a6076d9ef46d1f6ea75bc3d8e7e96ab3.ppt
- Количество слайдов: 57
 Flow Cytometry and its Applications
Flow Cytometry and its Applications
 I can not trust FACS data on multicolor experiment, because……
I can not trust FACS data on multicolor experiment, because……
 1. What is Flow Cytometry? 2. How does a Flow Cytometer work? Fluidics and Optics 3. Dye and Single Color Compensation 4. Sample Preparation for Flow Cytometry 5. Applications
1. What is Flow Cytometry? 2. How does a Flow Cytometer work? Fluidics and Optics 3. Dye and Single Color Compensation 4. Sample Preparation for Flow Cytometry 5. Applications
 What is Flow Cytometry? • Cytometry refers to the measurement of physical/chemical characteristics of cells or other biological particles. • Flow Cytometry is the process whereby such measurements are made upon cells/particles as they pass through a measuring apparatus (hopefully in single file) suspended in a fluid stream. 1968, Wolfgang Gohde from the University of Munster (Patent No. DE 1815352) named ‘pulse cytophotometry” 1978, the name was changed to ‘flow cytometry’ • Flow Sorting (Flow Cytometric Cell Sorting) extends flow cytometry with the additional capacity to divert and collect cells exhibiting an identifiable set of characteristics either mechanically or by electrical means (Flow Cytometric Analysis). • FACS - Fluorescence Activated Cell Sorting? FACS is a trademark of Becton Dickinson Immunocytometry Systems (BDIS). All FACS instruments are BDIS systems, but not all cytometers are FACS. Adapted from ppt by Dr. EDWARD F. SROUR
What is Flow Cytometry? • Cytometry refers to the measurement of physical/chemical characteristics of cells or other biological particles. • Flow Cytometry is the process whereby such measurements are made upon cells/particles as they pass through a measuring apparatus (hopefully in single file) suspended in a fluid stream. 1968, Wolfgang Gohde from the University of Munster (Patent No. DE 1815352) named ‘pulse cytophotometry” 1978, the name was changed to ‘flow cytometry’ • Flow Sorting (Flow Cytometric Cell Sorting) extends flow cytometry with the additional capacity to divert and collect cells exhibiting an identifiable set of characteristics either mechanically or by electrical means (Flow Cytometric Analysis). • FACS - Fluorescence Activated Cell Sorting? FACS is a trademark of Becton Dickinson Immunocytometry Systems (BDIS). All FACS instruments are BDIS systems, but not all cytometers are FACS. Adapted from ppt by Dr. EDWARD F. SROUR
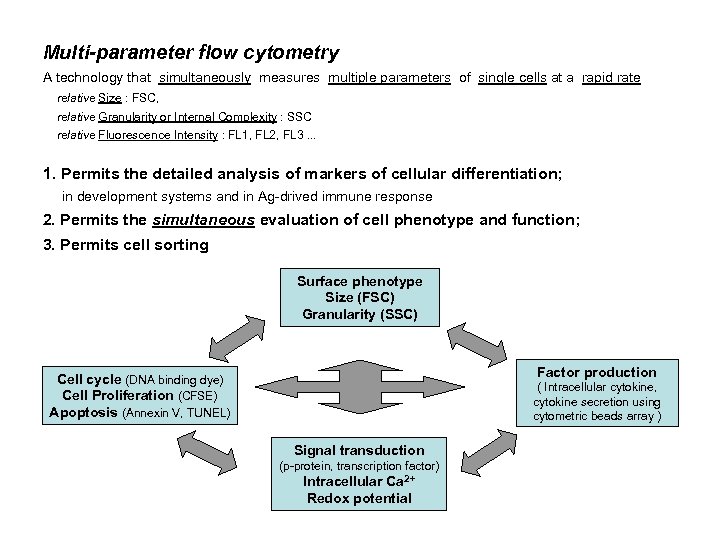 Multi-parameter flow cytometry A technology that simultaneously measures multiple parameters of single cells at a rapid rate relative Size : FSC, relative Granularity or Internal Complexity : SSC relative Fluorescence Intensity : FL 1, FL 2, FL 3. . . 1. Permits the detailed analysis of markers of cellular differentiation; in development systems and in Ag-drived immune response 2. Permits the simultaneous evaluation of cell phenotype and function; 3. Permits cell sorting Surface phenotype Size (FSC) Granularity (SSC) Factor production Cell cycle (DNA binding dye) Cell Proliferation (CFSE) Apoptosis (Annexin V, TUNEL) ( Intracellular cytokine, cytokine secretion using cytometric beads array ) Signal transduction (p-protein, transcription factor) Intracellular Ca 2+ Redox potential
Multi-parameter flow cytometry A technology that simultaneously measures multiple parameters of single cells at a rapid rate relative Size : FSC, relative Granularity or Internal Complexity : SSC relative Fluorescence Intensity : FL 1, FL 2, FL 3. . . 1. Permits the detailed analysis of markers of cellular differentiation; in development systems and in Ag-drived immune response 2. Permits the simultaneous evaluation of cell phenotype and function; 3. Permits cell sorting Surface phenotype Size (FSC) Granularity (SSC) Factor production Cell cycle (DNA binding dye) Cell Proliferation (CFSE) Apoptosis (Annexin V, TUNEL) ( Intracellular cytokine, cytokine secretion using cytometric beads array ) Signal transduction (p-protein, transcription factor) Intracellular Ca 2+ Redox potential
 How does a Flow Cytometer work? (Fluidics and Optics)
How does a Flow Cytometer work? (Fluidics and Optics)
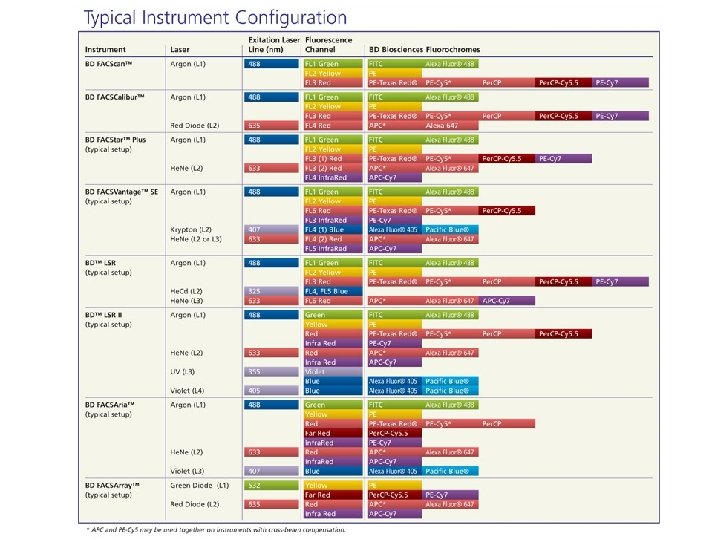
