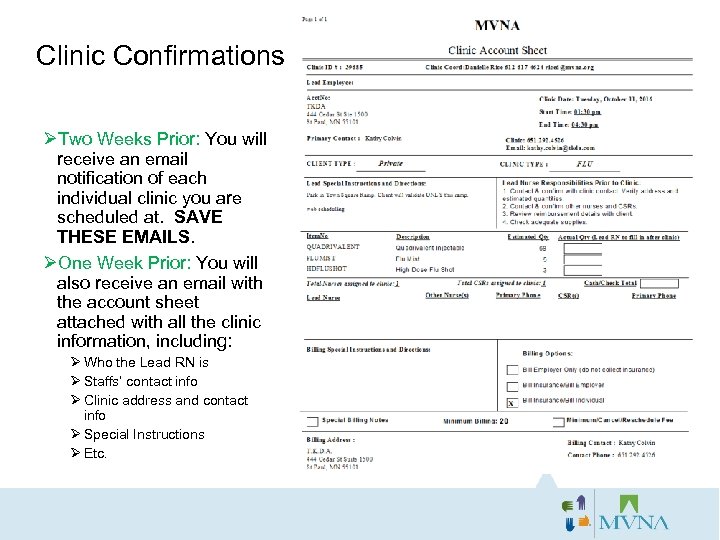 Clinic Confirmations ØTwo Weeks Prior: You will receive an email notification of each individual clinic you are scheduled at. SAVE THESE EMAILS. ØOne Week Prior: You will also receive an email with the account sheet attached with all the clinic information, including: Ø Who the Lead RN is Ø Staffs’ contact info Ø Clinic address and contact info Ø Special Instructions Ø Etc.
Clinic Confirmations ØTwo Weeks Prior: You will receive an email notification of each individual clinic you are scheduled at. SAVE THESE EMAILS. ØOne Week Prior: You will also receive an email with the account sheet attached with all the clinic information, including: Ø Who the Lead RN is Ø Staffs’ contact info Ø Clinic address and contact info Ø Special Instructions Ø Etc.
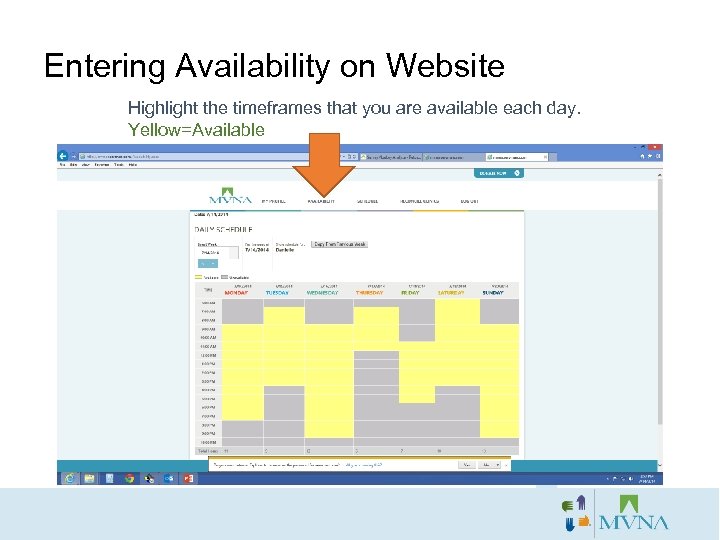 Entering Availability on Website Highlight the timeframes that you are available each day. Yellow=Available
Entering Availability on Website Highlight the timeframes that you are available each day. Yellow=Available
 Entering your Availability • You are required to enter your availability at least 3 full weeks in advance. • The schedule will be created based on your availability. If you make changes to your online availability less than 3 weeks out, the office staff may not see the change. You will be expected to work for each clinic assignment that appears on your schedule. • If you can not work a clinic that is on your schedule, you must contact Danielle Rice at Danielle. Rice@hcmed. org ASAP.
Entering your Availability • You are required to enter your availability at least 3 full weeks in advance. • The schedule will be created based on your availability. If you make changes to your online availability less than 3 weeks out, the office staff may not see the change. You will be expected to work for each clinic assignment that appears on your schedule. • If you can not work a clinic that is on your schedule, you must contact Danielle Rice at Danielle. Rice@hcmed. org ASAP.
 Entering your Availability ØYou are committing to your availability as indicated on the calendar, and you will be scheduled accordingly. For example, if you submit that you are available all week, expect to be scheduled heavily (20 -30 hours). If you only want to work 15 hours a week, only submit about 20 hours of availability. ØOnce you submit your availability, you have committed to work those hours (cancelling must be limited to an emergency or illness).
Entering your Availability ØYou are committing to your availability as indicated on the calendar, and you will be scheduled accordingly. For example, if you submit that you are available all week, expect to be scheduled heavily (20 -30 hours). If you only want to work 15 hours a week, only submit about 20 hours of availability. ØOnce you submit your availability, you have committed to work those hours (cancelling must be limited to an emergency or illness).
 Check schedule board üYou are responsible for checking your schedule board frequently and closely as changes may occur. üContact Danielle Rice at 612 -617 -4624 immediately about conflicts/errors/questions.
Check schedule board üYou are responsible for checking your schedule board frequently and closely as changes may occur. üContact Danielle Rice at 612 -617 -4624 immediately about conflicts/errors/questions.
 Reconciling Clinics Purpose: Each clinic will need to be reconciled through the Wellness Staff Login site by the lead nurse. By reconciling the clinic, the lead nurse verifies the number of vaccinations or screenings performed. If there were time and/or staffing changes, the lead should leave the clinic incomplete and describe the change that needs to be made.
Reconciling Clinics Purpose: Each clinic will need to be reconciled through the Wellness Staff Login site by the lead nurse. By reconciling the clinic, the lead nurse verifies the number of vaccinations or screenings performed. If there were time and/or staffing changes, the lead should leave the clinic incomplete and describe the change that needs to be made.
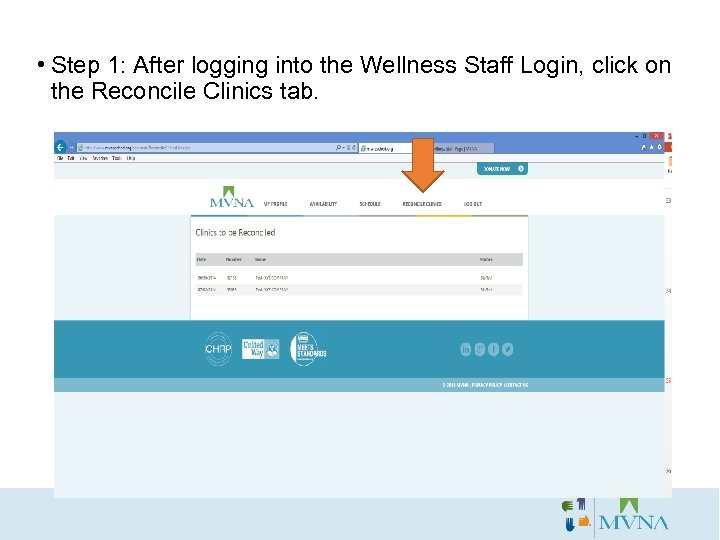 • Step 1: After logging into the Wellness Staff Login, click on the Reconcile Clinics tab.
• Step 1: After logging into the Wellness Staff Login, click on the Reconcile Clinics tab.
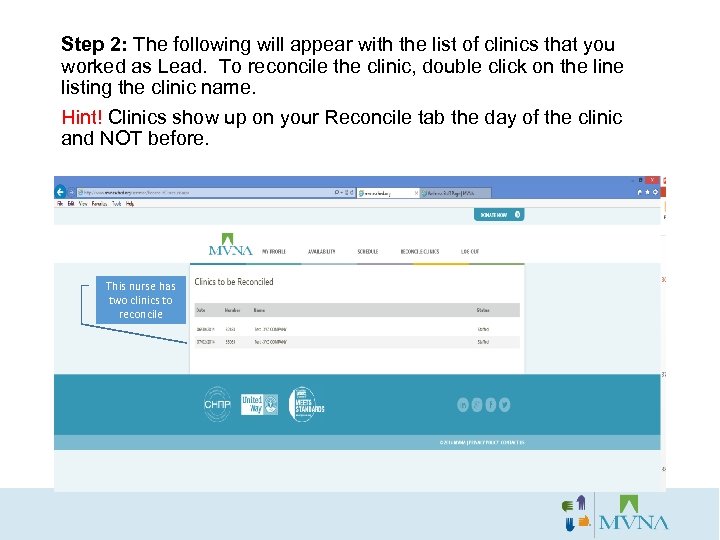 Step 2: The following will appear with the list of clinics that you worked as Lead. To reconcile the clinic, double click on the line listing the clinic name. Hint! Clinics show up on your Reconcile tab the day of the clinic and NOT before. This nurse has two clinics to reconcile
Step 2: The following will appear with the list of clinics that you worked as Lead. To reconcile the clinic, double click on the line listing the clinic name. Hint! Clinics show up on your Reconcile tab the day of the clinic and NOT before. This nurse has two clinics to reconcile
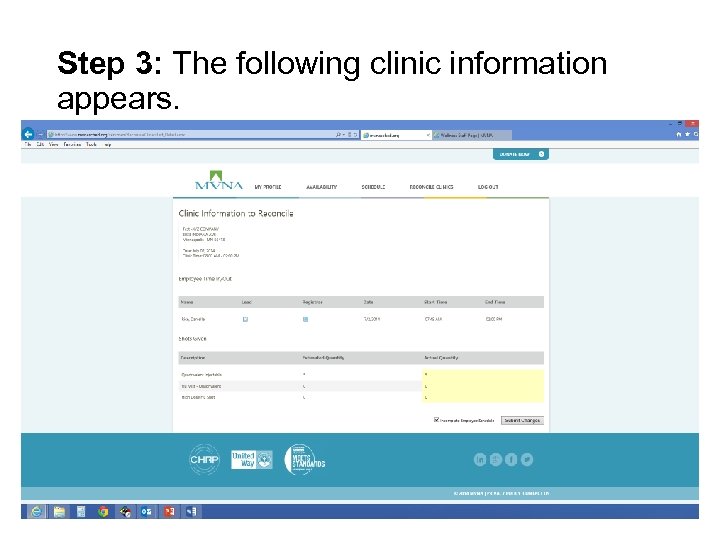 Step 3: The following clinic information appears.
Step 3: The following clinic information appears.
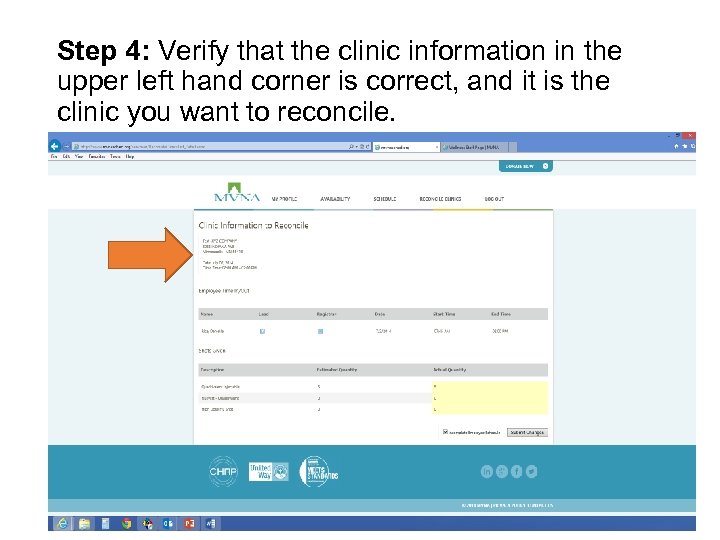 Step 4: Verify that the clinic information in the upper left hand corner is correct, and it is the clinic you want to reconcile.
Step 4: Verify that the clinic information in the upper left hand corner is correct, and it is the clinic you want to reconcile.
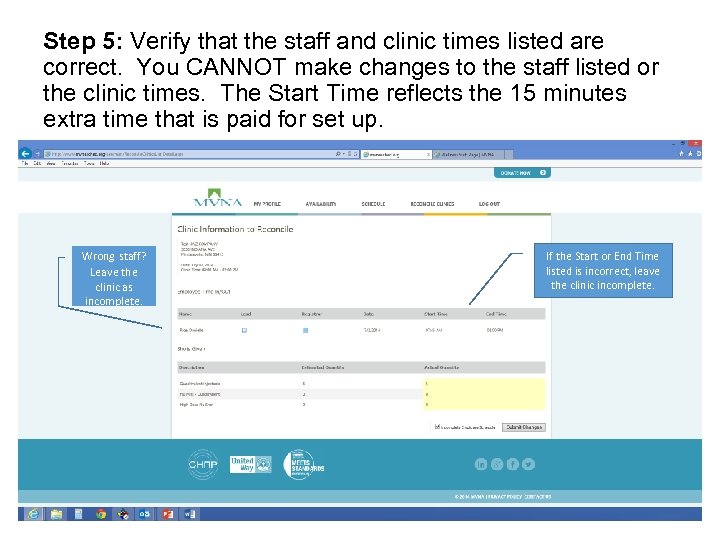 Step 5: Verify that the staff and clinic times listed are correct. You CANNOT make changes to the staff listed or the clinic times. The Start Time reflects the 15 minutes extra time that is paid for set up. Wrong staff? Leave the clinic as incomplete. If the Start or End Time listed is incorrect, leave the clinic incomplete.
Step 5: Verify that the staff and clinic times listed are correct. You CANNOT make changes to the staff listed or the clinic times. The Start Time reflects the 15 minutes extra time that is paid for set up. Wrong staff? Leave the clinic as incomplete. If the Start or End Time listed is incorrect, leave the clinic incomplete.