 Flow Cytometer Beckman Coulter Epics Altra BD FACS Calibur 2 laser 4 colors BD FACS Canto II > 2 laser > 6 -7 colors BD LSRII and LSRII Green 4 laser LSRII; 488, 633, 405, 355 LSRII Green; 405, 488, 532, 635 12 colors *DPSS (diode-pumped solid state) 532 nm
Flow Cytometer Beckman Coulter Epics Altra BD FACS Calibur 2 laser 4 colors BD FACS Canto II > 2 laser > 6 -7 colors BD LSRII and LSRII Green 4 laser LSRII; 488, 633, 405, 355 LSRII Green; 405, 488, 532, 635 12 colors *DPSS (diode-pumped solid state) 532 nm
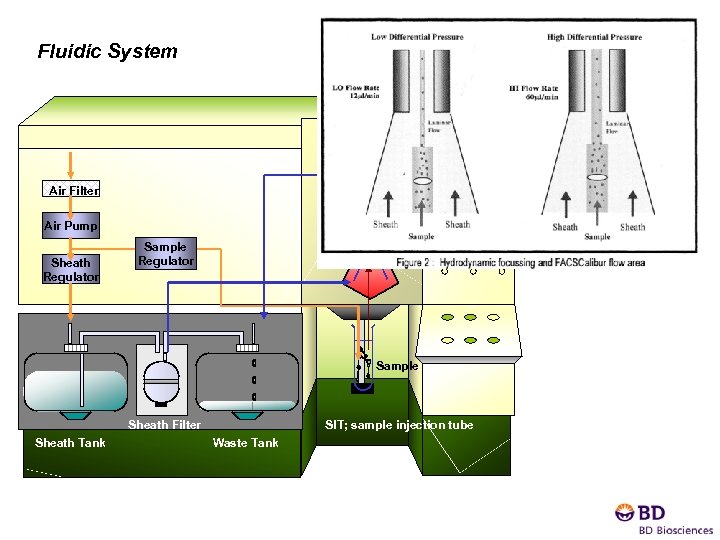 Fluidic System Air Filter Air Pump Sheath Regulator Flow Cell Sample Regulator Sample Sheath Filter Sheath Tank SIT; sample injection tube Waste Tank
Fluidic System Air Filter Air Pump Sheath Regulator Flow Cell Sample Regulator Sample Sheath Filter Sheath Tank SIT; sample injection tube Waste Tank
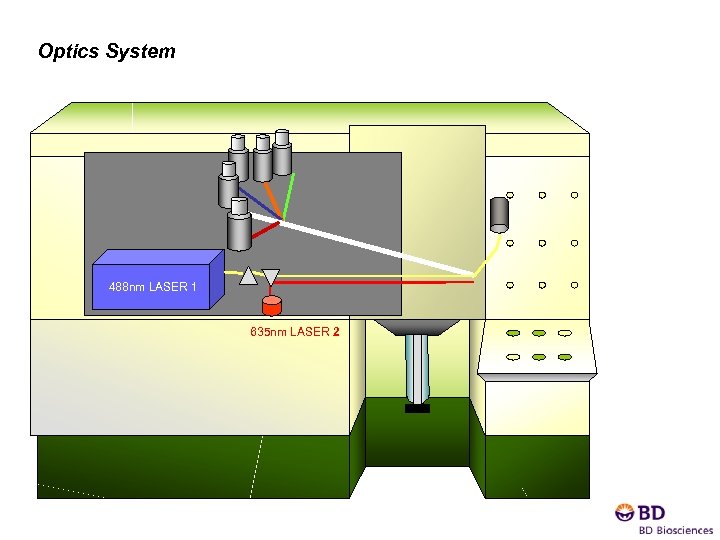 Optics System 488 nm LASER 1 635 nm LASER 2
Optics System 488 nm LASER 1 635 nm LASER 2
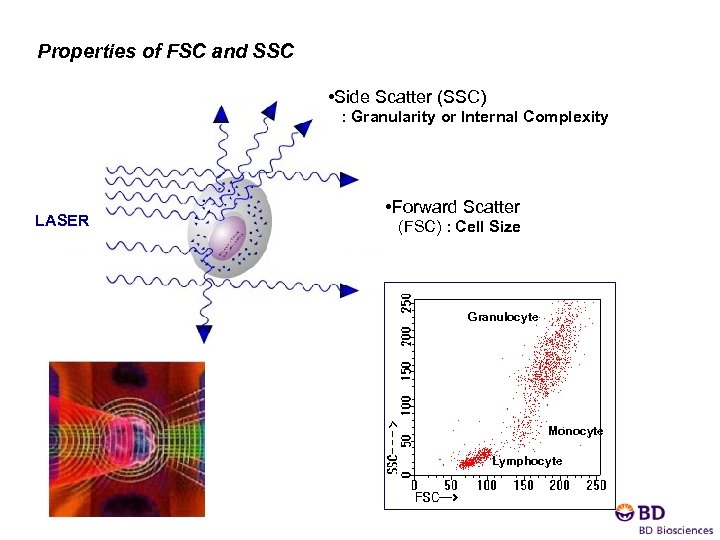 Properties of FSC and SSC : Granularity or Internal Complexity • Side Scatter (SSC) : Granularity or Internal Complexity LASER • Forward Scatter : size (FSC) : Cell Size Granulocyte Monocyte Lymphocyte
Properties of FSC and SSC : Granularity or Internal Complexity • Side Scatter (SSC) : Granularity or Internal Complexity LASER • Forward Scatter : size (FSC) : Cell Size Granulocyte Monocyte Lymphocyte
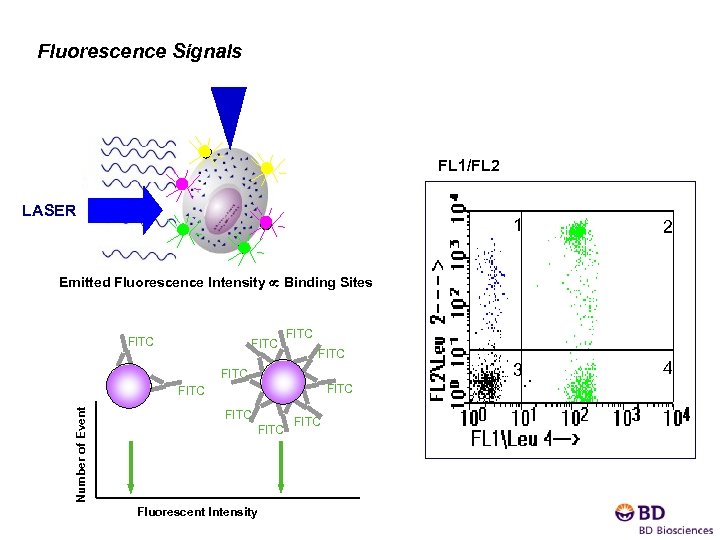 Fluorescence Signals FL 1/FL 2 LASER 1 2 3 4 Emitted Fluorescence Intensity Binding Sites FITC FITC Number of Event FITC Fluorescent Intensity FITC
Fluorescence Signals FL 1/FL 2 LASER 1 2 3 4 Emitted Fluorescence Intensity Binding Sites FITC FITC Number of Event FITC Fluorescent Intensity FITC
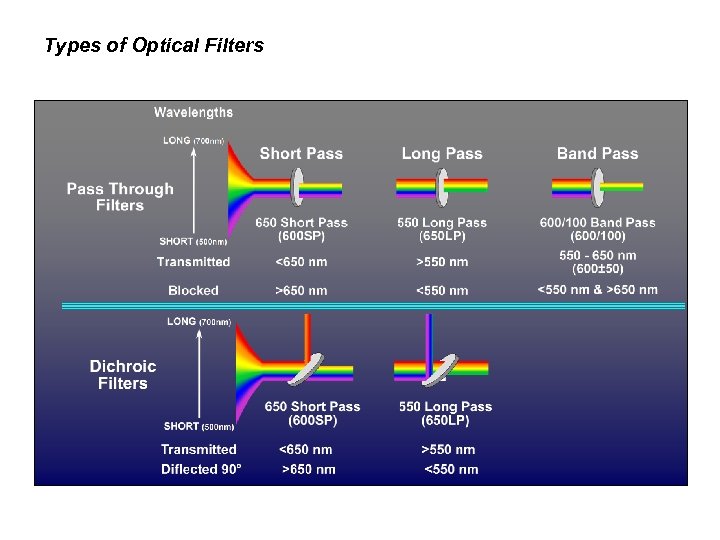 Types of Optical Filters
Types of Optical Filters
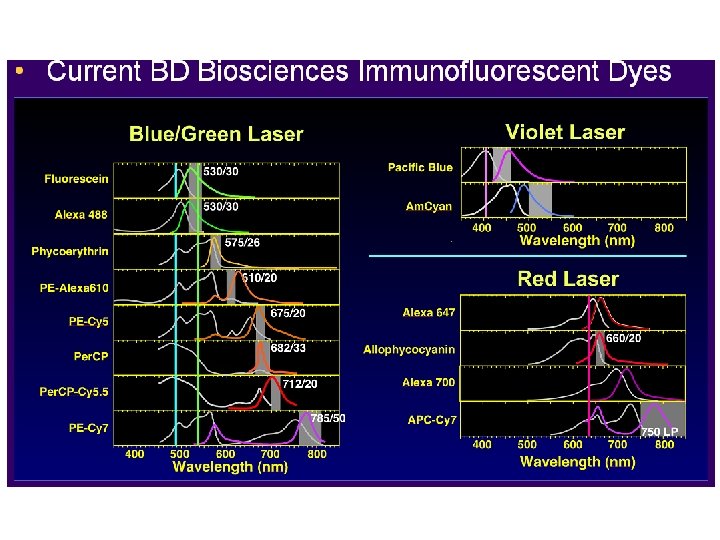
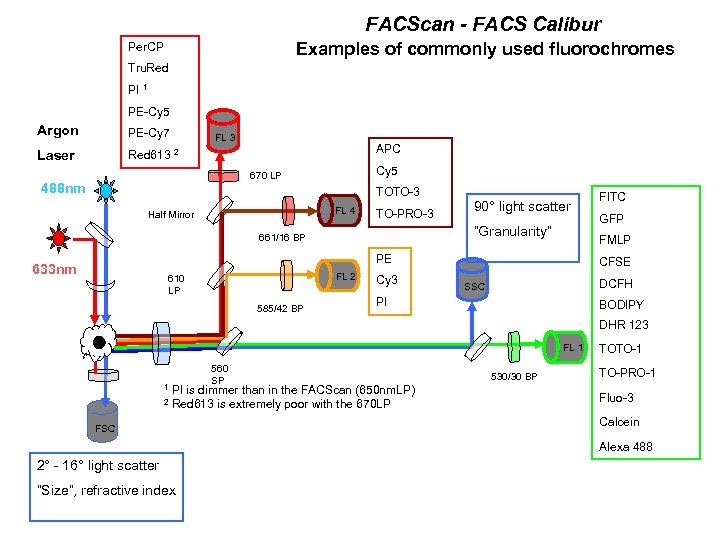 FACScan - FACS Calibur Examples of commonly used fluorochromes Per. CP Tru. Red PI 1 PE-Cy 5 Argon PE-Cy 7 Laser Red 613 FL 3 APC 2 Cy 5 670 LP 488 nm TOTO-3 FL 4 Half Mirror TO-PRO-3 90° light scatter “Granularity” 661/16 BP FL 2 610 LP 585/42 BP Cy 3 GFP FMLP PE 633 nm FITC CFSE DCFH SSC PI BODIPY DHR 123 FL 1 1 2 560 SP PI is dimmer than in the FACScan (650 nm. LP) Red 613 is extremely poor with the 670 LP FSC 530/30 BP TOTO-1 TO-PRO-1 Fluo-3 Calcein Alexa 488 2° - 16° light scatter “Size”, refractive index
FACScan - FACS Calibur Examples of commonly used fluorochromes Per. CP Tru. Red PI 1 PE-Cy 5 Argon PE-Cy 7 Laser Red 613 FL 3 APC 2 Cy 5 670 LP 488 nm TOTO-3 FL 4 Half Mirror TO-PRO-3 90° light scatter “Granularity” 661/16 BP FL 2 610 LP 585/42 BP Cy 3 GFP FMLP PE 633 nm FITC CFSE DCFH SSC PI BODIPY DHR 123 FL 1 1 2 560 SP PI is dimmer than in the FACScan (650 nm. LP) Red 613 is extremely poor with the 670 LP FSC 530/30 BP TOTO-1 TO-PRO-1 Fluo-3 Calcein Alexa 488 2° - 16° light scatter “Size”, refractive index
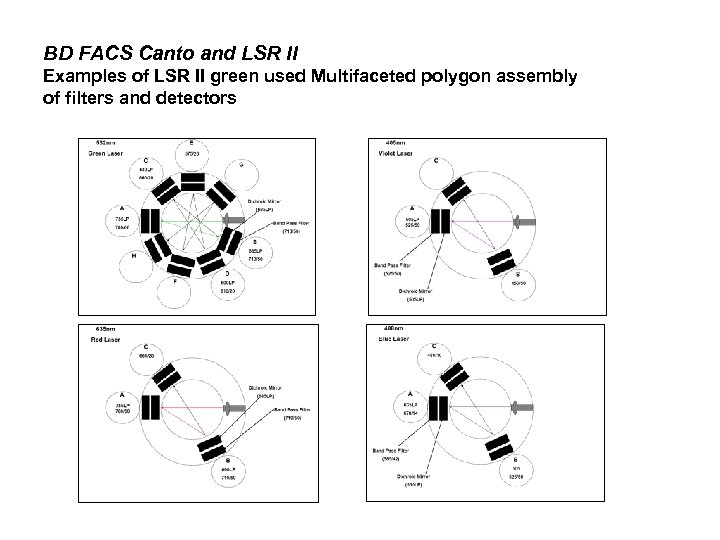 BD FACS Canto and LSR II Examples of LSR II green used Multifaceted polygon assembly of filters and detectors
BD FACS Canto and LSR II Examples of LSR II green used Multifaceted polygon assembly of filters and detectors
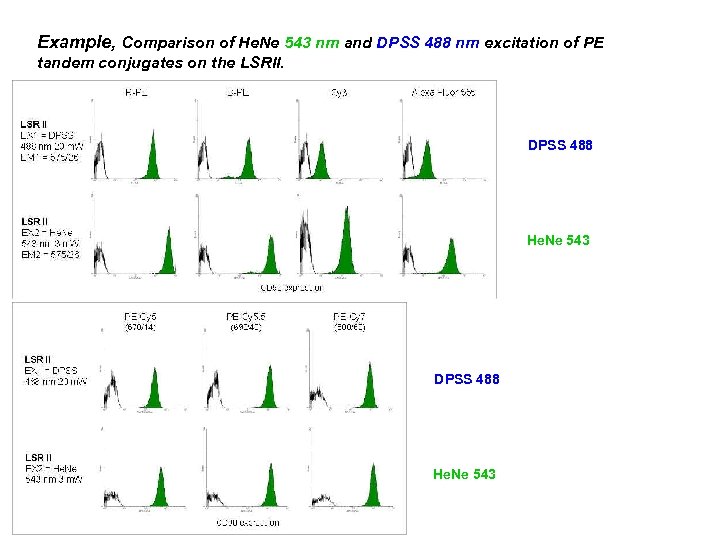 Example, Comparison of He. Ne 543 nm and DPSS 488 nm excitation of PE tandem conjugates on the LSRII. DPSS 488 He. Ne 543
Example, Comparison of He. Ne 543 nm and DPSS 488 nm excitation of PE tandem conjugates on the LSRII. DPSS 488 He. Ne 543
 Dye & Single Color Compensation
Dye & Single Color Compensation
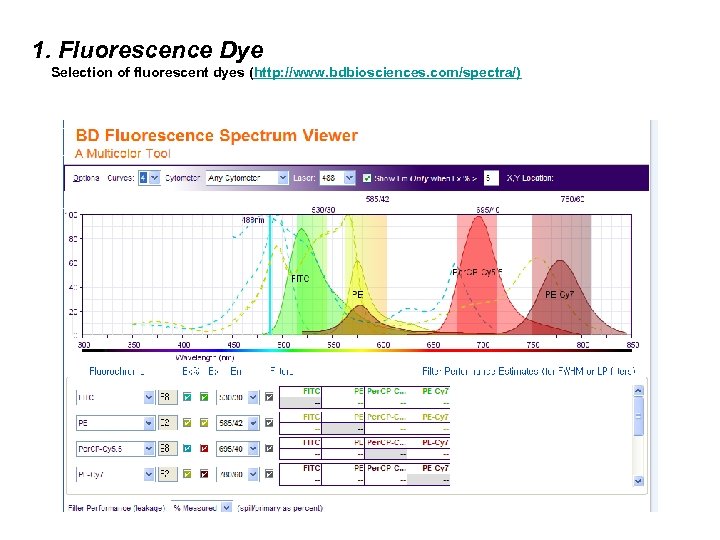 1. Fluorescence Dye Selection of fluorescent dyes (http: //www. bdbiosciences. com/spectra/)
1. Fluorescence Dye Selection of fluorescent dyes (http: //www. bdbiosciences. com/spectra/)
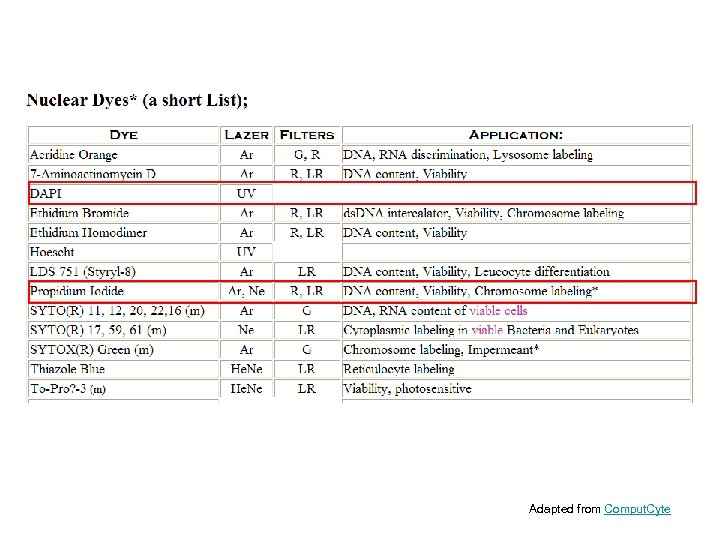 Adapted from Comput. Cyte
Adapted from Comput. Cyte
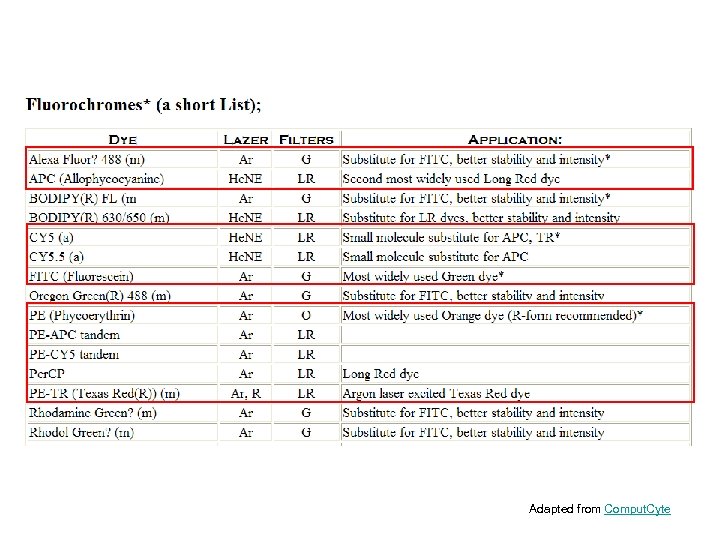 Adapted from Comput. Cyte
Adapted from Comput. Cyte
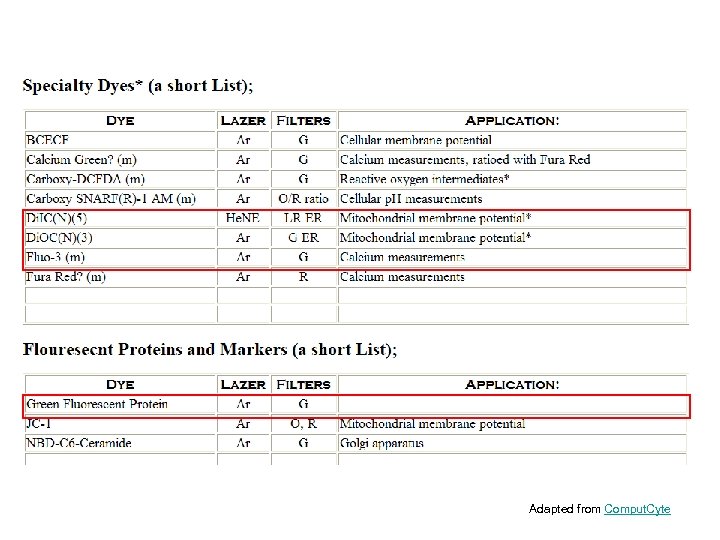 Adapted from Comput. Cyte
Adapted from Comput. Cyte
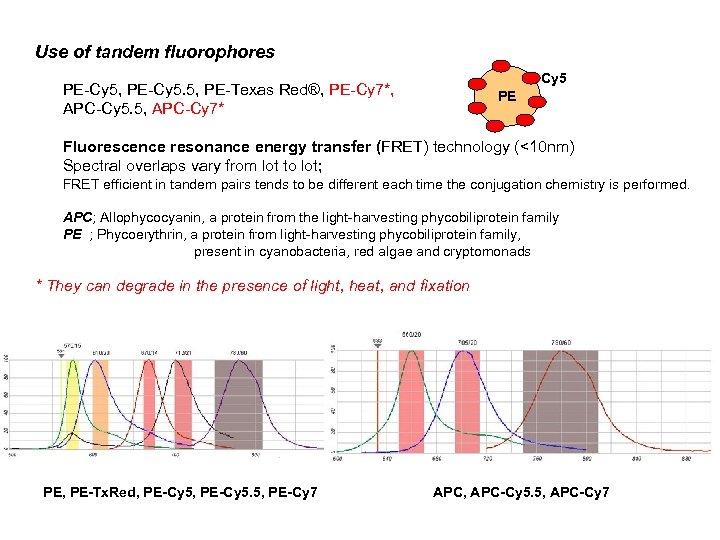 Use of tandem fluorophores Cy 5 PE-Cy 5, PE-Cy 5. 5, PE-Texas Red®, PE-Cy 7*, APC-Cy 5. 5, APC-Cy 7* PE Fluorescence resonance energy transfer (FRET) technology (<10 nm) Spectral overlaps vary from lot to lot; FRET efficient in tandem pairs tends to be different each time the conjugation chemistry is performed. APC; Allophycocyanin, a protein from the light-harvesting phycobiliprotein family PE ; Phycoerythrin, a protein from light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptomonads * They can degrade in the presence of light, heat, and fixation PE, PE-Tx. Red, PE-Cy 5. 5, PE-Cy 7 APC, APC-Cy 5. 5, APC-Cy 7
Use of tandem fluorophores Cy 5 PE-Cy 5, PE-Cy 5. 5, PE-Texas Red®, PE-Cy 7*, APC-Cy 5. 5, APC-Cy 7* PE Fluorescence resonance energy transfer (FRET) technology (<10 nm) Spectral overlaps vary from lot to lot; FRET efficient in tandem pairs tends to be different each time the conjugation chemistry is performed. APC; Allophycocyanin, a protein from the light-harvesting phycobiliprotein family PE ; Phycoerythrin, a protein from light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptomonads * They can degrade in the presence of light, heat, and fixation PE, PE-Tx. Red, PE-Cy 5. 5, PE-Cy 7 APC, APC-Cy 5. 5, APC-Cy 7
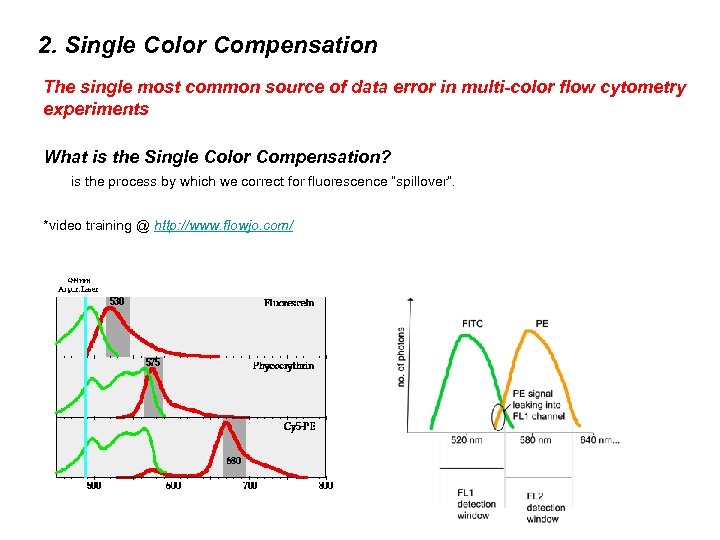 2. Single Color Compensation The single most common source of data error in multi-color flow cytometry experiments What is the Single Color Compensation? is the process by which we correct for fluorescence “spillover”. *video training @ http: //www. flowjo. com/
2. Single Color Compensation The single most common source of data error in multi-color flow cytometry experiments What is the Single Color Compensation? is the process by which we correct for fluorescence “spillover”. *video training @ http: //www. flowjo. com/
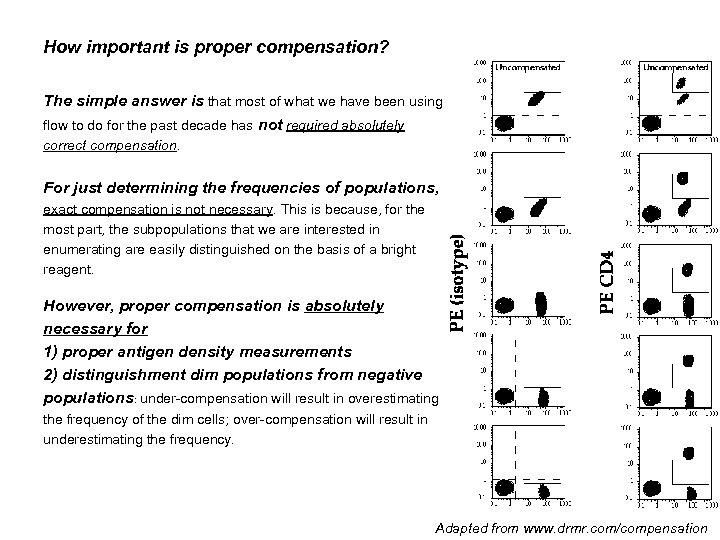 How important is proper compensation? The simple answer is that most of what we have been using flow to do for the past decade has not required absolutely correct compensation. For just determining the frequencies of populations, exact compensation is not necessary. This is because, for the most part, the subpopulations that we are interested in enumerating are easily distinguished on the basis of a bright reagent. However, proper compensation is absolutely necessary for 1) proper antigen density measurements 2) distinguishment dim populations from negative populations: under-compensation will result in overestimating the frequency of the dim cells; over-compensation will result in underestimating the frequency. Adapted from www. drmr. com/compensation
How important is proper compensation? The simple answer is that most of what we have been using flow to do for the past decade has not required absolutely correct compensation. For just determining the frequencies of populations, exact compensation is not necessary. This is because, for the most part, the subpopulations that we are interested in enumerating are easily distinguished on the basis of a bright reagent. However, proper compensation is absolutely necessary for 1) proper antigen density measurements 2) distinguishment dim populations from negative populations: under-compensation will result in overestimating the frequency of the dim cells; over-compensation will result in underestimating the frequency. Adapted from www. drmr. com/compensation
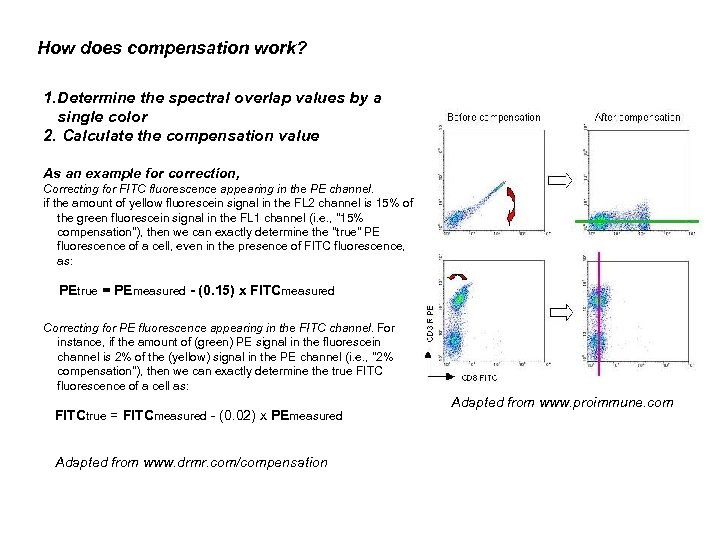 How does compensation work? 1. Determine the spectral overlap values by a single color 2. Calculate the compensation value As an example for correction, Correcting for FITC fluorescence appearing in the PE channel. if the amount of yellow fluorescein signal in the FL 2 channel is 15% of the green fluorescein signal in the FL 1 channel (i. e. , "15% compensation"), then we can exactly determine the "true" PE fluorescence of a cell, even in the presence of FITC fluorescence, as: PEtrue = PEmeasured - (0. 15) x FITCmeasured Correcting for PE fluorescence appearing in the FITC channel. For instance, if the amount of (green) PE signal in the fluorescein channel is 2% of the (yellow) signal in the PE channel (i. e. , "2% compensation"), then we can exactly determine the true FITC fluorescence of a cell as: FITCtrue = FITCmeasured - (0. 02) x PEmeasured Adapted from www. drmr. com/compensation Adapted from www. proimmune. com
How does compensation work? 1. Determine the spectral overlap values by a single color 2. Calculate the compensation value As an example for correction, Correcting for FITC fluorescence appearing in the PE channel. if the amount of yellow fluorescein signal in the FL 2 channel is 15% of the green fluorescein signal in the FL 1 channel (i. e. , "15% compensation"), then we can exactly determine the "true" PE fluorescence of a cell, even in the presence of FITC fluorescence, as: PEtrue = PEmeasured - (0. 15) x FITCmeasured Correcting for PE fluorescence appearing in the FITC channel. For instance, if the amount of (green) PE signal in the fluorescein channel is 2% of the (yellow) signal in the PE channel (i. e. , "2% compensation"), then we can exactly determine the true FITC fluorescence of a cell as: FITCtrue = FITCmeasured - (0. 02) x PEmeasured Adapted from www. drmr. com/compensation Adapted from www. proimmune. com