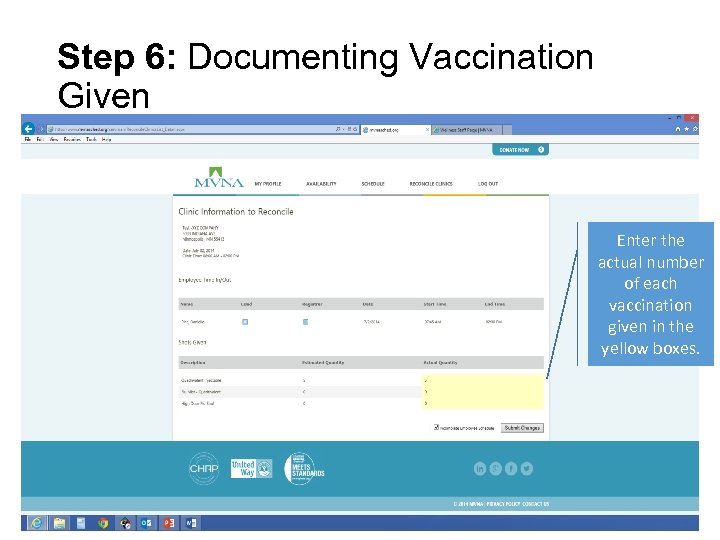 Step 6: Documenting Vaccination Given Enter the actual number of each vaccination given in the yellow boxes.
Step 6: Documenting Vaccination Given Enter the actual number of each vaccination given in the yellow boxes.
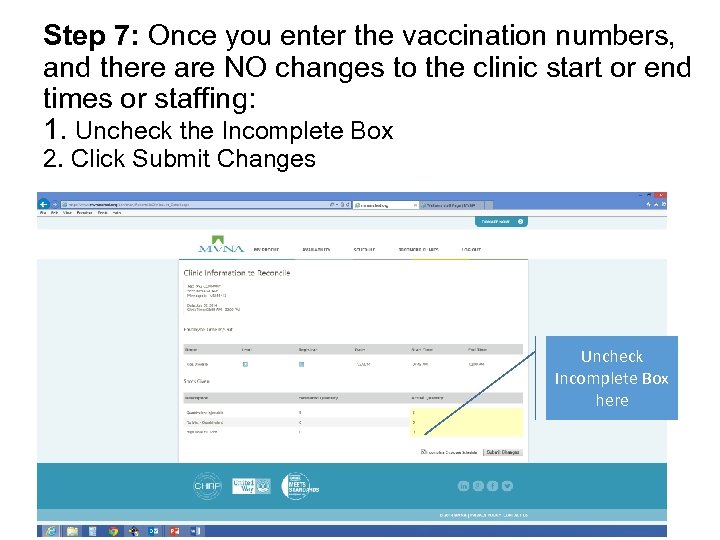 Step 7: Once you enter the vaccination numbers, and there are NO changes to the clinic start or end times or staffing: 1. Uncheck the Incomplete Box 2. Click Submit Changes Uncheck Incomplete Box here
Step 7: Once you enter the vaccination numbers, and there are NO changes to the clinic start or end times or staffing: 1. Uncheck the Incomplete Box 2. Click Submit Changes Uncheck Incomplete Box here
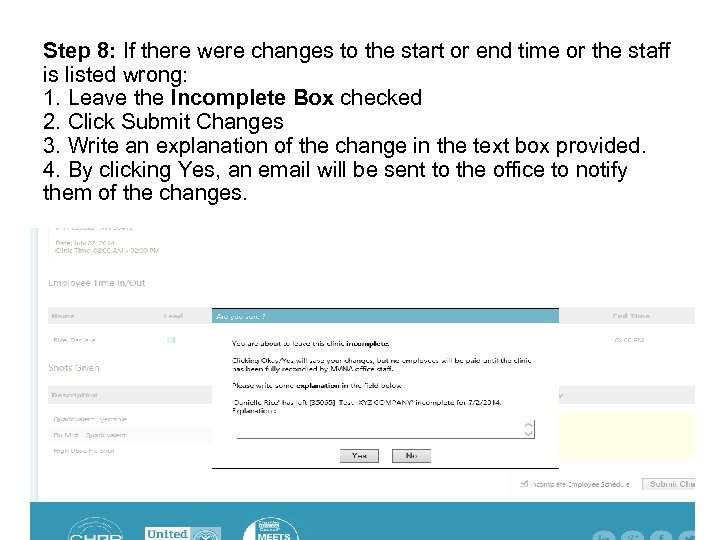 Step 8: If there were changes to the start or end time or the staff is listed wrong: 1. Leave the Incomplete Box checked 2. Click Submit Changes 3. Write an explanation of the change in the text box provided. 4. By clicking Yes, an email will be sent to the office to notify them of the changes.
Step 8: If there were changes to the start or end time or the staff is listed wrong: 1. Leave the Incomplete Box checked 2. Click Submit Changes 3. Write an explanation of the change in the text box provided. 4. By clicking Yes, an email will be sent to the office to notify them of the changes.
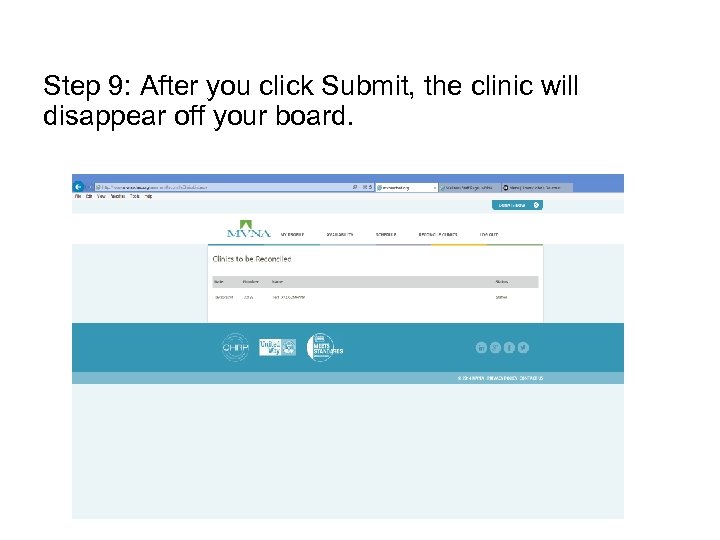 Step 9: After you click Submit, the clinic will disappear off your board.
Step 9: After you click Submit, the clinic will disappear off your board.
 PAYROLL/TIMESHEETS
PAYROLL/TIMESHEETS
 Timesheet & Mileage Log
Timesheet & Mileage Log
 Mileage and Drive Time in-between work assignments (including clinics and supply pick-ups): • You are paid drive-time in between assignments. Please refer to guidelines below: • . 1 – 15 miles between clients: 15 minutes of travel time allotted • 16. 0 – 35 miles between clients: 30 minutes of travel time allotted • 36. 0 – 55 miles between clients: 45 minutes of travel time allotted Mileage between work assignments (including clinics and supply pick-ups): • You are paid for the mileage between your clinics (including your appointment for supplies at MVNA). • Track your miles and record on your timesheet. Mileage/time getting to your first clinic/getting home from your last clinic: • No mileage or drive time is paid to get to your first assignment or to return home after your last assignment, unless the assignment is over 30 miles. • If you are traveling more than 30 miles, you will be paid mileage and drive time for the difference above 30 miles. • Mileage reimbursement needs to be submitted within 60 days of occurance. Anything submitted after 60 days will not be paid.
Mileage and Drive Time in-between work assignments (including clinics and supply pick-ups): • You are paid drive-time in between assignments. Please refer to guidelines below: • . 1 – 15 miles between clients: 15 minutes of travel time allotted • 16. 0 – 35 miles between clients: 30 minutes of travel time allotted • 36. 0 – 55 miles between clients: 45 minutes of travel time allotted Mileage between work assignments (including clinics and supply pick-ups): • You are paid for the mileage between your clinics (including your appointment for supplies at MVNA). • Track your miles and record on your timesheet. Mileage/time getting to your first clinic/getting home from your last clinic: • No mileage or drive time is paid to get to your first assignment or to return home after your last assignment, unless the assignment is over 30 miles. • If you are traveling more than 30 miles, you will be paid mileage and drive time for the difference above 30 miles. • Mileage reimbursement needs to be submitted within 60 days of occurance. Anything submitted after 60 days will not be paid.
 Lead RN ONLY Supply Pick up and Drop off: • You should plan to come to MVNA to drop off and pick up supplies once every 5 -10 days. • Each appointment will be 15 minutes long. • Record 15 minutes on your time sheet. Contacting the client and other staff before the clinic: • Whenever possible, use the time you have between clinics to make these contacts. • Keep a folder in your car of your account sheets for the next week – the account sheets list all the contact information for the clinic contacts and other staff. • Our expectation is that your time spent on making these contacts not exceed: • 1 -5 clinics: 15 minutes • 6+ clinics: 30 minutes
Lead RN ONLY Supply Pick up and Drop off: • You should plan to come to MVNA to drop off and pick up supplies once every 5 -10 days. • Each appointment will be 15 minutes long. • Record 15 minutes on your time sheet. Contacting the client and other staff before the clinic: • Whenever possible, use the time you have between clinics to make these contacts. • Keep a folder in your car of your account sheets for the next week – the account sheets list all the contact information for the clinic contacts and other staff. • Our expectation is that your time spent on making these contacts not exceed: • 1 -5 clinics: 15 minutes • 6+ clinics: 30 minutes
 Clinic Set-up: • All staff should arrive 15 minutes before the start of the clinic and help set up the clinic. • The lead should report to MVNA if anyone does not arrive 15 minutes before the clinic start time on the reconcile tab of the Wellness Staff Website. Leave the clinic incomplete and write a message to the office. • All staff should include this 15 minutes on their timesheets. • You will only be paid to arrive 15 minutes before the clinic. Any exception needs to be pre-approved by the office staff.
Clinic Set-up: • All staff should arrive 15 minutes before the start of the clinic and help set up the clinic. • The lead should report to MVNA if anyone does not arrive 15 minutes before the clinic start time on the reconcile tab of the Wellness Staff Website. Leave the clinic incomplete and write a message to the office. • All staff should include this 15 minutes on their timesheets. • You will only be paid to arrive 15 minutes before the clinic. Any exception needs to be pre-approved by the office staff.
 Sample Timesheet/Mileage Log 45
Sample Timesheet/Mileage Log 45
 Submitting your timesheets: • You are required to have your timesheets submitted to Angela by the due date. If your timesheet is late, your time will be paid on the next paycheck. No exceptions. • Turn in one of the following ways: • Email: angela. thao@hcmed. org • Fax: 612 -617 -4772 • Mail: Attn: Angela Thao, 2000 Summer St. NE, Minneapolis MN 55413 • In person at the office • HINT: Take a picture with your phone and email the picture to Angela! • It is your responsibility to turn in your time. You will not be paid or reimbursed for time/miles/postage related to submitting your timesheets.
Submitting your timesheets: • You are required to have your timesheets submitted to Angela by the due date. If your timesheet is late, your time will be paid on the next paycheck. No exceptions. • Turn in one of the following ways: • Email: angela. thao@hcmed. org • Fax: 612 -617 -4772 • Mail: Attn: Angela Thao, 2000 Summer St. NE, Minneapolis MN 55413 • In person at the office • HINT: Take a picture with your phone and email the picture to Angela! • It is your responsibility to turn in your time. You will not be paid or reimbursed for time/miles/postage related to submitting your timesheets.
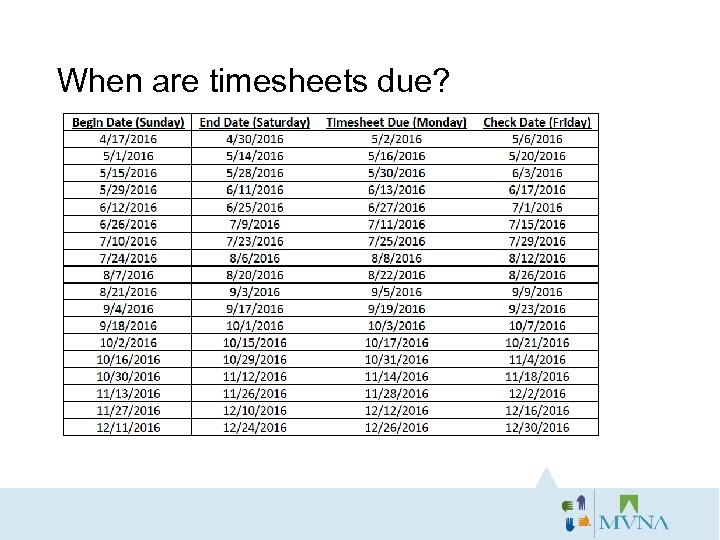 When are timesheets due?
When are timesheets due?
 Reimbursement for Parking (or other expenses, such as postage) • Complete and submit the reimbursement form (available on the Wellness Staff Site)
Reimbursement for Parking (or other expenses, such as postage) • Complete and submit the reimbursement form (available on the Wellness Staff Site)
 Training: • For any training, either in person or online, the time expectation will be communicated. We are not approved to pay for anything exceeding the maximum time.
Training: • For any training, either in person or online, the time expectation will be communicated. We are not approved to pay for anything exceeding the maximum time.
 Need help on timesheets? • First, check the material posted on the Wellness Staff Website: • • Payroll Timeline Timesheets 101 Timesheet & Mileage Log And Instructions on accessing your timesheets/paystubs online • If you still need help, contact: • Angela Thao, Principal Office Specialist • Angela. Thao@hcmed. org • 612 -617 -4706
Need help on timesheets? • First, check the material posted on the Wellness Staff Website: • • Payroll Timeline Timesheets 101 Timesheet & Mileage Log And Instructions on accessing your timesheets/paystubs online • If you still need help, contact: • Angela Thao, Principal Office Specialist • Angela. Thao@hcmed. org • 612 -617 -4706
 • We are now part of a larger system in which we have a fiduciary responsibility to be accountable for all dollars in and out. We are expected to be accountable for adhering to federal laws and general regulations, and adhering to protocol. • Every part of our program is subject to audits by Hennepin Health System to insure accuracy and integrity. • Please be mindful of this when you are completing your timesheets and expense reports. • Purposeful fraudulence is grounds for immediate dismissal.