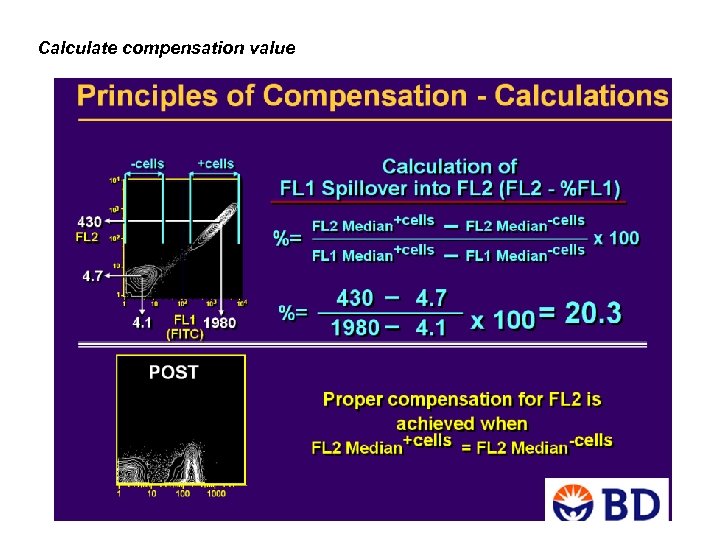 Calculate compensation value
Calculate compensation value
 Myths about setting compensation 1) You should set your compensation on the same tissue you are going to analyze 2) Compensation controls should be the same intensity as the reagent to be use Bright reagents require more compensation than dim (dull) reagents 3) Compensation can be set by eye 4) Compensation settings can be saved and used from day-to-day 5) Improper compensation doesn’t affect the data very much
Myths about setting compensation 1) You should set your compensation on the same tissue you are going to analyze 2) Compensation controls should be the same intensity as the reagent to be use Bright reagents require more compensation than dim (dull) reagents 3) Compensation can be set by eye 4) Compensation settings can be saved and used from day-to-day 5) Improper compensation doesn’t affect the data very much
 Requirements for Proper Compensation 1. The fluorescence spectrum (% spillover) of the compensation control reagent should be identical to the reagent used in the experiment Critical for tandem reagents even similar fluorochromes like FITC/Alexa 488 or APC/C 5 2. The negative and positive populations must have the same autofluorescence don’t use CD 3+ lymphocytes and CD 3 - monocytes 3. The positive population should be a bright as possible 4. Take enough events to get statically accurate numbers • Type of Single color control - BD Cali. BRITE™ beads, - Single-stained cellular controls; popular - Single-stained BD™ Comp. Beads ; > provide a convenient method of presenting single color control > Best match of spectra
Requirements for Proper Compensation 1. The fluorescence spectrum (% spillover) of the compensation control reagent should be identical to the reagent used in the experiment Critical for tandem reagents even similar fluorochromes like FITC/Alexa 488 or APC/C 5 2. The negative and positive populations must have the same autofluorescence don’t use CD 3+ lymphocytes and CD 3 - monocytes 3. The positive population should be a bright as possible 4. Take enough events to get statically accurate numbers • Type of Single color control - BD Cali. BRITE™ beads, - Single-stained cellular controls; popular - Single-stained BD™ Comp. Beads ; > provide a convenient method of presenting single color control > Best match of spectra
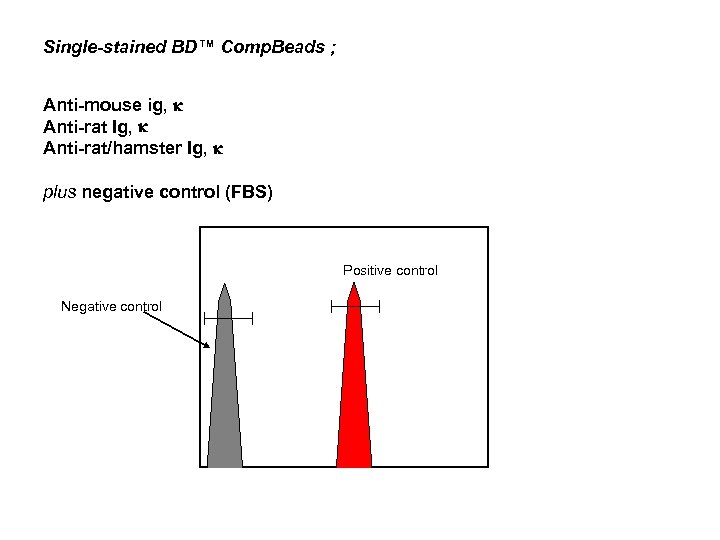 Single-stained BD™ Comp. Beads ; Anti-mouse ig, k Anti-rat Ig, k Anti-rat/hamster Ig, k plus negative control (FBS) Positive control Negative control
Single-stained BD™ Comp. Beads ; Anti-mouse ig, k Anti-rat Ig, k Anti-rat/hamster Ig, k plus negative control (FBS) Positive control Negative control
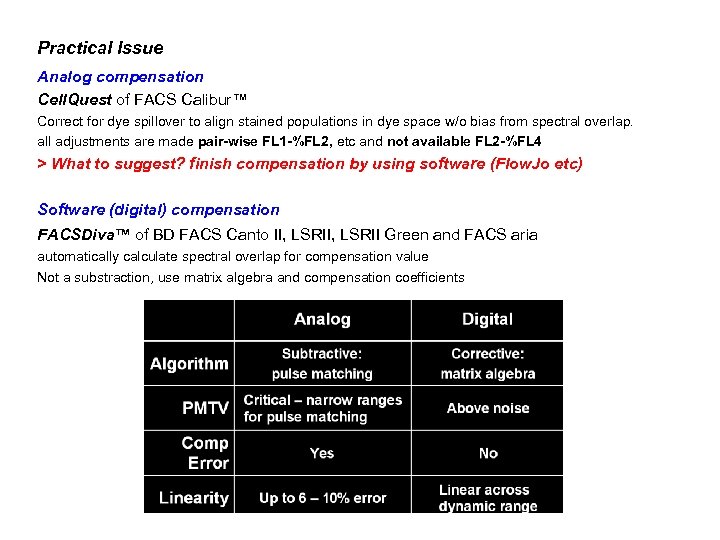 Practical Issue Analog compensation Cell. Quest of FACS Calibur™ Correct for dye spillover to align stained populations in dye space w/o bias from spectral overlap. all adjustments are made pair-wise FL 1 -%FL 2, etc and not available FL 2 -%FL 4 > What to suggest? finish compensation by using software (Flow. Jo etc) Software (digital) compensation FACSDiva™ of BD FACS Canto II, LSRII Green and FACS aria automatically calculate spectral overlap for compensation value Not a substraction, use matrix algebra and compensation coefficients
Practical Issue Analog compensation Cell. Quest of FACS Calibur™ Correct for dye spillover to align stained populations in dye space w/o bias from spectral overlap. all adjustments are made pair-wise FL 1 -%FL 2, etc and not available FL 2 -%FL 4 > What to suggest? finish compensation by using software (Flow. Jo etc) Software (digital) compensation FACSDiva™ of BD FACS Canto II, LSRII Green and FACS aria automatically calculate spectral overlap for compensation value Not a substraction, use matrix algebra and compensation coefficients
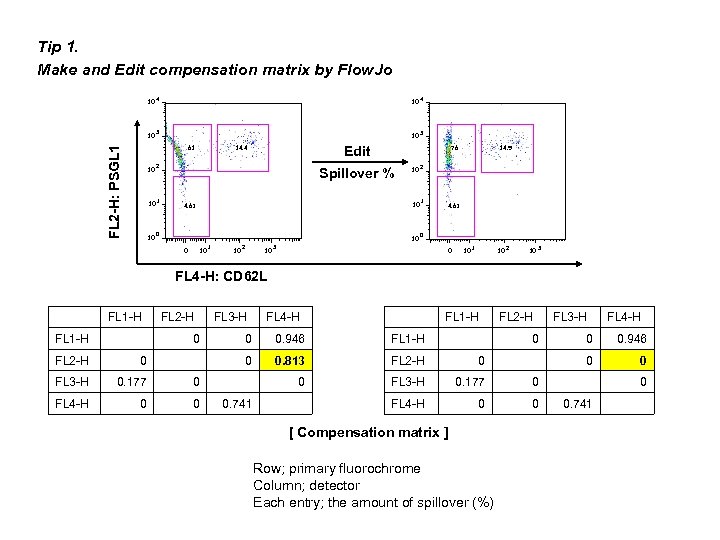 Tip 1. Make and Edit compensation matrix by Flow. Jo 10 10 4 10 3 10 FL 2 -H: PSGL 1 61 10 10 10 4 3 Edit 14. 4 2 76 4. 63 0 0 10 1 10 2 1 10 10 Spillover % 14. 9 0 3 4. 63 0 10 1 10 2 10 3 FL 4 -H: CD 62 L FL 1 -H FL 2 -H FL 3 -H 0 FL 4 -H FL 1 -H FL 2 -H 0 FL 3 -H 0. 177 0 FL 4 -H 0 0 0. 946 FL 1 -H 0 FL 2 -H 0 0. 813 FL 2 -H 0 0 FL 3 -H 0. 177 0 FL 4 -H 0 0 0. 741 FL 3 -H 0 [ Compensation matrix ] Row; primary fluorochrome Column; detector Each entry; the amount of spillover (%) FL 4 -H 0 0 0. 946 0 0 0. 741
Tip 1. Make and Edit compensation matrix by Flow. Jo 10 10 4 10 3 10 FL 2 -H: PSGL 1 61 10 10 10 4 3 Edit 14. 4 2 76 4. 63 0 0 10 1 10 2 1 10 10 Spillover % 14. 9 0 3 4. 63 0 10 1 10 2 10 3 FL 4 -H: CD 62 L FL 1 -H FL 2 -H FL 3 -H 0 FL 4 -H FL 1 -H FL 2 -H 0 FL 3 -H 0. 177 0 FL 4 -H 0 0 0. 946 FL 1 -H 0 FL 2 -H 0 0. 813 FL 2 -H 0 0 FL 3 -H 0. 177 0 FL 4 -H 0 0 0. 741 FL 3 -H 0 [ Compensation matrix ] Row; primary fluorochrome Column; detector Each entry; the amount of spillover (%) FL 4 -H 0 0 0. 946 0 0 0. 741
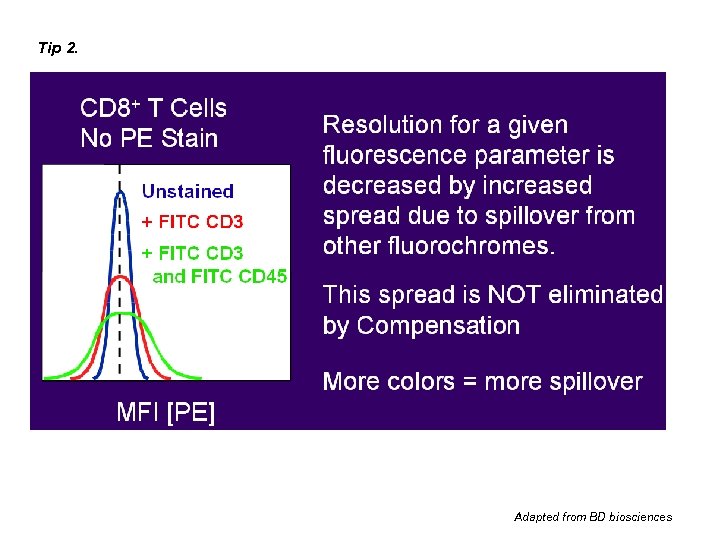 Tip 2. Adapted from BD biosciences
Tip 2. Adapted from BD biosciences
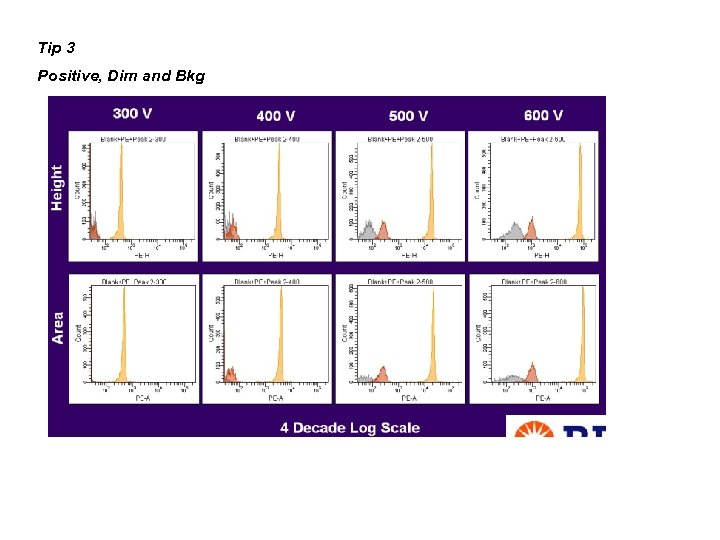 Tip 3 Positive, Dim and Bkg
Tip 3 Positive, Dim and Bkg
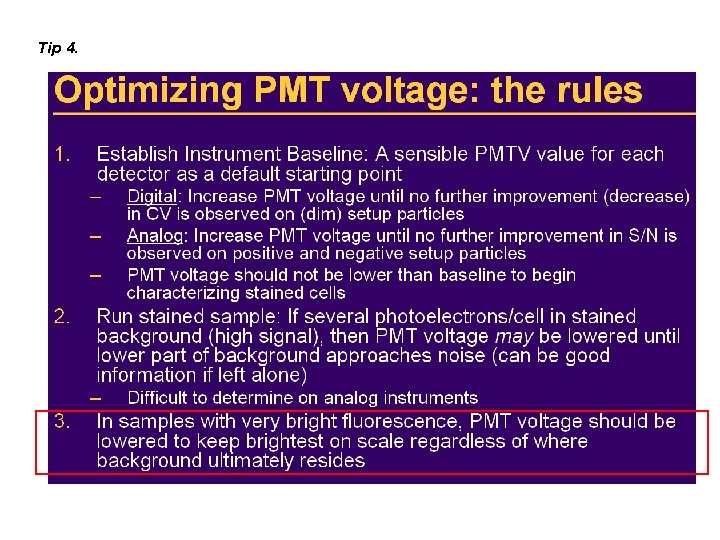 Tip 4.
Tip 4.
 Sample Preparation (cells and staining) 1. By density gradient: Ficoll-Hypaque, Percoll etc 2. By Ab-based methods other than magnetic beads & FACS 3. By Ab-coated magnetic beads 4. By FACS (fluorescence-activated cell sorter) 5. etc adapted from KAIST lecture
Sample Preparation (cells and staining) 1. By density gradient: Ficoll-Hypaque, Percoll etc 2. By Ab-based methods other than magnetic beads & FACS 3. By Ab-coated magnetic beads 4. By FACS (fluorescence-activated cell sorter) 5. etc adapted from KAIST lecture
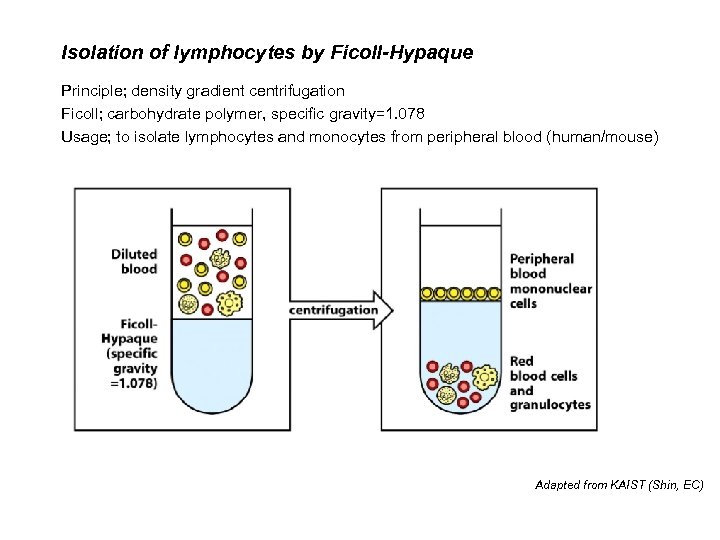 Isolation of lymphocytes by Ficoll-Hypaque Principle; density gradient centrifugation Ficoll; carbohydrate polymer, specific gravity=1. 078 Usage; to isolate lymphocytes and monocytes from peripheral blood (human/mouse) Adapted from KAIST (Shin, EC)
Isolation of lymphocytes by Ficoll-Hypaque Principle; density gradient centrifugation Ficoll; carbohydrate polymer, specific gravity=1. 078 Usage; to isolate lymphocytes and monocytes from peripheral blood (human/mouse) Adapted from KAIST (Shin, EC)
 NH 4 Cl, Hyptonic solution To get ride of RBC from spleen 0. 75% CH 3 Coo. H 0. 84% NH 4 Cl Water Ab-based methods other than magnetic beads & FACS 1. Panning (positive and negative) 2. Removal by complement 3. Column; similar to affinity chromatography Adapted from KAIST (Shin, EC)
NH 4 Cl, Hyptonic solution To get ride of RBC from spleen 0. 75% CH 3 Coo. H 0. 84% NH 4 Cl Water Ab-based methods other than magnetic beads & FACS 1. Panning (positive and negative) 2. Removal by complement 3. Column; similar to affinity chromatography Adapted from KAIST (Shin, EC)
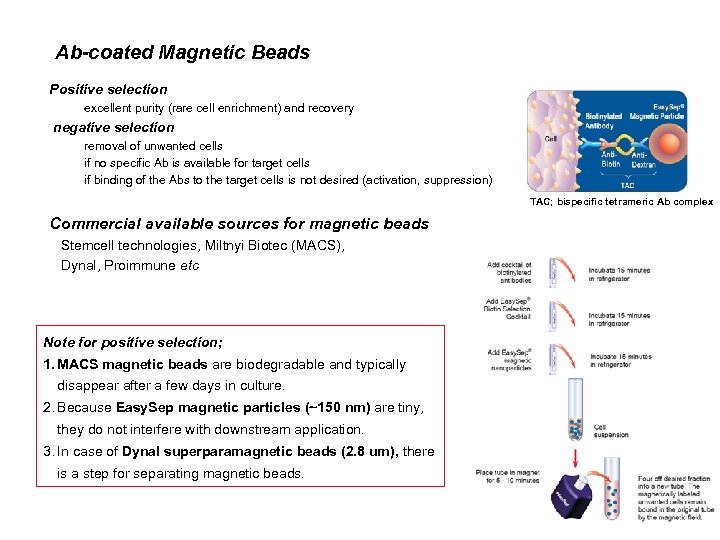 Ab-coated Magnetic Beads Positive selection excellent purity (rare cell enrichment) and recovery negative selection removal of unwanted cells if no specific Ab is available for target cells if binding of the Abs to the target cells is not desired (activation, suppression) TAC; bispecific tetrameric Ab complex Commercial available sources for magnetic beads Stemcell technologies, Miltnyi Biotec (MACS), Dynal, Proimmune etc Note for positive selection; 1. MACS magnetic beads are biodegradable and typically disappear after a few days in culture. 2. Because Easy. Sep magnetic particles (~150 nm) are tiny, they do not interfere with downstream application. 3. In case of Dynal superparamagnetic beads (2. 8 um), there is a step for separating magnetic beads.
Ab-coated Magnetic Beads Positive selection excellent purity (rare cell enrichment) and recovery negative selection removal of unwanted cells if no specific Ab is available for target cells if binding of the Abs to the target cells is not desired (activation, suppression) TAC; bispecific tetrameric Ab complex Commercial available sources for magnetic beads Stemcell technologies, Miltnyi Biotec (MACS), Dynal, Proimmune etc Note for positive selection; 1. MACS magnetic beads are biodegradable and typically disappear after a few days in culture. 2. Because Easy. Sep magnetic particles (~150 nm) are tiny, they do not interfere with downstream application. 3. In case of Dynal superparamagnetic beads (2. 8 um), there is a step for separating magnetic beads.