• We are now part of a larger system in which we have a fiduciary responsibility to be accountable for all dollars in and out. We are expected to be accountable for adhering to federal laws and general regulations, and adhering to protocol. • Every part of our program is subject to audits by Hennepin Health System to insure accuracy and integrity. • Please be mindful of this when you are completing your timesheets and expense reports. • Purposeful fraudulence is grounds for immediate dismissal.
 Consent Forms
Consent Forms
 Consent Form The form is 2 sided and needs to be fully completed
Consent Form The form is 2 sided and needs to be fully completed
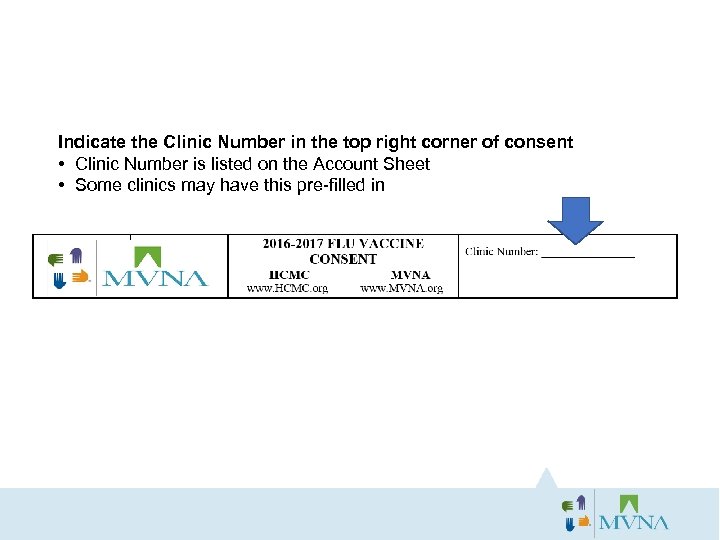 Indicate the Clinic Number in the top right corner of consent • Clinic Number is listed on the Account Sheet • Some clinics may have this pre-filled in
Indicate the Clinic Number in the top right corner of consent • Clinic Number is listed on the Account Sheet • Some clinics may have this pre-filled in
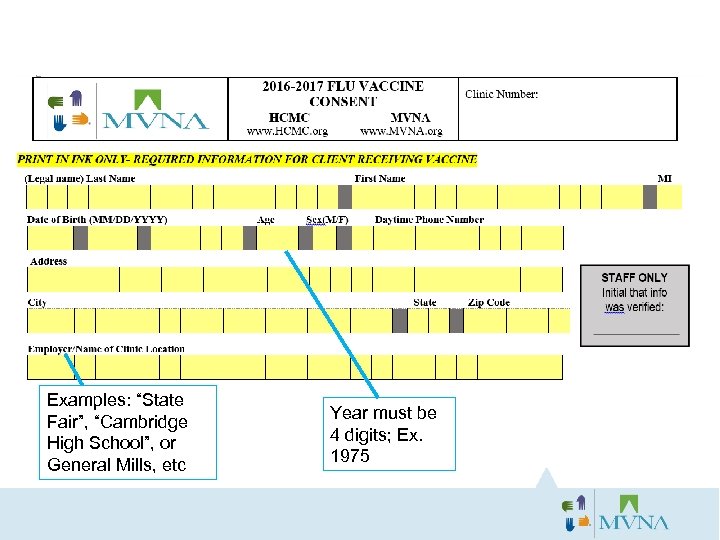 Examples: “State Fair”, “Cambridge High School”, or General Mills, etc Year must be 4 digits; Ex. 1975
Examples: “State Fair”, “Cambridge High School”, or General Mills, etc Year must be 4 digits; Ex. 1975
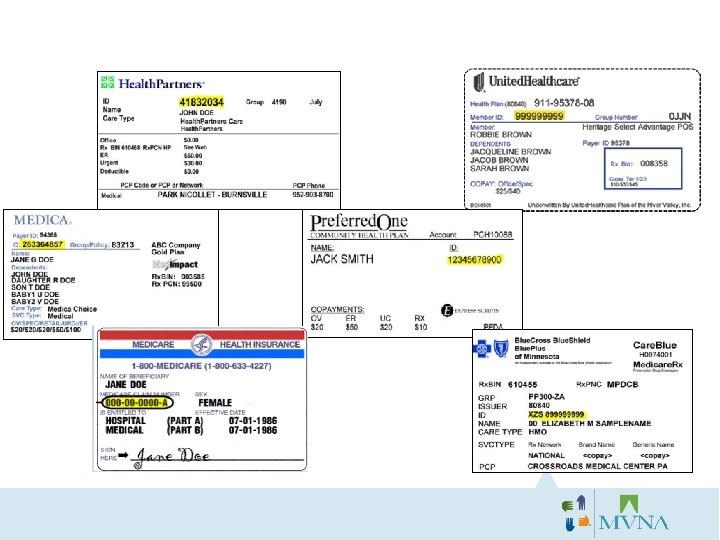
 “Does my insurance cover the flu shot? ” “Is the flu shot free if I have insurance? ” “How do I know if you accept my insurance? ” • NEVER say the flu shot is free! • Your response to questions about insurance coverage should always be: “MVNA/HCMC can bill any insurance provider for your vaccination, but it is the individuals’ responsibility to know if the flu shot is a covered benefit. ”
“Does my insurance cover the flu shot? ” “Is the flu shot free if I have insurance? ” “How do I know if you accept my insurance? ” • NEVER say the flu shot is free! • Your response to questions about insurance coverage should always be: “MVNA/HCMC can bill any insurance provider for your vaccination, but it is the individuals’ responsibility to know if the flu shot is a covered benefit. ”
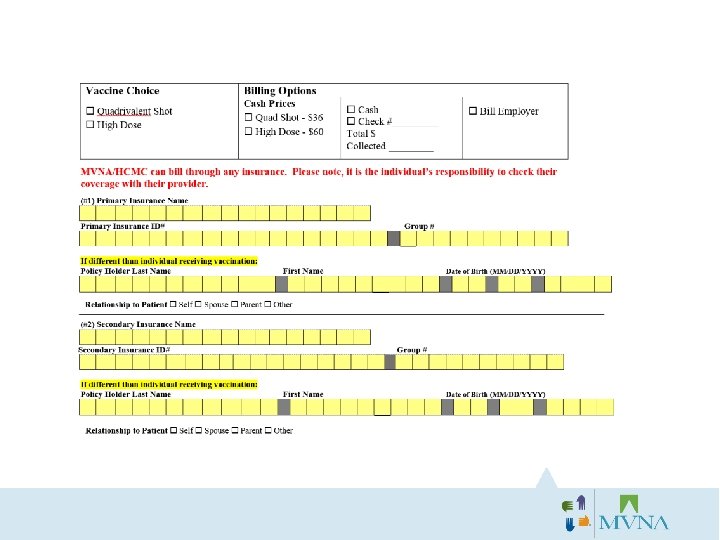
 Bill Insurance/Bill Employer Consent: Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only
Bill Insurance/Bill Employer Consent: Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only
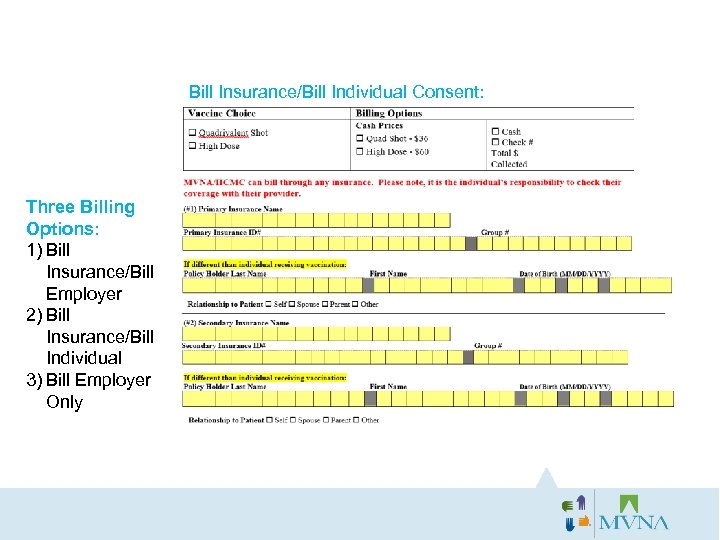 Bill Insurance/Bill Individual Consent: Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only
Bill Insurance/Bill Individual Consent: Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only
 Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only Consent:
Three Billing Options: 1) Bill Insurance/Bill Employer 2) Bill Insurance/Bill Individual 3) Bill Employer Only Consent:
 What if an employee, student, or resident knows that they need a record of their flu shot? How can they get this record? They have two options at the clinic: 1) You can give them a signed receipt of the vaccination. This is often sufficient proof of vaccination. These are packed in the RNs’ supply bins! 2) If they would like a full copy of the completed consent form released, they should initial on the bottom of the front page of the consent (pictured below) and indicate who they would like the record released to. They can release the record to themselves or directly to an employer/doctor’s office/school. Please make sure they fill in the name AND address, so we know where to send the record. They should allow 3 -4 weeks to receive the record. LEAD RNs: Please let the flu room staff know if you are returning a packet with participants that need a released medical record.
What if an employee, student, or resident knows that they need a record of their flu shot? How can they get this record? They have two options at the clinic: 1) You can give them a signed receipt of the vaccination. This is often sufficient proof of vaccination. These are packed in the RNs’ supply bins! 2) If they would like a full copy of the completed consent form released, they should initial on the bottom of the front page of the consent (pictured below) and indicate who they would like the record released to. They can release the record to themselves or directly to an employer/doctor’s office/school. Please make sure they fill in the name AND address, so we know where to send the record. They should allow 3 -4 weeks to receive the record. LEAD RNs: Please let the flu room staff know if you are returning a packet with participants that need a released medical record.
 Vaccine Receipt • Some patients may request verification that they received a vaccination from MVNA • Complete a Vaccine Receipt
Vaccine Receipt • Some patients may request verification that they received a vaccination from MVNA • Complete a Vaccine Receipt

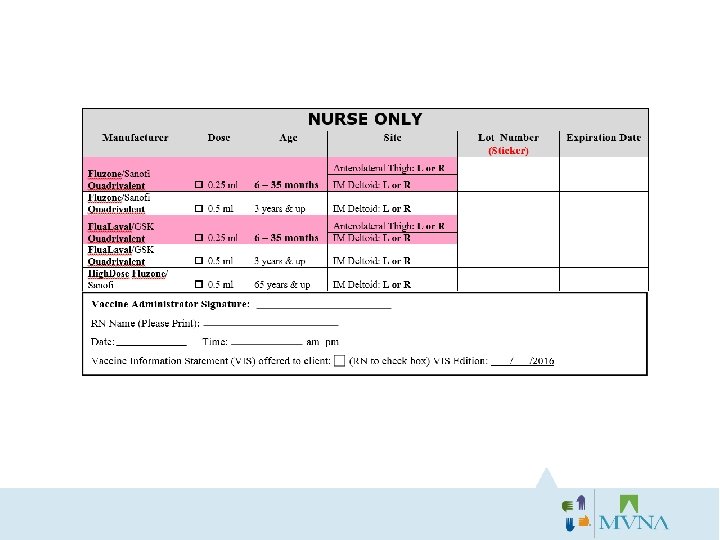
 The Client and the RN MUST both sign the consent form! You can NOT give a vaccination without signed consent. If either signature is missing, it is your responsibility to correct this. If you forget to sign your consent forms, you will be asked to return to the office to complete on your own time.
The Client and the RN MUST both sign the consent form! You can NOT give a vaccination without signed consent. If either signature is missing, it is your responsibility to correct this. If you forget to sign your consent forms, you will be asked to return to the office to complete on your own time.
 Why a photo ID for Insurance? Red Flag Law: Enacted to prevent identification fraud • Ask to see a picture ID (Drivers’ License or Work ID Badge) and confirm it matches the Consent/Insurance Card
Why a photo ID for Insurance? Red Flag Law: Enacted to prevent identification fraud • Ask to see a picture ID (Drivers’ License or Work ID Badge) and confirm it matches the Consent/Insurance Card
 Consent Form Policies & Procedures • During the clinic: • Each completed consent form must be placed out of site, in a folder or envelope, before the next patient enters the area. • At no time are the consent forms to be left unattended. • If any of the nurses have to leave their shot station, they are to give their folder or envelope of consent forms to another nurse or the CSR for safe keeping while away. • After the clinic: • Return the completed clinic packet, to include the completed consent forms, to MVNA as soon as possible. The same day is most desirable. • During all transport of completed consent forms, they should be locked in the trunk of your vehicle, until you either return them to MVNA or go home, whichever should come first. • If you are not going directly home but to another clinic site, you must bring them into the next location with you. • At no time should the consent forms be left unattended in your vehicle. • Once home, do not leave them in your vehicle but bring them inside your home to a secure and private area. • Until they are returned to MVNA, they are to be kept in a safe and private area in your home. • You should be returning consent forms/clinic packets to MVNA on a weekly basis, at the least, and returned as soon as possible. • For Clinics over 400 shots: • The consent forms for these larger clinics must be returned to MVNA on the same day as the clinic. • Arrangements may be made with internal flu staff for pick up.
Consent Form Policies & Procedures • During the clinic: • Each completed consent form must be placed out of site, in a folder or envelope, before the next patient enters the area. • At no time are the consent forms to be left unattended. • If any of the nurses have to leave their shot station, they are to give their folder or envelope of consent forms to another nurse or the CSR for safe keeping while away. • After the clinic: • Return the completed clinic packet, to include the completed consent forms, to MVNA as soon as possible. The same day is most desirable. • During all transport of completed consent forms, they should be locked in the trunk of your vehicle, until you either return them to MVNA or go home, whichever should come first. • If you are not going directly home but to another clinic site, you must bring them into the next location with you. • At no time should the consent forms be left unattended in your vehicle. • Once home, do not leave them in your vehicle but bring them inside your home to a secure and private area. • Until they are returned to MVNA, they are to be kept in a safe and private area in your home. • You should be returning consent forms/clinic packets to MVNA on a weekly basis, at the least, and returned as soon as possible. • For Clinics over 400 shots: • The consent forms for these larger clinics must be returned to MVNA on the same day as the clinic. • Arrangements may be made with internal flu staff for pick up.