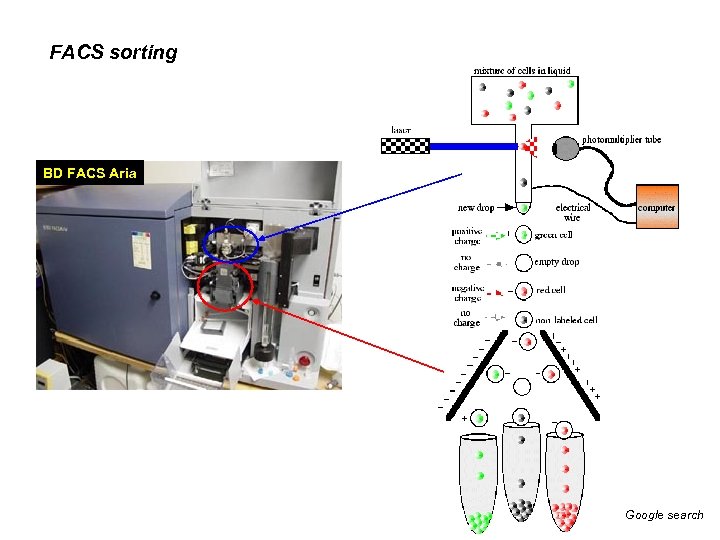 FACS sorting BD FACS Aria Google search
FACS sorting BD FACS Aria Google search
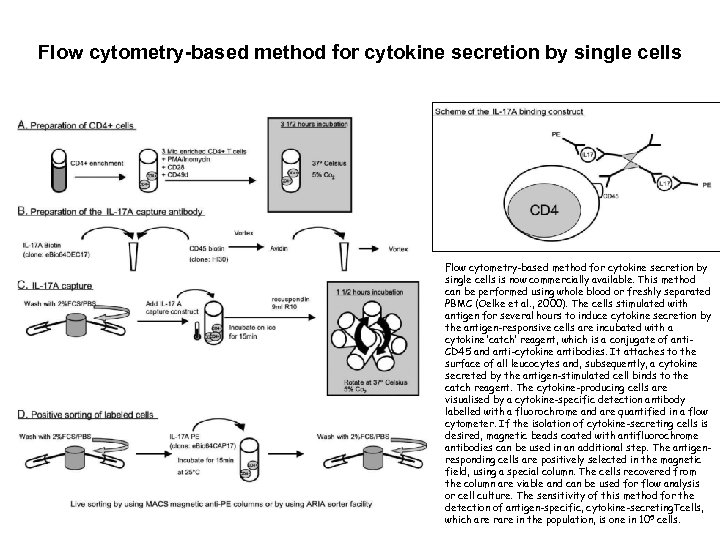
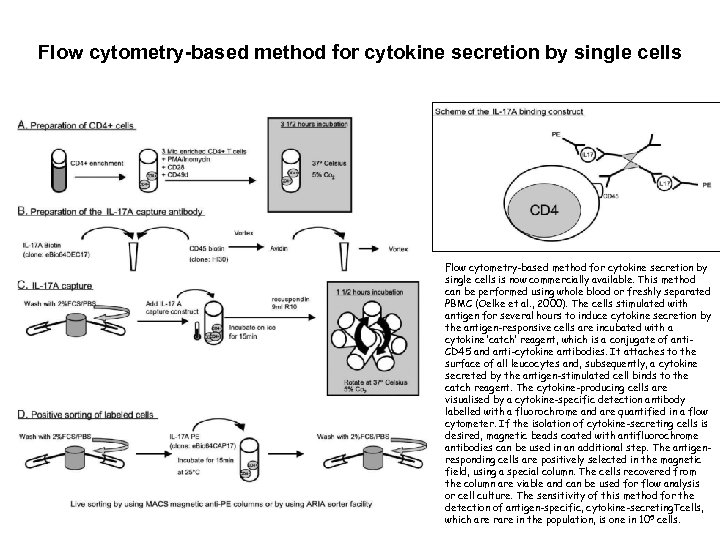 Flow cytometry-based method for cytokine secretion by single cells is now commercially available. This method can be performed using whole blood or freshly separated PBMC (Oelke et al. , 2000). The cells stimulated with antigen for several hours to induce cytokine secretion by the antigen-responsive cells are incubated with a cytokine ‘catch’ reagent, which is a conjugate of anti. CD 45 and anti-cytokine antibodies. It attaches to the surface of all leucocytes and, subsequently, a cytokine secreted by the antigen-stimulated cell binds to the catch reagent. The cytokine-producing cells are visualised by a cytokine-specific detection antibody labelled with a fluorochrome and are quantified in a flow cytometer. If the isolation of cytokine-secreting cells is desired, magnetic beads coated with antifluorochrome antibodies can be used in an additional step. The antigenresponding cells are positively selected in the magnetic field, using a special column. The cells recovered from the column are viable and can be used for flow analysis or cell culture. The sensitivity of this method for the detection of antigen-specific, cytokine-secreting. Tcells, which are rare in the population, is one in 105 cells.
Flow cytometry-based method for cytokine secretion by single cells is now commercially available. This method can be performed using whole blood or freshly separated PBMC (Oelke et al. , 2000). The cells stimulated with antigen for several hours to induce cytokine secretion by the antigen-responsive cells are incubated with a cytokine ‘catch’ reagent, which is a conjugate of anti. CD 45 and anti-cytokine antibodies. It attaches to the surface of all leucocytes and, subsequently, a cytokine secreted by the antigen-stimulated cell binds to the catch reagent. The cytokine-producing cells are visualised by a cytokine-specific detection antibody labelled with a fluorochrome and are quantified in a flow cytometer. If the isolation of cytokine-secreting cells is desired, magnetic beads coated with antifluorochrome antibodies can be used in an additional step. The antigenresponding cells are positively selected in the magnetic field, using a special column. The cells recovered from the column are viable and can be used for flow analysis or cell culture. The sensitivity of this method for the detection of antigen-specific, cytokine-secreting. Tcells, which are rare in the population, is one in 105 cells.
 Applications
Applications
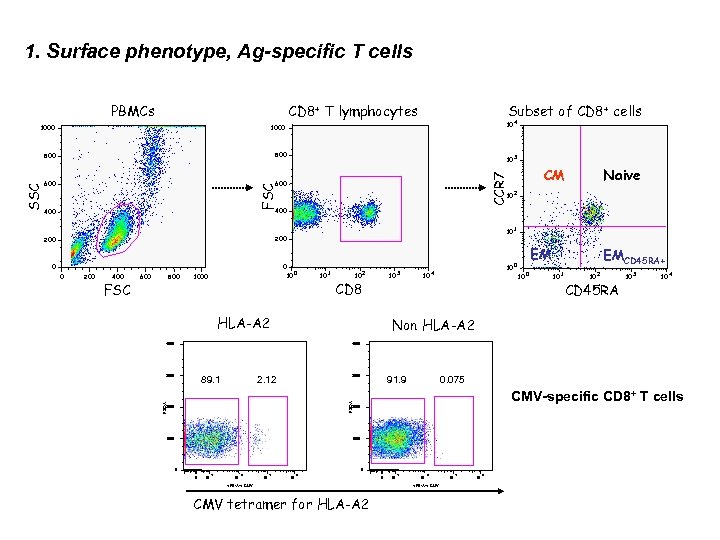 1. Surface phenotype, Ag-specific T cells CD 8+ T lymphocytes PBMCs 600 3 CM CCR 7 600 4 10 800 FSC 800 10 10 1 10 0 Naive 2 400 200 0 0 10 0 200 400 FSC 600 800 10 1 10 2 10 CD 8 HLA-A 2 3 10 4 EM 10 0 EMCD 45 RA+ 10 1 10 2 CD 45 RA 10 3 10 4 Non HLA-A 2 4000 3000 89. 1 3000 2. 12 0. 075 91. 9 CMV-specific CD 8+ T cells FSC-A 0 FSC-A SSC 1000 Subset of CD 8+ cells 2000 1000 0 0 0 10 2 10 3 10 4 10 5
1. Surface phenotype, Ag-specific T cells CD 8+ T lymphocytes PBMCs 600 3 CM CCR 7 600 4 10 800 FSC 800 10 10 1 10 0 Naive 2 400 200 0 0 10 0 200 400 FSC 600 800 10 1 10 2 10 CD 8 HLA-A 2 3 10 4 EM 10 0 EMCD 45 RA+ 10 1 10 2 CD 45 RA 10 3 10 4 Non HLA-A 2 4000 3000 89. 1 3000 2. 12 0. 075 91. 9 CMV-specific CD 8+ T cells FSC-A 0 FSC-A SSC 1000 Subset of CD 8+ cells 2000 1000 0 0 0 10 2 10 3 10 4 10 5
 2. Cytokine productions Fixative; PFA Perm; Sapoinin, PEG (BD Perm II solution for human) Adapted from KAIST (Shin, EC)
2. Cytokine productions Fixative; PFA Perm; Sapoinin, PEG (BD Perm II solution for human) Adapted from KAIST (Shin, EC)
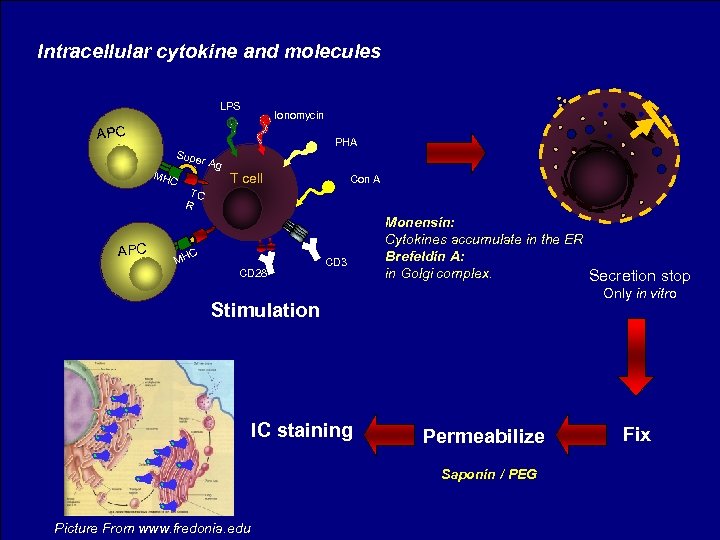 Intracellular cytokine and molecules LPS Ionomycin APC Supe MHC APC PHA r Ag TC R T cell C MH CD 28 Con A CD 3 Stimulation IC staining Monensin: Cytokines accumulate in the ER Brefeldin A: in Golgi complex. Secretion stop Only in vitro Permeabilize Saponin / PEG Picture From www. fredonia. edu Fix
Intracellular cytokine and molecules LPS Ionomycin APC Supe MHC APC PHA r Ag TC R T cell C MH CD 28 Con A CD 3 Stimulation IC staining Monensin: Cytokines accumulate in the ER Brefeldin A: in Golgi complex. Secretion stop Only in vitro Permeabilize Saponin / PEG Picture From www. fredonia. edu Fix
 Flow cytometry-based method for cytokine secretion by single cells is now commercially available. This method can be performed using whole blood or freshly separated PBMC (Oelke et al. , 2000). The cells stimulated with antigen for several hours to induce cytokine secretion by the antigen-responsive cells are incubated with a cytokine ‘catch’ reagent, which is a conjugate of anti. CD 45 and anti-cytokine antibodies. It attaches to the surface of all leucocytes and, subsequently, a cytokine secreted by the antigen-stimulated cell binds to the catch reagent. The cytokine-producing cells are visualised by a cytokine-specific detection antibody labelled with a fluorochrome and are quantified in a flow cytometer. If the isolation of cytokine-secreting cells is desired, magnetic beads coated with antifluorochrome antibodies can be used in an additional step. The antigenresponding cells are positively selected in the magnetic field, using a special column. The cells recovered from the column are viable and can be used for flow analysis or cell culture. The sensitivity of this method for the detection of antigen-specific, cytokine-secreting. Tcells, which are rare in the population, is one in 105 cells.