 OTHER FORMS & DOCUMENTS
OTHER FORMS & DOCUMENTS
 Kohls Consent Forms • Never accept cash/check at a Kohls clinic • If someone does not have insurance, the Kohl’s Grant will pay
Kohls Consent Forms • Never accept cash/check at a Kohls clinic • If someone does not have insurance, the Kohl’s Grant will pay
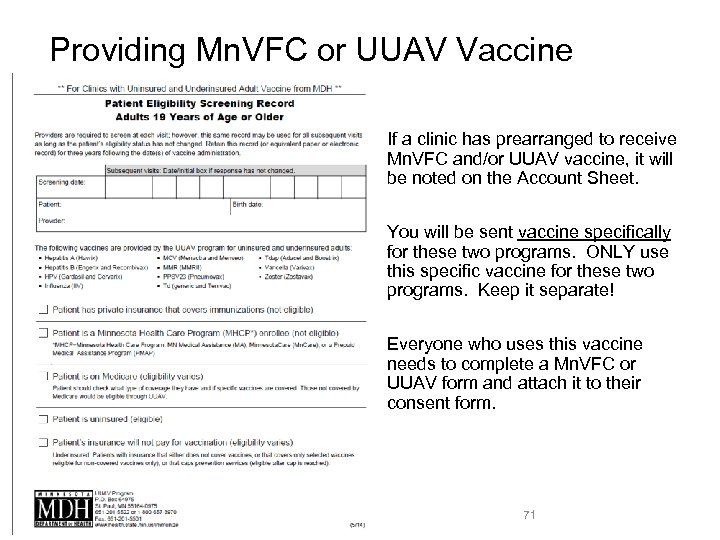 Providing Mn. VFC or UUAV Vaccine • If a clinic has prearranged to receive Mn. VFC and/or UUAV vaccine, it will be noted on the Account Sheet. • You will be sent vaccine specifically for these two programs. ONLY use this specific vaccine for these two programs. Keep it separate! • Everyone who uses this vaccine needs to complete a Mn. VFC or UUAV form and attach it to their consent form. 71
Providing Mn. VFC or UUAV Vaccine • If a clinic has prearranged to receive Mn. VFC and/or UUAV vaccine, it will be noted on the Account Sheet. • You will be sent vaccine specifically for these two programs. ONLY use this specific vaccine for these two programs. Keep it separate! • Everyone who uses this vaccine needs to complete a Mn. VFC or UUAV form and attach it to their consent form. 71
 • Mn. VFC (Minnesota Vaccine for Children) is a program for children 18 and under to receive a vaccine if they do not have insurance. • Children need to meet two criteria. • 18 and under • One of the three criteria listed on the screening form. • An eligibility screening form must be completed for each Mn. VFC vaccine provided and be returned with the consent form. • Uninsured • Minnesota Health Care Program enrollee; MN Medical Assistance (MA, Minnesota. Care, or a Prepaid Medical Assistance Program. • American Indican or Alaskan Native • Eligibility screening form: • Parents complete the form, including the name, etc, and which of the three screening criteria is appropriate. Even if the patient has MA or one of the listed insurances under the eligibility criteria, indicate MNVFC on the consent form. • Attach the eligibility screening form to the consent and return to the office as usual. 72
• Mn. VFC (Minnesota Vaccine for Children) is a program for children 18 and under to receive a vaccine if they do not have insurance. • Children need to meet two criteria. • 18 and under • One of the three criteria listed on the screening form. • An eligibility screening form must be completed for each Mn. VFC vaccine provided and be returned with the consent form. • Uninsured • Minnesota Health Care Program enrollee; MN Medical Assistance (MA, Minnesota. Care, or a Prepaid Medical Assistance Program. • American Indican or Alaskan Native • Eligibility screening form: • Parents complete the form, including the name, etc, and which of the three screening criteria is appropriate. Even if the patient has MA or one of the listed insurances under the eligibility criteria, indicate MNVFC on the consent form. • Attach the eligibility screening form to the consent and return to the office as usual. 72
 Uninsured and Underinsured Adult Vaccine (UUAV) Program UUAV program is a special program for adults 19 and older. To qualify adults must meet all criteria: -19 and above -Uninsured, underinsured 73
Uninsured and Underinsured Adult Vaccine (UUAV) Program UUAV program is a special program for adults 19 and older. To qualify adults must meet all criteria: -19 and above -Uninsured, underinsured 73
 Health Insurance Portability and Accountability Act (HIPAA) – copy in folder HIPAA Compliance includes: • Medical history discussed between nurse & client. • Completed consent forms kept out of view and are returned to MVNA as soon as possible. • An obligation to protect the health information of those we serve. § MVNA distributes a Notice of Privacy Practices Form at clinics.
Health Insurance Portability and Accountability Act (HIPAA) – copy in folder HIPAA Compliance includes: • Medical history discussed between nurse & client. • Completed consent forms kept out of view and are returned to MVNA as soon as possible. • An obligation to protect the health information of those we serve. § MVNA distributes a Notice of Privacy Practices Form at clinics.
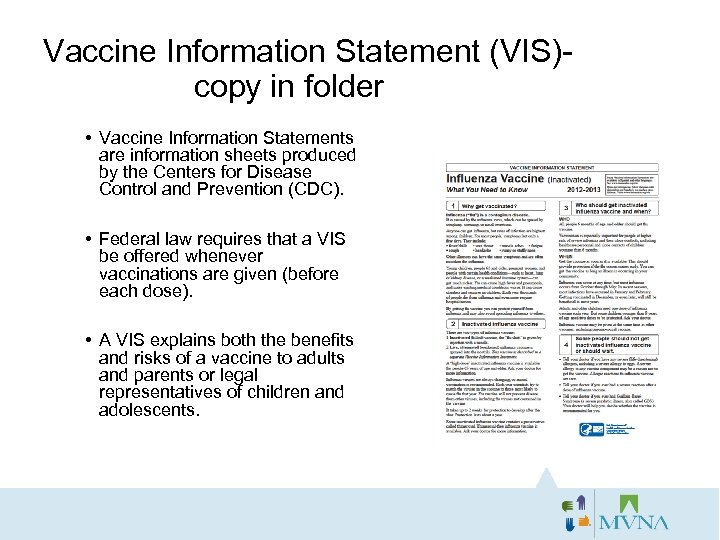 Vaccine Information Statement (VIS)- copy in folder • Vaccine Information Statements are information sheets produced by the Centers for Disease Control and Prevention (CDC). • Federal law requires that a VIS be offered whenever vaccinations are given (before each dose). • A VIS explains both the benefits and risks of a vaccine to adults and parents or legal representatives of children and adolescents.
Vaccine Information Statement (VIS)- copy in folder • Vaccine Information Statements are information sheets produced by the Centers for Disease Control and Prevention (CDC). • Federal law requires that a VIS be offered whenever vaccinations are given (before each dose). • A VIS explains both the benefits and risks of a vaccine to adults and parents or legal representatives of children and adolescents.
 MVNA Reportable Occurrence (formerly Incident Report) – copy in folder 76
MVNA Reportable Occurrence (formerly Incident Report) – copy in folder 76
 MVNA Wellness Program Policy and Procedure Manual v A copy of the MVNA Wellness Program Policy and Procedure Manual can be found in your gray bin. Please keep the manual in your bin for use as a quick reference guide when at a clinic. v All clinic forms referenced within this powerpoint, may also be found in your gray bin (blue folder) v Please review all materials before your first clinic
MVNA Wellness Program Policy and Procedure Manual v A copy of the MVNA Wellness Program Policy and Procedure Manual can be found in your gray bin. Please keep the manual in your bin for use as a quick reference guide when at a clinic. v All clinic forms referenced within this powerpoint, may also be found in your gray bin (blue folder) v Please review all materials before your first clinic
 What’s in the forms folder? Blue Folder: ü MVNA Notice of Privacy (HIPAA) ü Vaccine screening questions ü VAERS for reporting ü MVNA Reportable Occurrence (formerly “incident report”) ü Mn. VFC information/UUAV information ü Instructions for Epi Ampule ü Thermometer Instructions ü Temperature logs for cooler (hourly) and fridge (twice daily) ü Injection instructions ü Contact list/phone numbers (nurses/CSR) ü Timesheet/Miles log (nurses/CSR) ü Consent forms (nurses/CSR ü Copy of Powerpoint • You should have this folder with you at all times when working. • You are expected to be familiar with all the forms and what they mean.
What’s in the forms folder? Blue Folder: ü MVNA Notice of Privacy (HIPAA) ü Vaccine screening questions ü VAERS for reporting ü MVNA Reportable Occurrence (formerly “incident report”) ü Mn. VFC information/UUAV information ü Instructions for Epi Ampule ü Thermometer Instructions ü Temperature logs for cooler (hourly) and fridge (twice daily) ü Injection instructions ü Contact list/phone numbers (nurses/CSR) ü Timesheet/Miles log (nurses/CSR) ü Consent forms (nurses/CSR ü Copy of Powerpoint • You should have this folder with you at all times when working. • You are expected to be familiar with all the forms and what they mean.
 2016 FLU VACCINES
2016 FLU VACCINES
 Vaccines, Manufacturers & Age Groups 3 Types of Inactivated Vaccines: v. Flu. Zone: is Quadrivalent vaccine. Manufacturer is Sanofi Pasteur/Vaxserve. For ages 6 months and up. v. Flulaval: is Quadrivalent vaccine. Manufacturer is GSK. For ages 6 months and up. v. Flu. Zone High Dose: is High Dose Trivalent. Manufacturer is Sanofi. For ages 65 years & older. v. Flu. Mist will not be offered this year based on the CDC’s advice.
Vaccines, Manufacturers & Age Groups 3 Types of Inactivated Vaccines: v. Flu. Zone: is Quadrivalent vaccine. Manufacturer is Sanofi Pasteur/Vaxserve. For ages 6 months and up. v. Flulaval: is Quadrivalent vaccine. Manufacturer is GSK. For ages 6 months and up. v. Flu. Zone High Dose: is High Dose Trivalent. Manufacturer is Sanofi. For ages 65 years & older. v. Flu. Mist will not be offered this year based on the CDC’s advice.
 Flu Vaccine Strains The World Health Organization (WHO) recommends the following strains for the Quadrivalent vaccine flu season, based on its advisory group's in-depth analysis of the most recent circulating strains and patterns. v. The 2016 -2017 Quadrivalent Vaccine Strains: • • A/California/7/2009(H 1 N 1)pdm 09 -like virus; A/Hong Kong/4801/2014(H 3 N 2)-like virus; B/Brisbane/60/2008 -like virus. B/Phuket/3073/2013 -like virus
Flu Vaccine Strains The World Health Organization (WHO) recommends the following strains for the Quadrivalent vaccine flu season, based on its advisory group's in-depth analysis of the most recent circulating strains and patterns. v. The 2016 -2017 Quadrivalent Vaccine Strains: • • A/California/7/2009(H 1 N 1)pdm 09 -like virus; A/Hong Kong/4801/2014(H 3 N 2)-like virus; B/Brisbane/60/2008 -like virus. B/Phuket/3073/2013 -like virus
 High Dose Flu Vaccine 65 years of age and older v. Clients 65 and over have the option to receive Fluzone High-Dose which contains 4 times the antigen of the standard dose vaccine. v. If you are asked (nurse) for a recommendation from the patient, you can now recommend High Dose over the QIV if you have HD with you at that clinic. v. Keep in mind that any flu shot is better than no flu shot. If you only have QIV, then don’t bring it up (unless by the patient). v. It is a Trivalent vaccine and covers the same strains that the WHO has approved for all manufacturers to produce in Trivalent vaccine: * an A/California/7/2009 (H 1 N 1)pdm 09 -like virus; * an A/Hong Kong/4801/2014 (H 3 N 2)-like virus; * a B/Brisbane/60/2008 -like virus (B/Victoria lineage).
High Dose Flu Vaccine 65 years of age and older v. Clients 65 and over have the option to receive Fluzone High-Dose which contains 4 times the antigen of the standard dose vaccine. v. If you are asked (nurse) for a recommendation from the patient, you can now recommend High Dose over the QIV if you have HD with you at that clinic. v. Keep in mind that any flu shot is better than no flu shot. If you only have QIV, then don’t bring it up (unless by the patient). v. It is a Trivalent vaccine and covers the same strains that the WHO has approved for all manufacturers to produce in Trivalent vaccine: * an A/California/7/2009 (H 1 N 1)pdm 09 -like virus; * an A/Hong Kong/4801/2014 (H 3 N 2)-like virus; * a B/Brisbane/60/2008 -like virus (B/Victoria lineage).
 Mock Clinic Video https: //youtu. be/Zg 7 lkq. V 01 TY
Mock Clinic Video https: //youtu. be/Zg 7 lkq. V 01 TY
 Questions?
Questions?
 SUPPLIES & SUPPLY ROOM PICK-UP Everything you need for your clinic
SUPPLIES & SUPPLY ROOM PICK-UP Everything you need for your clinic
 Please pick up your Flu Bin after Training!
Please pick up your Flu Bin after Training!
 Supply Room Hours q. Monday: 10 am-6 pm q. Tuesday: 8 am-4 pm q. Wednesday: 8 am-2 pm q. Thursday: 7: 30 am-3: 30 pm q. Friday: 7: 30 am-3 pm Supply Room hours will fluctuate during the slower parts of the season.
Supply Room Hours q. Monday: 10 am-6 pm q. Tuesday: 8 am-4 pm q. Wednesday: 8 am-2 pm q. Thursday: 7: 30 am-3: 30 pm q. Friday: 7: 30 am-3 pm Supply Room hours will fluctuate during the slower parts of the season.