Flow cytometry-based method for cytokine secretion by single cells is now commercially available. This method can be performed using whole blood or freshly separated PBMC (Oelke et al. , 2000). The cells stimulated with antigen for several hours to induce cytokine secretion by the antigen-responsive cells are incubated with a cytokine ‘catch’ reagent, which is a conjugate of anti. CD 45 and anti-cytokine antibodies. It attaches to the surface of all leucocytes and, subsequently, a cytokine secreted by the antigen-stimulated cell binds to the catch reagent. The cytokine-producing cells are visualised by a cytokine-specific detection antibody labelled with a fluorochrome and are quantified in a flow cytometer. If the isolation of cytokine-secreting cells is desired, magnetic beads coated with antifluorochrome antibodies can be used in an additional step. The antigenresponding cells are positively selected in the magnetic field, using a special column. The cells recovered from the column are viable and can be used for flow analysis or cell culture. The sensitivity of this method for the detection of antigen-specific, cytokine-secreting. Tcells, which are rare in the population, is one in 105 cells.
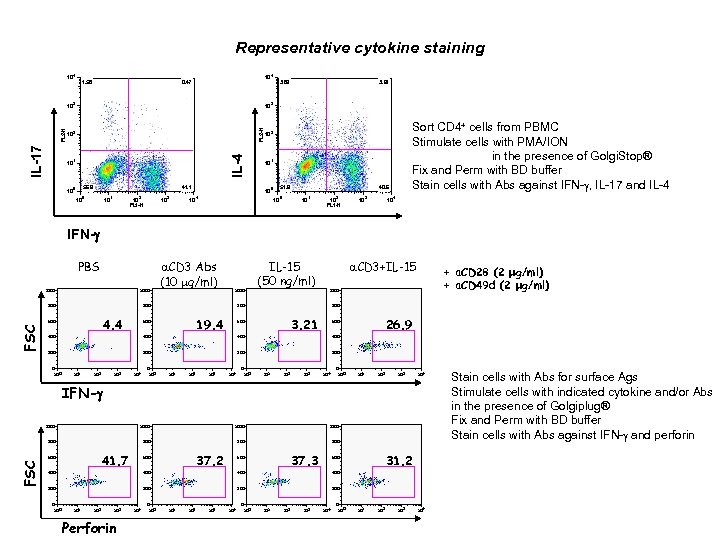 Representative cytokine staining 3 10 2 10 1 10 0 10 41. 1 0 10 1 2 10 FL 1 -H 10 3 10 10 2 1 10 FL 2 -H 56. 9 10 3 10 0. 47 4 10 1. 58 IL-4 IL-17 4 10 FL 2 -H 10 0 4 3. 89 3. 61 40. 6 51. 9 10 0 Sort CD 4+ cells from PBMC Stimulate cells with PMA/ION in the presence of Golgi. Stop® Fix and Perm with BD buffer Stain cells with Abs against IFN-g, IL-17 and IL-4 10 1 2 10 FL 1 -H 10 3 10 4 IFN-g PBS 1000 800 4. 4 600 FSC a. CD 3 Abs (10 mg/ml) 1000 IL-15 (50 ng/ml) a. CD 3+IL-15 1000 800 19. 4 600 + a. CD 28 (2 mg/ml) + a. CD 49 d (2 mg/ml) 800 3. 21 600 26. 9 600 400 400 200 200 0 100 101 10 2 10 3 10 4 IFN-g 1000 800 800 41. 7 FSC 600 37. 2 600 37. 3 600 31. 2 600 400 400 200 200 0 10 1 10 2 10 3 Perforin 10 4 0 100 101 10 2 10 3 10 4 0 10 1 10 2 10 3 10 4 Stain cells with Abs for surface Ags Stimulate cells with indicated cytokine and/or Abs in the presence of Golgiplug® Fix and Perm with BD buffer Stain cells with Abs against IFN-g and perforin
Representative cytokine staining 3 10 2 10 1 10 0 10 41. 1 0 10 1 2 10 FL 1 -H 10 3 10 10 2 1 10 FL 2 -H 56. 9 10 3 10 0. 47 4 10 1. 58 IL-4 IL-17 4 10 FL 2 -H 10 0 4 3. 89 3. 61 40. 6 51. 9 10 0 Sort CD 4+ cells from PBMC Stimulate cells with PMA/ION in the presence of Golgi. Stop® Fix and Perm with BD buffer Stain cells with Abs against IFN-g, IL-17 and IL-4 10 1 2 10 FL 1 -H 10 3 10 4 IFN-g PBS 1000 800 4. 4 600 FSC a. CD 3 Abs (10 mg/ml) 1000 IL-15 (50 ng/ml) a. CD 3+IL-15 1000 800 19. 4 600 + a. CD 28 (2 mg/ml) + a. CD 49 d (2 mg/ml) 800 3. 21 600 26. 9 600 400 400 200 200 0 100 101 10 2 10 3 10 4 IFN-g 1000 800 800 41. 7 FSC 600 37. 2 600 37. 3 600 31. 2 600 400 400 200 200 0 10 1 10 2 10 3 Perforin 10 4 0 100 101 10 2 10 3 10 4 0 10 1 10 2 10 3 10 4 Stain cells with Abs for surface Ags Stimulate cells with indicated cytokine and/or Abs in the presence of Golgiplug® Fix and Perm with BD buffer Stain cells with Abs against IFN-g and perforin
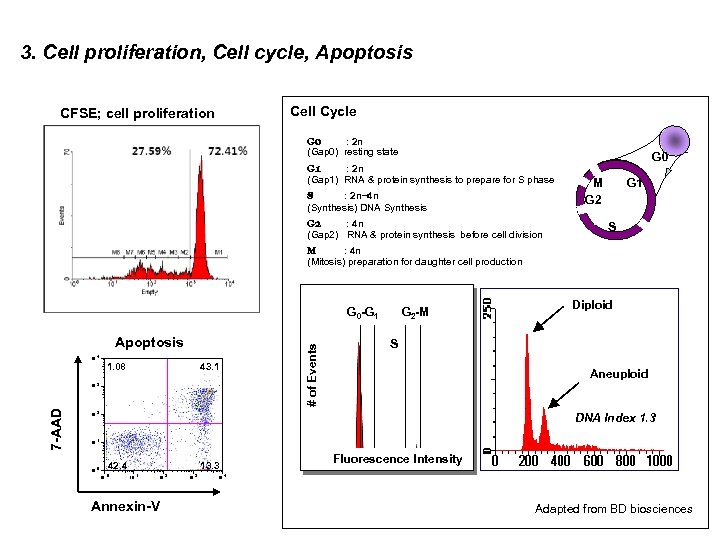 3. Cell proliferation, Cell cycle, Apoptosis Cell Cycle CFSE; cell proliferation G 0 : 2 n (Gap 0) resting state G 1 : 2 n (Gap 1) RNA & protein synthesis to prepare for S phase S : 2 n~4 n (Synthesis) DNA Synthesis G 2 : 4 n (Gap 2) RNA & protein synthesis before cell division G 0 M G 2 G 1 S M : 4 n (Mitosis) preparation for daughter cell production Apoptosis 10 4 1. 08 43. 1 7 -AAD 10 3 # of Events G 0 -G 1 G 2 -M Diploid S Aneuploid 10 2 DNA Index 1. 3 10 1 10 0 42. 4 10 0 13. 3 10 1 10 2 Annexin-V 10 3 Fluorescence Intensity 10 4 Adapted from BD biosciences
3. Cell proliferation, Cell cycle, Apoptosis Cell Cycle CFSE; cell proliferation G 0 : 2 n (Gap 0) resting state G 1 : 2 n (Gap 1) RNA & protein synthesis to prepare for S phase S : 2 n~4 n (Synthesis) DNA Synthesis G 2 : 4 n (Gap 2) RNA & protein synthesis before cell division G 0 M G 2 G 1 S M : 4 n (Mitosis) preparation for daughter cell production Apoptosis 10 4 1. 08 43. 1 7 -AAD 10 3 # of Events G 0 -G 1 G 2 -M Diploid S Aneuploid 10 2 DNA Index 1. 3 10 1 10 0 42. 4 10 0 13. 3 10 1 10 2 Annexin-V 10 3 Fluorescence Intensity 10 4 Adapted from BD biosciences
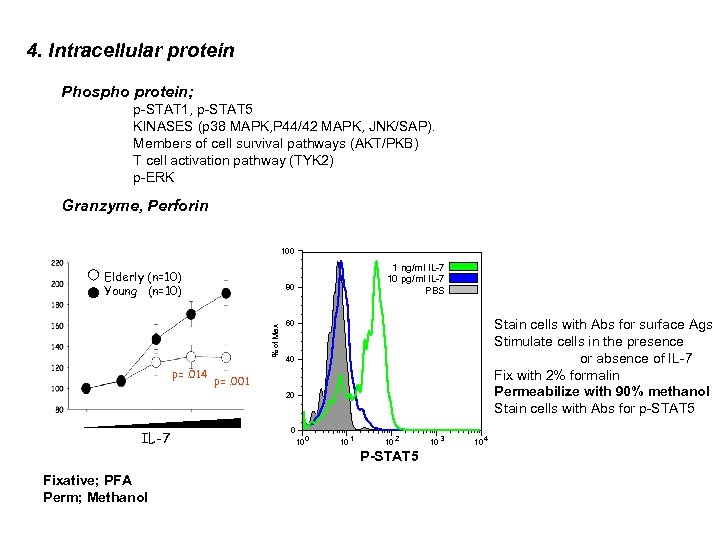 4. Intracellular protein Phospho protein; p-STAT 1, p-STAT 5 KINASES (p 38 MAPK, P 44/42 MAPK, JNK/SAP). Members of cell survival pathways (AKT/PKB) T cell activation pathway (TYK 2) p-ERK Granzyme, Perforin 100 Elderly (n=10) Young (n=10) 80 % of Max p=. 014 1 ng/ml IL-7 10 pg/ml IL-7 PBS Stain cells with Abs for surface Ags Stimulate cells in the presence or absence of IL-7 Fix with 2% formalin Permeabilize with 90% methanol Stain cells with Abs for p-STAT 5 60 40 p=. 001 20 IL-7 0 10 1 10 2 P-STAT 5 Fixative; PFA Perm; Methanol 10 3 10 4
4. Intracellular protein Phospho protein; p-STAT 1, p-STAT 5 KINASES (p 38 MAPK, P 44/42 MAPK, JNK/SAP). Members of cell survival pathways (AKT/PKB) T cell activation pathway (TYK 2) p-ERK Granzyme, Perforin 100 Elderly (n=10) Young (n=10) 80 % of Max p=. 014 1 ng/ml IL-7 10 pg/ml IL-7 PBS Stain cells with Abs for surface Ags Stimulate cells in the presence or absence of IL-7 Fix with 2% formalin Permeabilize with 90% methanol Stain cells with Abs for p-STAT 5 60 40 p=. 001 20 IL-7 0 10 1 10 2 P-STAT 5 Fixative; PFA Perm; Methanol 10 3 10 4