 Supply Room Pick Up Lead nurses need to schedule an appointment to pick up supplies for their clinic(s) at least 2 full business days prior to the day you want to pick up. The system will NOT allow you to schedule an appointment less than 2 full days before. Plan Ahead! Step 1: After receiving an email stating that you are confirmed as the lead nurse to a clinic, log onto the Wellness Employee site. On the home page is a link to “Supply Pickup. ”
Supply Room Pick Up Lead nurses need to schedule an appointment to pick up supplies for their clinic(s) at least 2 full business days prior to the day you want to pick up. The system will NOT allow you to schedule an appointment less than 2 full days before. Plan Ahead! Step 1: After receiving an email stating that you are confirmed as the lead nurse to a clinic, log onto the Wellness Employee site. On the home page is a link to “Supply Pickup. ”
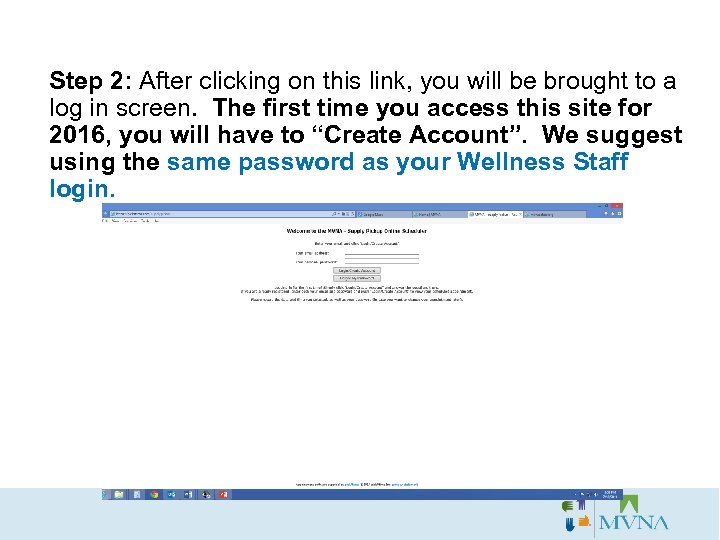 Step 2: After clicking on this link, you will be brought to a log in screen. The first time you access this site for 2016, you will have to “Create Account”. We suggest using the same password as your Wellness Staff login.
Step 2: After clicking on this link, you will be brought to a log in screen. The first time you access this site for 2016, you will have to “Create Account”. We suggest using the same password as your Wellness Staff login.
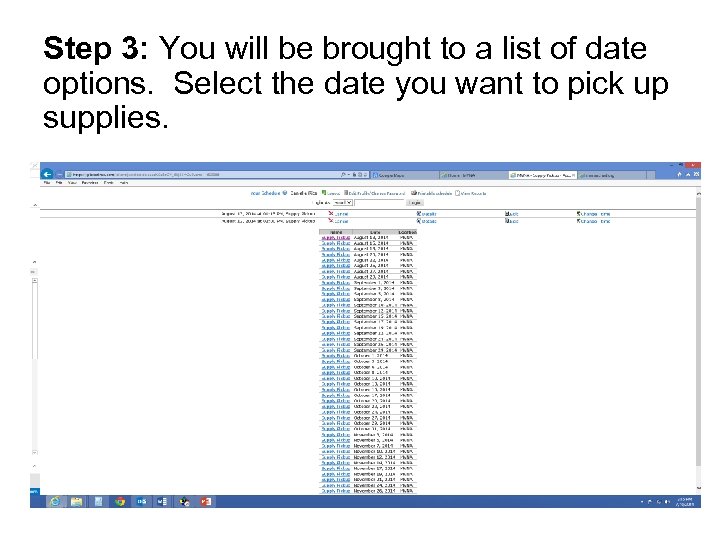 Step 3: You will be brought to a list of date options. Select the date you want to pick up supplies.
Step 3: You will be brought to a list of date options. Select the date you want to pick up supplies.
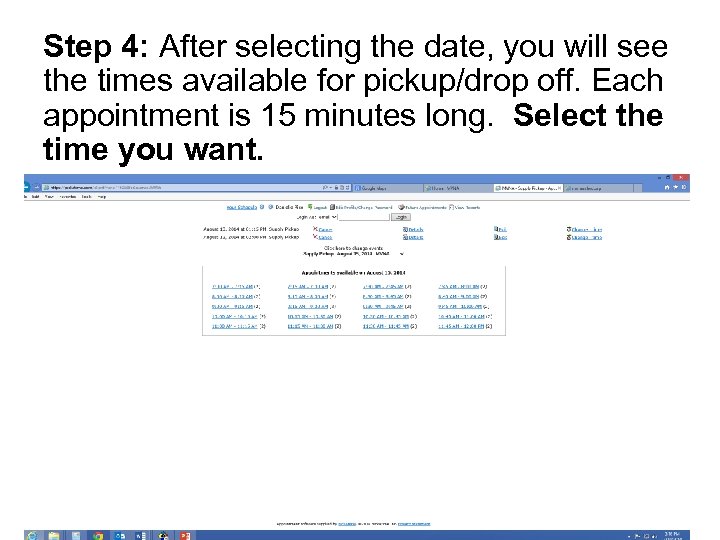 Step 4: After selecting the date, you will see the times available for pickup/drop off. Each appointment is 15 minutes long. Select the time you want.
Step 4: After selecting the date, you will see the times available for pickup/drop off. Each appointment is 15 minutes long. Select the time you want.
 Step 5: Use the drop down boxes to identify if you are Picking Up Supplies and/or Returning Supplies. In the large text box, list the Clinic Numbers and the Clinic Dates for each clinic you are picking up. You can schedule when you want to get the reminder email. Click Create Appointment when done NOTE: Wrong/Incomplete information will delay your appointment!
Step 5: Use the drop down boxes to identify if you are Picking Up Supplies and/or Returning Supplies. In the large text box, list the Clinic Numbers and the Clinic Dates for each clinic you are picking up. You can schedule when you want to get the reminder email. Click Create Appointment when done NOTE: Wrong/Incomplete information will delay your appointment!
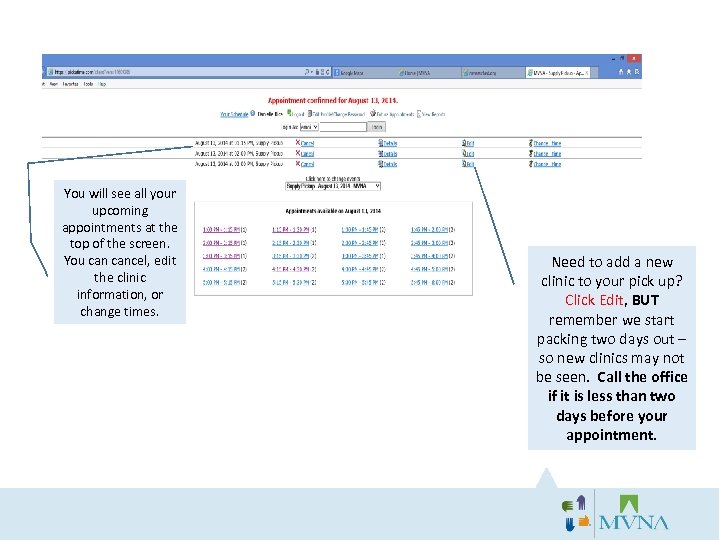 You will see all your upcoming appointments at the top of the screen. You cancel, edit the clinic information, or change times. Need to add a new clinic to your pick up? Click Edit, BUT remember we start packing two days out – so new clinics may not be seen. Call the office if it is less than two days before your appointment.
You will see all your upcoming appointments at the top of the screen. You cancel, edit the clinic information, or change times. Need to add a new clinic to your pick up? Click Edit, BUT remember we start packing two days out – so new clinics may not be seen. Call the office if it is less than two days before your appointment.
 Supply room protocol: Do you have everything? ü Clinic account sheet and special instructions ü Vaccines, syringes ü Gray bin (open house pick up) ü Epi Kit It is your responsibility to let the MVNA Flu Room staff know when you need additional supplies. Please do everything in your power to make and keep your appointment. Arriving early or late can cause issues with the flow of the flu room. Also, if you don’t have an appointment there may be no one here to hand out the vaccine. Please do not keep extra supplies or stock up. If you keep extra supplies or pick up extra supplies not needed, it causes many issues for office staff and your teammates. Plan accordingly, and inventory your bin often!!
Supply room protocol: Do you have everything? ü Clinic account sheet and special instructions ü Vaccines, syringes ü Gray bin (open house pick up) ü Epi Kit It is your responsibility to let the MVNA Flu Room staff know when you need additional supplies. Please do everything in your power to make and keep your appointment. Arriving early or late can cause issues with the flow of the flu room. Also, if you don’t have an appointment there may be no one here to hand out the vaccine. Please do not keep extra supplies or stock up. If you keep extra supplies or pick up extra supplies not needed, it causes many issues for office staff and your teammates. Plan accordingly, and inventory your bin often!!
 VACCINE STORAGE & TRANSPORT
VACCINE STORAGE & TRANSPORT
 Vaccine Storage All vaccines must be transported in a hard-sided cooler, with walls 2” thick, with proper insulation. Vaccine will be stored in this same cooler at onsite clinics. This cooler should be dedicated to vaccine only. *Medium sized cooler is usually sufficient. Flu Vaccine is stored at 2 -8⁰C. Aim of 6⁰ C
Vaccine Storage All vaccines must be transported in a hard-sided cooler, with walls 2” thick, with proper insulation. Vaccine will be stored in this same cooler at onsite clinics. This cooler should be dedicated to vaccine only. *Medium sized cooler is usually sufficient. Flu Vaccine is stored at 2 -8⁰C. Aim of 6⁰ C
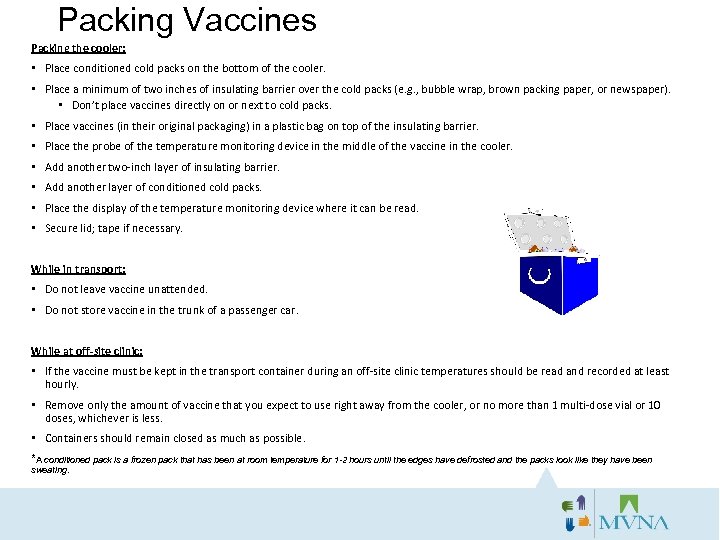 Packing Vaccines Packing the cooler: • Place conditioned cold packs on the bottom of the cooler. • Place a minimum of two inches of insulating barrier over the cold packs (e. g. , bubble wrap, brown packing paper, or newspaper). • Don’t place vaccines directly on or next to cold packs. • Place vaccines (in their original packaging) in a plastic bag on top of the insulating barrier. • Place the probe of the temperature monitoring device in the middle of the vaccine in the cooler. • Add another two-inch layer of insulating barrier. • Add another layer of conditioned cold packs. • Place the display of the temperature monitoring device where it can be read. • Secure lid; tape if necessary. While in transport: • Do not leave vaccine unattended. • Do not store vaccine in the trunk of a passenger car. While at off-site clinic: • If the vaccine must be kept in the transport container during an off-site clinic temperatures should be read and recorded at least hourly. • Remove only the amount of vaccine that you expect to use right away from the cooler, or no more than 1 multi-dose vial or 10 doses, whichever is less. • Containers should remain closed as much as possible. *A conditioned pack is a frozen pack that has been at room temperature for 1 -2 hours until the edges have defrosted and the packs look like they have been sweating.
Packing Vaccines Packing the cooler: • Place conditioned cold packs on the bottom of the cooler. • Place a minimum of two inches of insulating barrier over the cold packs (e. g. , bubble wrap, brown packing paper, or newspaper). • Don’t place vaccines directly on or next to cold packs. • Place vaccines (in their original packaging) in a plastic bag on top of the insulating barrier. • Place the probe of the temperature monitoring device in the middle of the vaccine in the cooler. • Add another two-inch layer of insulating barrier. • Add another layer of conditioned cold packs. • Place the display of the temperature monitoring device where it can be read. • Secure lid; tape if necessary. While in transport: • Do not leave vaccine unattended. • Do not store vaccine in the trunk of a passenger car. While at off-site clinic: • If the vaccine must be kept in the transport container during an off-site clinic temperatures should be read and recorded at least hourly. • Remove only the amount of vaccine that you expect to use right away from the cooler, or no more than 1 multi-dose vial or 10 doses, whichever is less. • Containers should remain closed as much as possible. *A conditioned pack is a frozen pack that has been at room temperature for 1 -2 hours until the edges have defrosted and the packs look like they have been sweating.
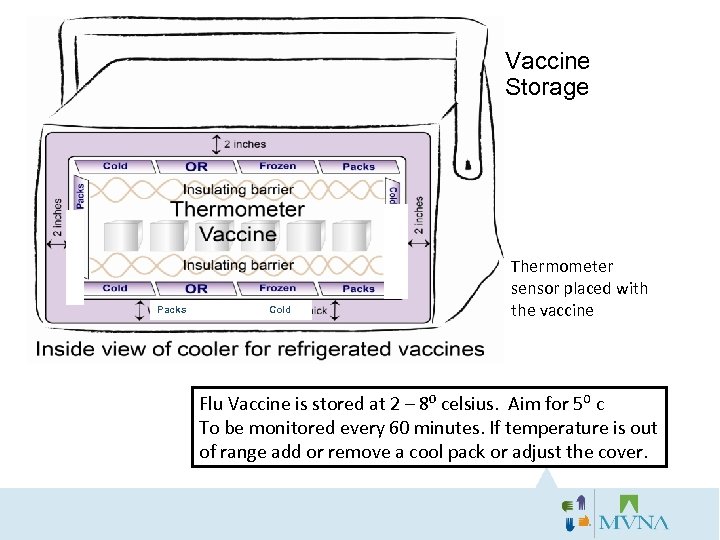 Vaccine Storage s k jf k h g d d Packs Cold Thermometer sensor placed with the vaccine Flu Vaccine is stored at 2 – 8⁰ celsius. Aim for 5⁰ c To be monitored every 60 minutes. If temperature is out of range add or remove a cool pack or adjust the cover.
Vaccine Storage s k jf k h g d d Packs Cold Thermometer sensor placed with the vaccine Flu Vaccine is stored at 2 – 8⁰ celsius. Aim for 5⁰ c To be monitored every 60 minutes. If temperature is out of range add or remove a cool pack or adjust the cover.
 Thermometer Flu Vaccine is stored at 2⁰-8⁰C. Aim of 6⁰ C To be monitored every 60 minutes. If temperature is out of range add or remove a cool pack. • There is the current temp reading and the min/max. The min/max can be cleared at any time to record only what happens after that point. • Always have the alarm on while the vaccine is in the cooler and while being stored at home. • Vaccine in a cooler must be checked and recorded every hour. Only the current temp is needed. • Vaccine in a fridge must be checked and recorded every AM and PM. You must record the min/max and the current actual temp. • Always keep the alarm on so you are alerted to temp fluxuations. Act accordingly (details next page). • For directions on how to use your thermometer please refer to instructions found in your folder/gray bin. • If therm alarms, the alarm will sound for 1 minute and issue a 3 second repeater beep every minute thereafter, for up to 12 hours. The alarm will continue to sound even if the current temp display returns to “in range” • To temporarily silence the alarm press either the HI or LO button on the back of the unit. . The unit is still active and the alarm will sound again if the temp goes out of range.
Thermometer Flu Vaccine is stored at 2⁰-8⁰C. Aim of 6⁰ C To be monitored every 60 minutes. If temperature is out of range add or remove a cool pack. • There is the current temp reading and the min/max. The min/max can be cleared at any time to record only what happens after that point. • Always have the alarm on while the vaccine is in the cooler and while being stored at home. • Vaccine in a cooler must be checked and recorded every hour. Only the current temp is needed. • Vaccine in a fridge must be checked and recorded every AM and PM. You must record the min/max and the current actual temp. • Always keep the alarm on so you are alerted to temp fluxuations. Act accordingly (details next page). • For directions on how to use your thermometer please refer to instructions found in your folder/gray bin. • If therm alarms, the alarm will sound for 1 minute and issue a 3 second repeater beep every minute thereafter, for up to 12 hours. The alarm will continue to sound even if the current temp display returns to “in range” • To temporarily silence the alarm press either the HI or LO button on the back of the unit. . The unit is still active and the alarm will sound again if the temp goes out of range.
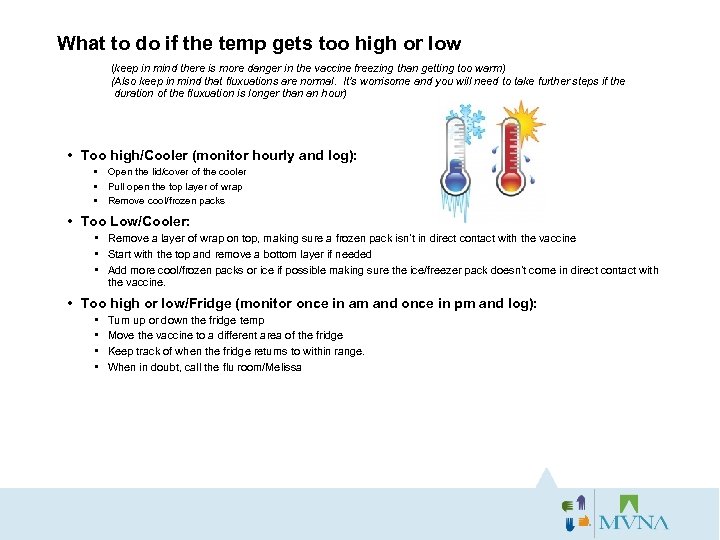 What to do if the temp gets too high or low (keep in mind there is more danger in the vaccine freezing than getting too warm) (Also keep in mind that fluxuations are normal. It’s worrisome and you will need to take further steps if the duration of the fluxuation is longer than an hour) • Too high/Cooler (monitor hourly and log): • Open the lid/cover of the cooler • Pull open the top layer of wrap • Remove cool/frozen packs • Too Low/Cooler: • Remove a layer of wrap on top, making sure a frozen pack isn’t in direct contact with the vaccine • Start with the top and remove a bottom layer if needed • Add more cool/frozen packs or ice if possible making sure the ice/freezer pack doesn’t come in direct contact with the vaccine. • Too high or low/Fridge (monitor once in am and once in pm and log): • • Turn up or down the fridge temp Move the vaccine to a different area of the fridge Keep track of when the fridge returns to within range. When in doubt, call the flu room/Melissa
What to do if the temp gets too high or low (keep in mind there is more danger in the vaccine freezing than getting too warm) (Also keep in mind that fluxuations are normal. It’s worrisome and you will need to take further steps if the duration of the fluxuation is longer than an hour) • Too high/Cooler (monitor hourly and log): • Open the lid/cover of the cooler • Pull open the top layer of wrap • Remove cool/frozen packs • Too Low/Cooler: • Remove a layer of wrap on top, making sure a frozen pack isn’t in direct contact with the vaccine • Start with the top and remove a bottom layer if needed • Add more cool/frozen packs or ice if possible making sure the ice/freezer pack doesn’t come in direct contact with the vaccine. • Too high or low/Fridge (monitor once in am and once in pm and log): • • Turn up or down the fridge temp Move the vaccine to a different area of the fridge Keep track of when the fridge returns to within range. When in doubt, call the flu room/Melissa
 Home Fridge Storage Protocol • Vaccine should be removed from the cooler and transferred to the fridge as soon as you get home. • Vaccine should be double-bagged. • Combination refridgerator/freezers are acceptable. Dorm sized fridges are never appropriate to use for vaccine storage. • You should clear a shelf for vaccine only. • If you have a freezer on the top part of your combination fridge, do not store the vaccine on the top shelf of the fridge. • Second shelf is optimal placement of vaccine, stacked at least an inch away from the walls. • Important: Place the vaccine sensor right next to the vaccine so thermometer is showing the temp of the unit where the vaccine is placed, with the unit on the outside of the fridge so you can hear if the alarm goes off. • Place thermometer unit outside the fridge with the alarm on! • If alarm sounds, based on it being too high or too low, you will need to either adjust the placement of the vaccine or the temp of your fridge. • If these immediate steps do not correct the problem, contact Melissa Beebe or Danielle Rice for next steps.
Home Fridge Storage Protocol • Vaccine should be removed from the cooler and transferred to the fridge as soon as you get home. • Vaccine should be double-bagged. • Combination refridgerator/freezers are acceptable. Dorm sized fridges are never appropriate to use for vaccine storage. • You should clear a shelf for vaccine only. • If you have a freezer on the top part of your combination fridge, do not store the vaccine on the top shelf of the fridge. • Second shelf is optimal placement of vaccine, stacked at least an inch away from the walls. • Important: Place the vaccine sensor right next to the vaccine so thermometer is showing the temp of the unit where the vaccine is placed, with the unit on the outside of the fridge so you can hear if the alarm goes off. • Place thermometer unit outside the fridge with the alarm on! • If alarm sounds, based on it being too high or too low, you will need to either adjust the placement of the vaccine or the temp of your fridge. • If these immediate steps do not correct the problem, contact Melissa Beebe or Danielle Rice for next steps.
 VACCINE ADMINISTRATION & DOCUMENTATION Child/Teen/Adult
VACCINE ADMINISTRATION & DOCUMENTATION Child/Teen/Adult
 Anaphylaxis/Epinephrine Admin Anaphylaxis Sudden or gradual onset of generalized itching, redness, or hives; swelling of the lips, face, or throat; severe wheezing; shortness of breath; shock; abdominal cramping; or cardiovascular collapse üAssess patient to determine if cause is allergic reaction vs. psychological fright. üUse Epi if a severe allergic reaction, with either sudden or gradual onset of: Shortness of breath, wheezing, itching, redness, hives, swelling of lips, throat and face, shock, abdominal cramping, or cardiovascular shock. üClinic Lead or delegate calls 911. üObtain a set of vital signs. üDraw up epinephrine according to instructions in Epi kit. Epi is given IM or SQ, SQ being the preferred method of delivery for our product. üClinic Lead stays with patient until transfer of care to the paramedics or other health professional. üIf other MVNA employees present, maintain crowd control. üCall the office, complete VAERS and MVNA Reportable Occurrence forms once client is in care of paramedics. Please review full Epinephrine protocol/standing orders in blue Forms Folder
Anaphylaxis/Epinephrine Admin Anaphylaxis Sudden or gradual onset of generalized itching, redness, or hives; swelling of the lips, face, or throat; severe wheezing; shortness of breath; shock; abdominal cramping; or cardiovascular collapse üAssess patient to determine if cause is allergic reaction vs. psychological fright. üUse Epi if a severe allergic reaction, with either sudden or gradual onset of: Shortness of breath, wheezing, itching, redness, hives, swelling of lips, throat and face, shock, abdominal cramping, or cardiovascular shock. üClinic Lead or delegate calls 911. üObtain a set of vital signs. üDraw up epinephrine according to instructions in Epi kit. Epi is given IM or SQ, SQ being the preferred method of delivery for our product. üClinic Lead stays with patient until transfer of care to the paramedics or other health professional. üIf other MVNA employees present, maintain crowd control. üCall the office, complete VAERS and MVNA Reportable Occurrence forms once client is in care of paramedics. Please review full Epinephrine protocol/standing orders in blue Forms Folder
 How to use Epi ampule • • 1 m. L of Epinephrine in every ampule. Place filtered needle on syringe. Clean ampule with alcohol using friction. Place gauze over ampule, grasp the neck of the ampule, snap the neck quickly and firmly away from hands. • Place the filtered needle in the ampule to draw up the epi, avoiding the rim. • When correct dosage is drawn, carefully cap the filtered needle and remove it, replacing it with needle used for injection. • Administer proper dosage to patient, IM or SQ (SQ being the preferred route for product) • Read complete instructions in Blue Folder • Video Demo/Mick • The Epi in ampules needs to be protected from light. • Be sure to keep in the brown bag within the Epi kit.
How to use Epi ampule • • 1 m. L of Epinephrine in every ampule. Place filtered needle on syringe. Clean ampule with alcohol using friction. Place gauze over ampule, grasp the neck of the ampule, snap the neck quickly and firmly away from hands. • Place the filtered needle in the ampule to draw up the epi, avoiding the rim. • When correct dosage is drawn, carefully cap the filtered needle and remove it, replacing it with needle used for injection. • Administer proper dosage to patient, IM or SQ (SQ being the preferred route for product) • Read complete instructions in Blue Folder • Video Demo/Mick • The Epi in ampules needs to be protected from light. • Be sure to keep in the brown bag within the Epi kit.