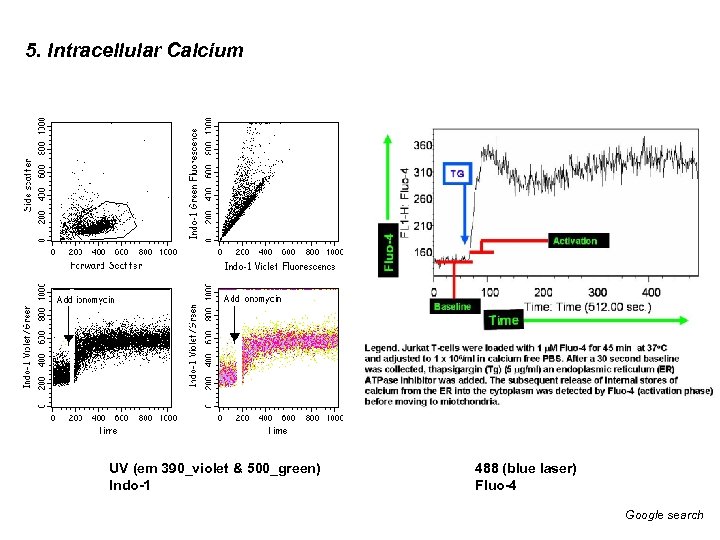 5. Intracellular Calcium UV (em 390_violet & 500_green) Indo-1 488 (blue laser) Fluo-4 Google search
5. Intracellular Calcium UV (em 390_violet & 500_green) Indo-1 488 (blue laser) Fluo-4 Google search
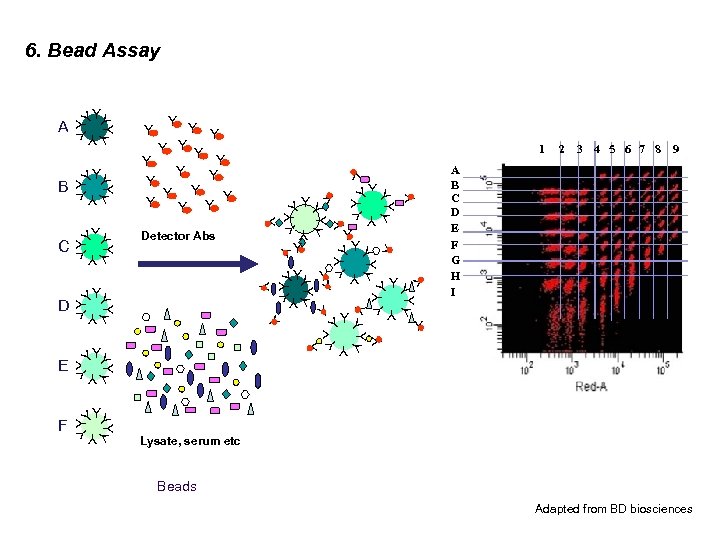 6. Bead Assay Y YY Y Y YY 2 3 4 5 6 7 8 9 A B C D E F G H I Y YY YY YYY Y Y YY Y Y Y YYY Y Y Y + YYY YY YYY YY Y Y 1 Detector Abs YY Y F Y Y Y Y YY E YY Y YY D YY Y YY C YY Y YY B Y YY A YY Lysate, serum etc Beads Adapted from BD biosciences
6. Bead Assay Y YY Y Y YY 2 3 4 5 6 7 8 9 A B C D E F G H I Y YY YY YYY Y Y YY Y Y Y YYY Y Y Y + YYY YY YYY YY Y Y 1 Detector Abs YY Y F Y Y Y Y YY E YY Y YY D YY Y YY C YY Y YY B Y YY A YY Lysate, serum etc Beads Adapted from BD biosciences
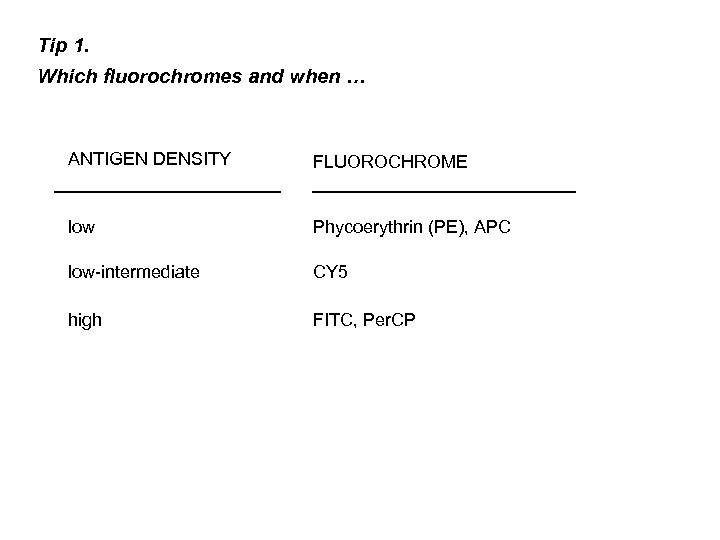 Tip 1. Which fluorochromes and when … ANTIGEN DENSITY FLUOROCHROME low Phycoerythrin (PE), APC low-intermediate CY 5 high FITC, Per. CP
Tip 1. Which fluorochromes and when … ANTIGEN DENSITY FLUOROCHROME low Phycoerythrin (PE), APC low-intermediate CY 5 high FITC, Per. CP
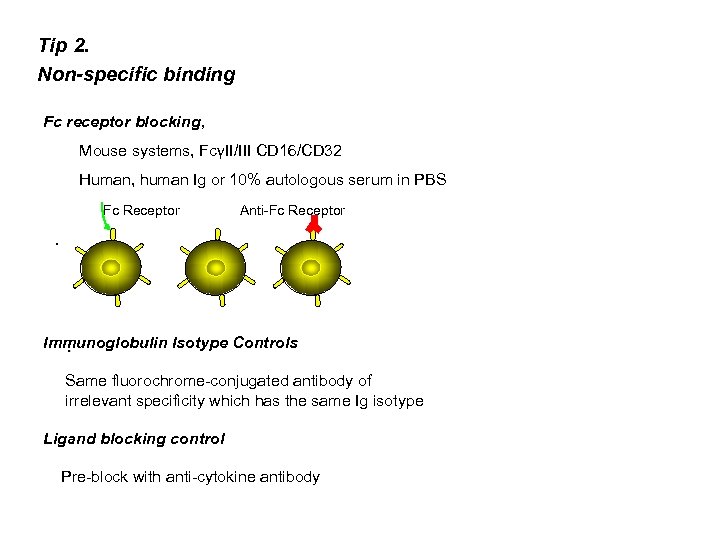 Tip 2. Non-specific binding Fc receptor blocking, Mouse systems, FcγII/III CD 16/CD 32 Human, human Ig or 10% autologous serum in PBS Fc Receptor Anti-Fc Receptor . Immunoglobulin Isotype Controls. Same fluorochrome-conjugated antibody of irrelevant specificity which has the same Ig isotype Ligand blocking control Pre-block with anti-cytokine antibody
Tip 2. Non-specific binding Fc receptor blocking, Mouse systems, FcγII/III CD 16/CD 32 Human, human Ig or 10% autologous serum in PBS Fc Receptor Anti-Fc Receptor . Immunoglobulin Isotype Controls. Same fluorochrome-conjugated antibody of irrelevant specificity which has the same Ig isotype Ligand blocking control Pre-block with anti-cytokine antibody
 Tip 3. Advantages/ Disadvantages of Using More Colors Advantages Save Time, Reagents, Samples (1) 6 -color stain = (15) 2 -color stains Exponential increase in information Data from (1) 6 -color stain » (15) 2 -color stains identify new/rare population (<0. 05%) internal controls Disadvantages Must carefully choose combinations of fluorochrome conjugates All reagents not available in all colors Greater potential for errors in compensation Proper controls required
Tip 3. Advantages/ Disadvantages of Using More Colors Advantages Save Time, Reagents, Samples (1) 6 -color stain = (15) 2 -color stains Exponential increase in information Data from (1) 6 -color stain » (15) 2 -color stains identify new/rare population (<0. 05%) internal controls Disadvantages Must carefully choose combinations of fluorochrome conjugates All reagents not available in all colors Greater potential for errors in compensation Proper controls required
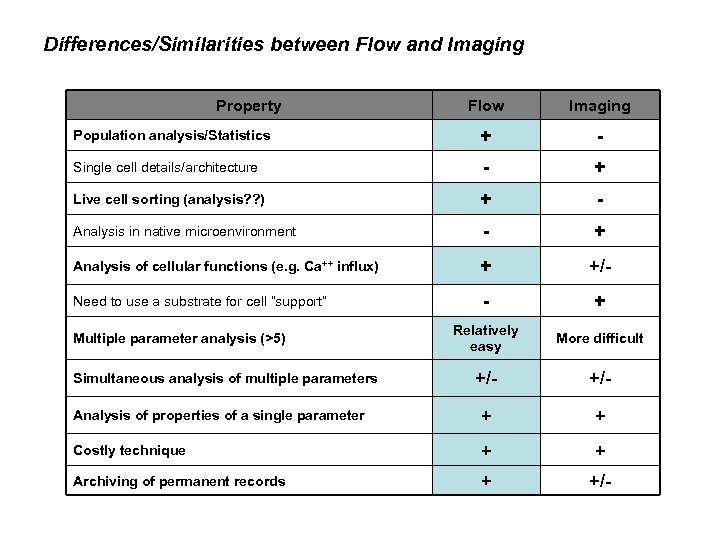 Differences/Similarities between Flow and Imaging Property Flow Imaging Population analysis/Statistics + - Single cell details/architecture - + Live cell sorting (analysis? ? ) + - Analysis in native microenvironment - + Analysis of cellular functions (e. g. Ca++ influx) + +/- Need to use a substrate for cell “support” - + Relatively easy More difficult +/- Analysis of properties of a single parameter + + Costly technique + + Archiving of permanent records + +/- Multiple parameter analysis (>5) Simultaneous analysis of multiple parameters
Differences/Similarities between Flow and Imaging Property Flow Imaging Population analysis/Statistics + - Single cell details/architecture - + Live cell sorting (analysis? ? ) + - Analysis in native microenvironment - + Analysis of cellular functions (e. g. Ca++ influx) + +/- Need to use a substrate for cell “support” - + Relatively easy More difficult +/- Analysis of properties of a single parameter + + Costly technique + + Archiving of permanent records + +/- Multiple parameter analysis (>5) Simultaneous analysis of multiple parameters
 Web Resource for Flow Cytometry General http: //flowcyt. salk. edu/sitelink. html (The Salk Institute CCMI) http: //www. aecom. yu. edu/facs/default. html (Albert Einstein) http: //www. drmr. com/compensation (Mario Roederer) http: //www. bdbiosciences. com/colors/ (BD Biosciences Inc. ) Fluorescent Dye http: //www. bdbiosciences. com/spectra/ (BD spectrum viewer) http: //probes. invitrogen. com/resources/spectraviewer/ (Invitrogen spectrum viewer) Analysis Software http: //www. flowjo. com (Flow. Jo) http: //www. denovosoftware. com (FCS Express) etc. http: //info. med. yale. edu/immuno/cytometry/ (Yale Cell Sorter Facility)
Web Resource for Flow Cytometry General http: //flowcyt. salk. edu/sitelink. html (The Salk Institute CCMI) http: //www. aecom. yu. edu/facs/default. html (Albert Einstein) http: //www. drmr. com/compensation (Mario Roederer) http: //www. bdbiosciences. com/colors/ (BD Biosciences Inc. ) Fluorescent Dye http: //www. bdbiosciences. com/spectra/ (BD spectrum viewer) http: //probes. invitrogen. com/resources/spectraviewer/ (Invitrogen spectrum viewer) Analysis Software http: //www. flowjo. com (Flow. Jo) http: //www. denovosoftware. com (FCS Express) etc. http: //info. med. yale. edu/immuno/cytometry/ (Yale Cell Sorter Facility)
 Questions Yale, Beinecke Rare Book
Questions Yale, Beinecke Rare Book