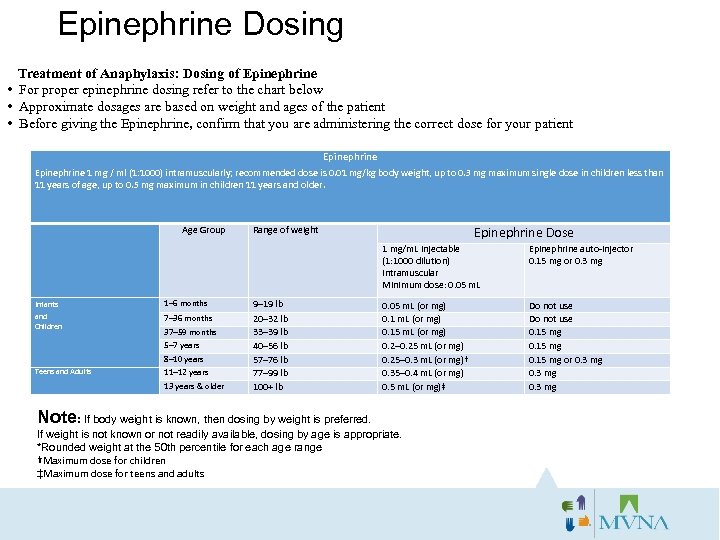 Epinephrine Dosing Treatment of Anaphylaxis: Dosing of Epinephrine • For proper epinephrine dosing refer to the chart below • Approximate dosages are based on weight and ages of the patient • Before giving the Epinephrine, confirm that you are administering the correct dose for your patient Epinephrine 1 mg / ml (1: 1000) intramuscularly; recommended dose is 0. 01 mg/kg body weight, up to 0. 3 mg maximum single dose in children less than 11 years of age, up to 0. 5 mg maximum in children 11 years and older. Age Group Epinephrine Dose Range of weight 1 mg/m. L injectable (1: 1000 dilution) intramuscular Minimum dose: 0. 05 m. L Infants 1– 6 months and Children 7– 36 months 37– 59 months 5– 7 years 8– 10 years Teens and Adults 11– 12 years 13 years & older 9– 19 lb 20– 32 lb 33– 39 lb 40– 56 lb 57– 76 lb 77– 99 lb 100+ lb Epinephrine auto-injector 0. 15 mg or 0. 3 mg 0. 05 m. L (or mg) 0. 15 m. L (or mg) 0. 2– 0. 25 m. L (or mg) 0. 25– 0. 3 m. L (or mg)† 0. 35– 0. 4 m. L (or mg) 0. 5 m. L (or mg)‡ Do not use 0. 15 mg or 0. 3 mg Note: If body weight is known, then dosing by weight is preferred. If weight is not known or not readily available, dosing by age is appropriate. *Rounded weight at the 50 th percentile for each age range †Maximum dose for children ‡Maximum dose for teens and adults
Epinephrine Dosing Treatment of Anaphylaxis: Dosing of Epinephrine • For proper epinephrine dosing refer to the chart below • Approximate dosages are based on weight and ages of the patient • Before giving the Epinephrine, confirm that you are administering the correct dose for your patient Epinephrine 1 mg / ml (1: 1000) intramuscularly; recommended dose is 0. 01 mg/kg body weight, up to 0. 3 mg maximum single dose in children less than 11 years of age, up to 0. 5 mg maximum in children 11 years and older. Age Group Epinephrine Dose Range of weight 1 mg/m. L injectable (1: 1000 dilution) intramuscular Minimum dose: 0. 05 m. L Infants 1– 6 months and Children 7– 36 months 37– 59 months 5– 7 years 8– 10 years Teens and Adults 11– 12 years 13 years & older 9– 19 lb 20– 32 lb 33– 39 lb 40– 56 lb 57– 76 lb 77– 99 lb 100+ lb Epinephrine auto-injector 0. 15 mg or 0. 3 mg 0. 05 m. L (or mg) 0. 15 m. L (or mg) 0. 2– 0. 25 m. L (or mg) 0. 25– 0. 3 m. L (or mg)† 0. 35– 0. 4 m. L (or mg) 0. 5 m. L (or mg)‡ Do not use 0. 15 mg or 0. 3 mg Note: If body weight is known, then dosing by weight is preferred. If weight is not known or not readily available, dosing by age is appropriate. *Rounded weight at the 50 th percentile for each age range †Maximum dose for children ‡Maximum dose for teens and adults
 Vaccine Handling • Inspect vaccine for damage or contamination prior to use. • Always check the label on the vial before removing the cap to make sure you have the correct vaccine type and check the expiration date. • Initial and date open vials, only if partial at end of clinic. • An open vial is good for 28 days after the date first used. Contaminated Vaccine: • Before you withdraw vaccine, you’ll need to shake the vial and visually inspect for particulate*. If it cannot be shaken into a relatively even suspension (i. e. it won’t disperse), it should not be used. • Label the vial with a red “waste” sticker (gray bin). Do not place in biohazard but return to MVNA. *Particulate in contaminated vaccine comes from incorrect temperature during storage
Vaccine Handling • Inspect vaccine for damage or contamination prior to use. • Always check the label on the vial before removing the cap to make sure you have the correct vaccine type and check the expiration date. • Initial and date open vials, only if partial at end of clinic. • An open vial is good for 28 days after the date first used. Contaminated Vaccine: • Before you withdraw vaccine, you’ll need to shake the vial and visually inspect for particulate*. If it cannot be shaken into a relatively even suspension (i. e. it won’t disperse), it should not be used. • Label the vial with a red “waste” sticker (gray bin). Do not place in biohazard but return to MVNA. *Particulate in contaminated vaccine comes from incorrect temperature during storage
 Screening Questions on Consent 107
Screening Questions on Consent 107
 Why those questions/how to assess? 1. If this is the first flu shot, the participant should stay in the area for 10 -15 minutes. 2. There is no evidence that acute illness reduces vaccine efficacy or increases vaccine adverse events. People with an acute febrile illness usually should not be vaccinated until their symptoms have improved. Minor illnesses with or with-out fever do not contraindicate use of influenza vaccine. Do not withhold vaccination if a person is taking antibiotics. 3. It is prudent to avoid vaccinating people who are not at high risk for severe influenza complications (see source 3) and who are known to have developed Guillain-Barré syndrome (GBS) within 6 weeks after receiving a previous influenza vaccination. As an alternative, physicians might consider using influenza antiviral chemoprophylaxis for these people. Although data are limited, the established benefits of influenza vaccination for the majority of people who have a history of GBS, and who are at high risk for severe complications from influenza, justify yearly vaccination. 4. Allergic reactions to any vaccine component can occur. The majority of reactions probably are caused by residual egg protein. Although most current influenza vaccines contain only a very small quantity of egg protein, this protein can induce immediate allergic reactions among people who have severe egg allergy. Some people who report allergy to egg might not be egg-allergic. If a person can eat lightly cooked eggs (e. g. , scrambled eggs), they are unlikely to have an egg allergy. However, people who can tolerate egg in baked products (e. g. , cake) might still have an egg allergy. With any doubt, refer them to their provider for a shot. Some inactivated influenza vaccines contain thimerosal as a preservative. Most people who had sensitivity to thimerosal when it was used in contact lens solution do not have reactions to thimerosal when it is used in vaccines. The multi-dose vials have thimerosal, but pre-filled syringes are preservative-free. In addition, some vaccines also contain latex in the prefilled syringe cap which may cause allergic reactions in latex sensitive people. A person with a latex allergy should get their shot from their provider. 5. Patients reporting a serious reaction to a previous dose of inactivated influenza vaccine should be asked to describe their symptoms. Immediate – presumably allergic – reactions are usually a contraindication to further vaccination against influenza. Fever, malaise, myalgia, and other systemic symptoms most often affect people who are first-time vaccinees. These mild-to-moderate local reactions are not a contraindication to future vaccination. Also, red eyes or mild upper facial swelling following vaccination with inactivated injectable influenza vaccine is most likely a coincidental event and not related to the vaccine; these people can receive injectable vaccine without further evaluation.
Why those questions/how to assess? 1. If this is the first flu shot, the participant should stay in the area for 10 -15 minutes. 2. There is no evidence that acute illness reduces vaccine efficacy or increases vaccine adverse events. People with an acute febrile illness usually should not be vaccinated until their symptoms have improved. Minor illnesses with or with-out fever do not contraindicate use of influenza vaccine. Do not withhold vaccination if a person is taking antibiotics. 3. It is prudent to avoid vaccinating people who are not at high risk for severe influenza complications (see source 3) and who are known to have developed Guillain-Barré syndrome (GBS) within 6 weeks after receiving a previous influenza vaccination. As an alternative, physicians might consider using influenza antiviral chemoprophylaxis for these people. Although data are limited, the established benefits of influenza vaccination for the majority of people who have a history of GBS, and who are at high risk for severe complications from influenza, justify yearly vaccination. 4. Allergic reactions to any vaccine component can occur. The majority of reactions probably are caused by residual egg protein. Although most current influenza vaccines contain only a very small quantity of egg protein, this protein can induce immediate allergic reactions among people who have severe egg allergy. Some people who report allergy to egg might not be egg-allergic. If a person can eat lightly cooked eggs (e. g. , scrambled eggs), they are unlikely to have an egg allergy. However, people who can tolerate egg in baked products (e. g. , cake) might still have an egg allergy. With any doubt, refer them to their provider for a shot. Some inactivated influenza vaccines contain thimerosal as a preservative. Most people who had sensitivity to thimerosal when it was used in contact lens solution do not have reactions to thimerosal when it is used in vaccines. The multi-dose vials have thimerosal, but pre-filled syringes are preservative-free. In addition, some vaccines also contain latex in the prefilled syringe cap which may cause allergic reactions in latex sensitive people. A person with a latex allergy should get their shot from their provider. 5. Patients reporting a serious reaction to a previous dose of inactivated influenza vaccine should be asked to describe their symptoms. Immediate – presumably allergic – reactions are usually a contraindication to further vaccination against influenza. Fever, malaise, myalgia, and other systemic symptoms most often affect people who are first-time vaccinees. These mild-to-moderate local reactions are not a contraindication to future vaccination. Also, red eyes or mild upper facial swelling following vaccination with inactivated injectable influenza vaccine is most likely a coincidental event and not related to the vaccine; these people can receive injectable vaccine without further evaluation.
 Immunization and Pregnancy CDC Recommendations • Influenza (the flu) is a serious illness, especially when you are pregnant. • FACT: The flu can cause serious illness in pregnant women. • Getting the flu can cause serious problems when you are pregnant. Even if you are generally healthy, changes in immune, heart, and lung functions during pregnancy make you more likely to get seriously ill from the flu. Pregnant women who get the flu are at higher risk of hospitalization, and even death, than non-pregnant women. Severe illness in the pregnant mother can also be dangerous to her fetus because it increases the chance for serious problems such as premature labor and delivery. • The flu shot is the best protection for you – and your baby. • FACT: Getting a flu shot is the first and most important step in protecting yourself against the flu. • When you get your flu shot, your body starts to make antibodies that help protect you against the flu. Antibodies can be passed on to your unborn baby, and help protect the baby for up to 6 months after he or she is born. This is important because babies younger than 6 months of age are too young to get a flu vaccine. If you breastfeed your infant, antibodies may also be passed in breast milk. • It takes about two weeks to make antibodies after getting flu vaccine. Talk to your doctor, nurse, or clinic about getting vaccinated as soon as you can. • The flu shot is safe for you and for your unborn child. • FACT: The flu shot is safe for pregnant and breastfeeding women and their infants. • You can receive the flu shot at any time, during any trimester, while you are pregnant. Millions of flu shots have been given to pregnant women over many years. Flu shots have not been shown to cause harm to pregnant women or their infants. • If you have your baby before getting your flu shot, you still need to get vaccinated. The flu is spread from person to person. You, or others who care for your baby, may get the flu, and pass it to the baby. Because babies younger than 6 months are too young to receive the vaccine, it is important that everyone who cares for your baby get a flu vaccine, including other household members, relatives, and babysitters. • FACT: The side effects of the flu vaccine are mild when compared to the disease itself. • After getting your flu shot, you may experience some mild side effects. The most common side effects include soreness, tenderness, redness and/or swelling where the shot was given. Sometimes you might have headache, muscle aches, fever, and nausea or feel tired.
Immunization and Pregnancy CDC Recommendations • Influenza (the flu) is a serious illness, especially when you are pregnant. • FACT: The flu can cause serious illness in pregnant women. • Getting the flu can cause serious problems when you are pregnant. Even if you are generally healthy, changes in immune, heart, and lung functions during pregnancy make you more likely to get seriously ill from the flu. Pregnant women who get the flu are at higher risk of hospitalization, and even death, than non-pregnant women. Severe illness in the pregnant mother can also be dangerous to her fetus because it increases the chance for serious problems such as premature labor and delivery. • The flu shot is the best protection for you – and your baby. • FACT: Getting a flu shot is the first and most important step in protecting yourself against the flu. • When you get your flu shot, your body starts to make antibodies that help protect you against the flu. Antibodies can be passed on to your unborn baby, and help protect the baby for up to 6 months after he or she is born. This is important because babies younger than 6 months of age are too young to get a flu vaccine. If you breastfeed your infant, antibodies may also be passed in breast milk. • It takes about two weeks to make antibodies after getting flu vaccine. Talk to your doctor, nurse, or clinic about getting vaccinated as soon as you can. • The flu shot is safe for you and for your unborn child. • FACT: The flu shot is safe for pregnant and breastfeeding women and their infants. • You can receive the flu shot at any time, during any trimester, while you are pregnant. Millions of flu shots have been given to pregnant women over many years. Flu shots have not been shown to cause harm to pregnant women or their infants. • If you have your baby before getting your flu shot, you still need to get vaccinated. The flu is spread from person to person. You, or others who care for your baby, may get the flu, and pass it to the baby. Because babies younger than 6 months are too young to receive the vaccine, it is important that everyone who cares for your baby get a flu vaccine, including other household members, relatives, and babysitters. • FACT: The side effects of the flu vaccine are mild when compared to the disease itself. • After getting your flu shot, you may experience some mild side effects. The most common side effects include soreness, tenderness, redness and/or swelling where the shot was given. Sometimes you might have headache, muscle aches, fever, and nausea or feel tired.
 Dosing for Influenza by Injection Pediatrics: • Age 6 -35 months, administer 0. 25 m. L vaccine IM • Age 3 years and older, administer 0. 5 m. L vaccine IM Children under the age of 9 who are receiving their first flu vaccine injection should be scheduled to return for a second dose of vaccine in one month. Adults: • Patients under the age of 65 administer 0. 5 m. L of vaccine IM • Age 65 and older, administer Fluzone High Dose 0. 5 m. L IM Recommended IM Site Selection: • Infants and young children: anterolateral aspect of the thigh • Older children and adults: the deltoid muscle
Dosing for Influenza by Injection Pediatrics: • Age 6 -35 months, administer 0. 25 m. L vaccine IM • Age 3 years and older, administer 0. 5 m. L vaccine IM Children under the age of 9 who are receiving their first flu vaccine injection should be scheduled to return for a second dose of vaccine in one month. Adults: • Patients under the age of 65 administer 0. 5 m. L of vaccine IM • Age 65 and older, administer Fluzone High Dose 0. 5 m. L IM Recommended IM Site Selection: • Infants and young children: anterolateral aspect of the thigh • Older children and adults: the deltoid muscle
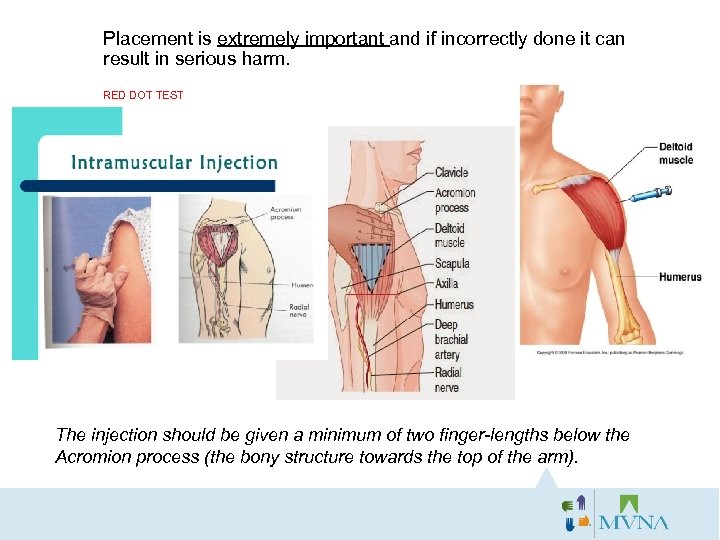 Placement is extremely important and if incorrectly done it can result in serious harm. RED DOT TEST The injection should be given a minimum of two finger-lengths below the Acromion process (the bony structure towards the top of the arm).
Placement is extremely important and if incorrectly done it can result in serious harm. RED DOT TEST The injection should be given a minimum of two finger-lengths below the Acromion process (the bony structure towards the top of the arm).
 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. • Landmark the injection site Prep site with alcohol swab and let air dry Draw up vaccine Use a needle long enough to reach deep into the muscle. Infants age 6 through 11 mos: 1"; 1 through 2 yrs: 1– 1¼"; children and adults 3 yrs and older: 1– 1½". With your left hand*, bunch up the muscle. With your right hand*, insert the needle at a 90° angle to the skin with a quick thrust. Push down on the plunger and inject the entire contents of the syringe using steady pressure. Needle will retract when still in the arm. There is no need to aspirate. Remove the needle and simultaneously apply pressure to the injection site with a dry cotton ball or gauze. Hold in place for several seconds. If there is any bleeding, cover the injection site with a bandage. Put the used syringe in a sharps container. Use the opposite hand if you are left-handed. Video (from Mick): • https: //www. youtube. com/watch? v=jdbo. I 3 SKg. R 0
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. • Landmark the injection site Prep site with alcohol swab and let air dry Draw up vaccine Use a needle long enough to reach deep into the muscle. Infants age 6 through 11 mos: 1"; 1 through 2 yrs: 1– 1¼"; children and adults 3 yrs and older: 1– 1½". With your left hand*, bunch up the muscle. With your right hand*, insert the needle at a 90° angle to the skin with a quick thrust. Push down on the plunger and inject the entire contents of the syringe using steady pressure. Needle will retract when still in the arm. There is no need to aspirate. Remove the needle and simultaneously apply pressure to the injection site with a dry cotton ball or gauze. Hold in place for several seconds. If there is any bleeding, cover the injection site with a bandage. Put the used syringe in a sharps container. Use the opposite hand if you are left-handed. Video (from Mick): • https: //www. youtube. com/watch? v=jdbo. I 3 SKg. R 0
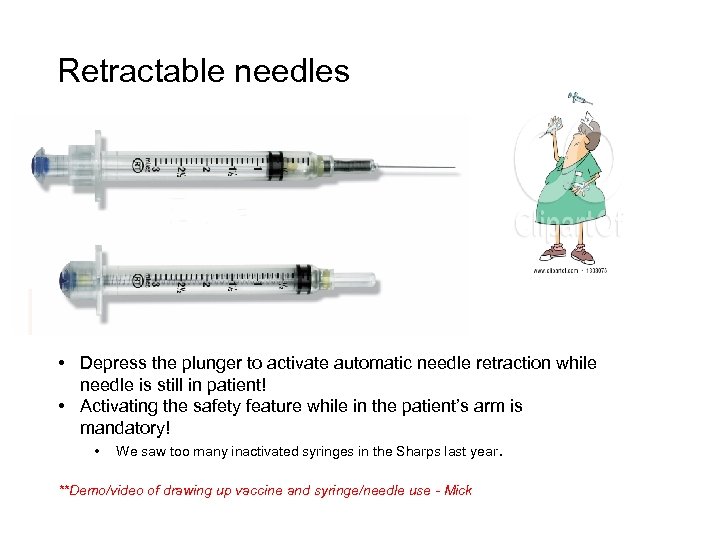 Retractable needles • Depress the plunger to activate automatic needle retraction while needle is still in patient! • Activating the safety feature while in the patient’s arm is mandatory! • We saw too many inactivated syringes in the Sharps last year. **Demo/video of drawing up vaccine and syringe/needle use - Mick
Retractable needles • Depress the plunger to activate automatic needle retraction while needle is still in patient! • Activating the safety feature while in the patient’s arm is mandatory! • We saw too many inactivated syringes in the Sharps last year. **Demo/video of drawing up vaccine and syringe/needle use - Mick
 Right Documentation After giving the immunization the nurse documents: üDose üSite üTime üVaccine lot number üVaccine expiration date üThat a VIS statement was offered to client üFull signature with credentials i. e. Nancy RN Nurse,
Right Documentation After giving the immunization the nurse documents: üDose üSite üTime üVaccine lot number üVaccine expiration date üThat a VIS statement was offered to client üFull signature with credentials i. e. Nancy RN Nurse,
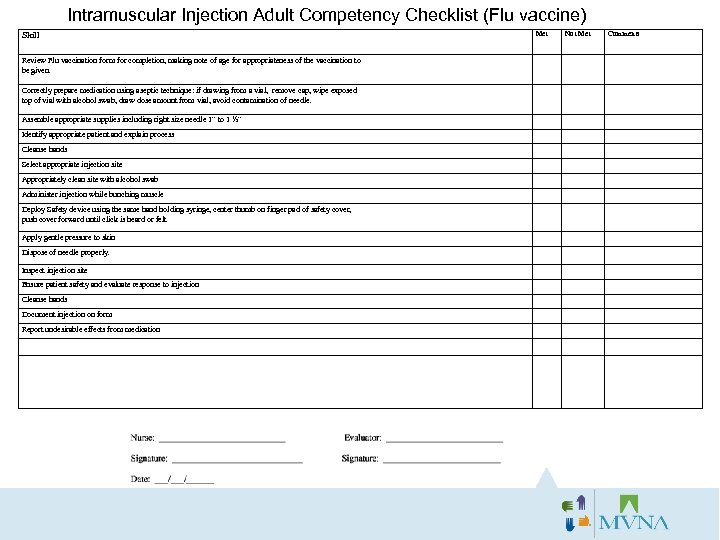 Intramuscular Injection Adult Competency Checklist (Flu vaccine) Skill Met Not Met Comments Review Flu vaccination form for completion, making note of age for appropriateness of the vaccination to be given. Correctly prepare medication using aseptic technique: if drawing from a vial, remove cap, wipe exposed top of vial with alcohol swab, draw dose amount from vial, avoid contamination of needle. Assemble appropriate supplies including right size needle 1” to 1 ½” Identify appropriate patient and explain process Cleanse hands Select appropriate injection site Appropriately clean site with alcohol swab Administer injection while bunching muscle Deploy Safety device using the same hand holding syringe, center thumb on finger pad of safety cover, push cover forward until click is heard or felt. Apply gentle pressure to skin Dispose of needle properly. Inspect injection site Ensure patient safety and evaluate response to injection Cleanse hands Document injection on form Report undesirable effects from medication
Intramuscular Injection Adult Competency Checklist (Flu vaccine) Skill Met Not Met Comments Review Flu vaccination form for completion, making note of age for appropriateness of the vaccination to be given. Correctly prepare medication using aseptic technique: if drawing from a vial, remove cap, wipe exposed top of vial with alcohol swab, draw dose amount from vial, avoid contamination of needle. Assemble appropriate supplies including right size needle 1” to 1 ½” Identify appropriate patient and explain process Cleanse hands Select appropriate injection site Appropriately clean site with alcohol swab Administer injection while bunching muscle Deploy Safety device using the same hand holding syringe, center thumb on finger pad of safety cover, push cover forward until click is heard or felt. Apply gentle pressure to skin Dispose of needle properly. Inspect injection site Ensure patient safety and evaluate response to injection Cleanse hands Document injection on form Report undesirable effects from medication
 Syringe Malfunction v. If unable to deploy vaccine after client has been stuck, explain situation and offer to vaccinate with another syringe. v. Document on consent that syringe malfunctioned. v. Complete MVNA Reportable Occurrence form. v. Return the Reportable Occurrence form and syringe to the office and ensure there is no danger for needle stick when returning the syringe.
Syringe Malfunction v. If unable to deploy vaccine after client has been stuck, explain situation and offer to vaccinate with another syringe. v. Document on consent that syringe malfunctioned. v. Complete MVNA Reportable Occurrence form. v. Return the Reportable Occurrence form and syringe to the office and ensure there is no danger for needle stick when returning the syringe.
 SHARPS • • • Never put your hand inside the container Only place sharps in the container • Alcohol, needle caps, gauze, etc. goes in regular garbage • Empty vaccine vials may go in regular garbage Do not over-fill Don’t shut and lock it before it is full Put your sharps in the trunk during transport – this is a DOT rule! If you experience a needlestick or sharps injury or were exposed to the blood or other body fluid of a patient during the course of your work, immediately follow these steps: Wash needlesticks and cuts with soap and water Flush splashes to the nose, mouth, or skin with water Irrigate eyes with clean water, saline, or sterile irrigants Report the incident to your supervisor Immediately seek medical treatment
SHARPS • • • Never put your hand inside the container Only place sharps in the container • Alcohol, needle caps, gauze, etc. goes in regular garbage • Empty vaccine vials may go in regular garbage Do not over-fill Don’t shut and lock it before it is full Put your sharps in the trunk during transport – this is a DOT rule! If you experience a needlestick or sharps injury or were exposed to the blood or other body fluid of a patient during the course of your work, immediately follow these steps: Wash needlesticks and cuts with soap and water Flush splashes to the nose, mouth, or skin with water Irrigate eyes with clean water, saline, or sterile irrigants Report the incident to your supervisor Immediately seek medical treatment
 Infection control is always essential! > Be aware of your needle at all times don’t uncap until ready to use. > Sanitize hands between clients, gloving is not required. > If visibly spoiled washing with soap and water is required.
Infection control is always essential! > Be aware of your needle at all times don’t uncap until ready to use. > Sanitize hands between clients, gloving is not required. > If visibly spoiled washing with soap and water is required.
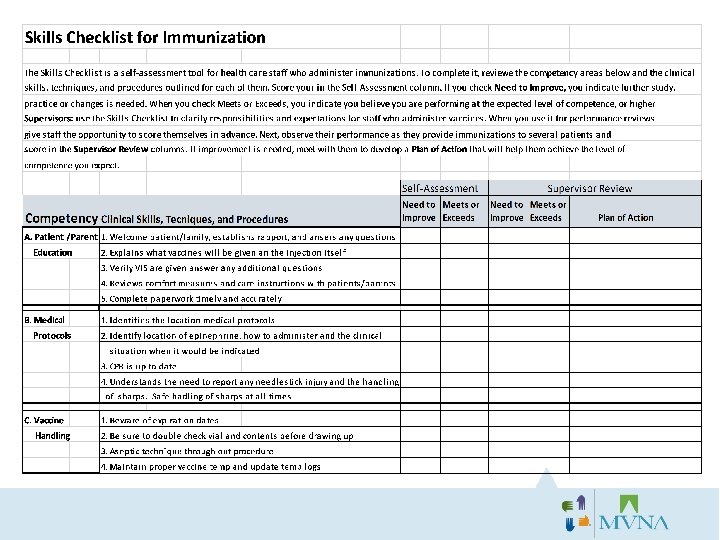
 Things to remember! • Do not pre-fill syringes • Fill every syringe in front of the patient • Do not pre-open syringes from package • Open the syringe in front of the patient • Do not uncap the needle until immediately before injection • Do not pull vaccine from one vial and put it in another vial • If there is not a full dose in one, pull the remainder from that vial and complete the proper dose with the next vial.
Things to remember! • Do not pre-fill syringes • Fill every syringe in front of the patient • Do not pre-open syringes from package • Open the syringe in front of the patient • Do not uncap the needle until immediately before injection • Do not pull vaccine from one vial and put it in another vial • If there is not a full dose in one, pull the remainder from that vial and complete the proper dose with the next vial.
 Mercury • There is a large body of scientific evidence on the safety of thimerosal. Data from several studies show the low doses of thimerosal in vaccines do not cause harm, and are only associated with minor local injection site reactions like redness and swelling at the injection site (CDC). • The type of mercury linked with nervous system damage is methyl mercury. Concerns over methyl mercury levels have led to recommendations that pregnant women avoid eating large amounts of certain types of fish, such as swordfish. • In contrast, thimerosal is an ethyl mercury compound. • Still, because the preservative raised controversy, especially over a now-disproven link to autism, it was taken out of almost all U. S. vaccines starting in 2001. • There is a tiny amount of thimerosal in the multidose bottles. The preservative ensures that no bacteria will grow in the vaccine. The individual-dose vaccines contain no thimerosal. • The nasal spray form of the flu vaccine contains no thimerosal.
Mercury • There is a large body of scientific evidence on the safety of thimerosal. Data from several studies show the low doses of thimerosal in vaccines do not cause harm, and are only associated with minor local injection site reactions like redness and swelling at the injection site (CDC). • The type of mercury linked with nervous system damage is methyl mercury. Concerns over methyl mercury levels have led to recommendations that pregnant women avoid eating large amounts of certain types of fish, such as swordfish. • In contrast, thimerosal is an ethyl mercury compound. • Still, because the preservative raised controversy, especially over a now-disproven link to autism, it was taken out of almost all U. S. vaccines starting in 2001. • There is a tiny amount of thimerosal in the multidose bottles. The preservative ensures that no bacteria will grow in the vaccine. The individual-dose vaccines contain no thimerosal. • The nasal spray form of the flu vaccine contains no thimerosal.
 Is there in vaccines? v. It is actually true that some vaccines contain porcine gelatin. They include Fluzone (only the regular product, not high-dose) and Flu. Mist. v. Because of the concerns about this from the global Muslim community about this and other medical products that use gelatin (e. g. , gel capsules), the WHO asked the Muslim scholar community to review this situation. They determined that the processing that occurs in the manufacturing transforms the pork, an impure or unclean product, into gelatin, a pure or clean product, so that it is acceptable for a practicing Muslim to use.
Is there in vaccines? v. It is actually true that some vaccines contain porcine gelatin. They include Fluzone (only the regular product, not high-dose) and Flu. Mist. v. Because of the concerns about this from the global Muslim community about this and other medical products that use gelatin (e. g. , gel capsules), the WHO asked the Muslim scholar community to review this situation. They determined that the processing that occurs in the manufacturing transforms the pork, an impure or unclean product, into gelatin, a pure or clean product, so that it is acceptable for a practicing Muslim to use.
 What’s in my gray bin? • Video
What’s in my gray bin? • Video
 Questions?
Questions?


