55f68887dbc1d43acc1a599bb780e5d8.ppt
- Количество слайдов: 25
 First-principles molecular dynamics studies of liquid and glasses Carlo Massobrio Institut de Physique et Chimie des Matériaux Strasbourg (France) (CNRS-Univ. L. Pasteur) Short and intermediate range order structural properties in disordered network-forming materials: AX 2, An X(1 -n) A=Ge, Si , X=Se Current Challenges in Liquid and Glasses Science Cosener’s House, Abingdon, United Kingdom, 10 -12/1/2007 Old Strasbourg: "Petite France"
First-principles molecular dynamics studies of liquid and glasses Carlo Massobrio Institut de Physique et Chimie des Matériaux Strasbourg (France) (CNRS-Univ. L. Pasteur) Short and intermediate range order structural properties in disordered network-forming materials: AX 2, An X(1 -n) A=Ge, Si , X=Se Current Challenges in Liquid and Glasses Science Cosener’s House, Abingdon, United Kingdom, 10 -12/1/2007 Old Strasbourg: "Petite France"
 First-principles molecular dynamics studies of liquid and glasses Carlo Massobrio Institut de Physique et Chimie des Matériaux Strasbourg (France) (CNRS-Univ. L. Pasteur) Short and intermediate range order structural properties in disordered network-forming materials: AX 2, An X(1 -n) A=Ge, Si , X=Se Current Challenges in Liquid and Glasses Science Cosener’s House, Abingdon, United Kingdom, 10 -12/1/2007 Liquid Ge. Se 2 at 1100 K
First-principles molecular dynamics studies of liquid and glasses Carlo Massobrio Institut de Physique et Chimie des Matériaux Strasbourg (France) (CNRS-Univ. L. Pasteur) Short and intermediate range order structural properties in disordered network-forming materials: AX 2, An X(1 -n) A=Ge, Si , X=Se Current Challenges in Liquid and Glasses Science Cosener’s House, Abingdon, United Kingdom, 10 -12/1/2007 Liquid Ge. Se 2 at 1100 K
 Our model: essential features First principles molecular dynamics Density functional theory: best with GGA…!! (case of Ge. Se 2) Periodic cell, Plane waves basis sets Norm conserving pseudopotentials How do we deal with space and time limitations. . ? Typical size of the periodic boxes: L=15 -20 Å (N=120, 144) kmin < 0. 4 Å-1 k. FSDP 1 Å-1 (intermediate range order scales) Length of equilibrium trajectories : Liquids : up to 100 ps (significant sampling ensured by diffusion) Glasses : Quench of uncorrelated liquid configurations (LC) followed by structural relaxation Ex: Ge. Se 2: for each LC, 22 ps down to T=600 K and 22 ps at T=300 K Computer code: norm-conserving version of ultra-soft FPMD written by A. Pasquarello (Lausanne) (PRL 69, 1982 (1992), PRB 47, 10142 (1993))
Our model: essential features First principles molecular dynamics Density functional theory: best with GGA…!! (case of Ge. Se 2) Periodic cell, Plane waves basis sets Norm conserving pseudopotentials How do we deal with space and time limitations. . ? Typical size of the periodic boxes: L=15 -20 Å (N=120, 144) kmin < 0. 4 Å-1 k. FSDP 1 Å-1 (intermediate range order scales) Length of equilibrium trajectories : Liquids : up to 100 ps (significant sampling ensured by diffusion) Glasses : Quench of uncorrelated liquid configurations (LC) followed by structural relaxation Ex: Ge. Se 2: for each LC, 22 ps down to T=600 K and 22 ps at T=300 K Computer code: norm-conserving version of ultra-soft FPMD written by A. Pasquarello (Lausanne) (PRL 69, 1982 (1992), PRB 47, 10142 (1993))
 Two issues of methodology: 1) Role of the generalized gradient approximation (GGA) within density functional theory (case of liquid Ge. Se 2) (CM, Alfredo Pasquarello, Roberto Car, JACS 121, 2943 (1999)) 2) System size and periodicity: are they compatible with IRO. . ? (CM, Alfredo Pasquarello, Roberto Car, PRB 64, 144205 (2001))
Two issues of methodology: 1) Role of the generalized gradient approximation (GGA) within density functional theory (case of liquid Ge. Se 2) (CM, Alfredo Pasquarello, Roberto Car, JACS 121, 2943 (1999)) 2) System size and periodicity: are they compatible with IRO. . ? (CM, Alfredo Pasquarello, Roberto Car, PRB 64, 144205 (2001))
 Correlation between structure and bonding properties: role of the GGA in DFT (case of liquid Ge. Se 2) GGA LDA Ionic character of bonding enhanced: GGA brings the FSDP together with the predominant occurrence of a tetrahedral order.
Correlation between structure and bonding properties: role of the GGA in DFT (case of liquid Ge. Se 2) GGA LDA Ionic character of bonding enhanced: GGA brings the FSDP together with the predominant occurrence of a tetrahedral order.
 Two issues of methodology: 1) Role of the generalized gradient approximation (GGA) within density functional theory (case of liquid Ge. Se 2) (CM, Alfredo Pasquarello, Roberto Car, JACS 121, 2943 (1999)) 2) System size and periodicity: are they compatible with intermediate range order (IRO) distances. . . ? 1) (CM, Alfredo Pasquarello, Roberto Car, PRB 64, 144205 (2001))
Two issues of methodology: 1) Role of the generalized gradient approximation (GGA) within density functional theory (case of liquid Ge. Se 2) (CM, Alfredo Pasquarello, Roberto Car, JACS 121, 2943 (1999)) 2) System size and periodicity: are they compatible with intermediate range order (IRO) distances. . . ? 1) (CM, Alfredo Pasquarello, Roberto Car, PRB 64, 144205 (2001))
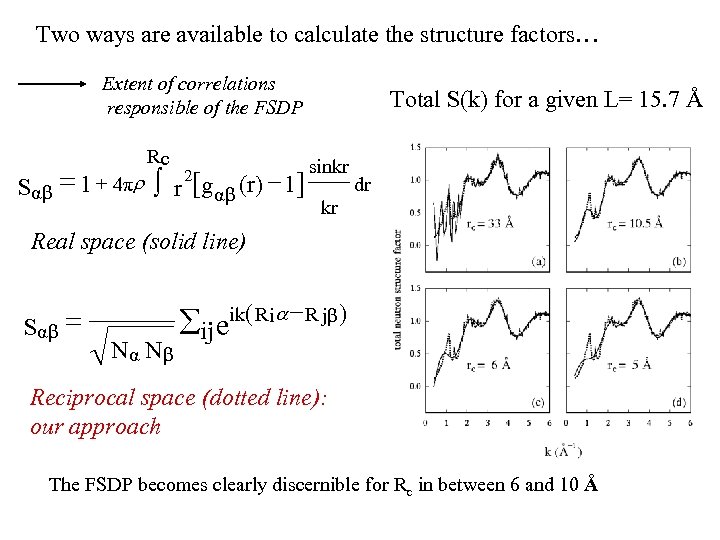 Two ways are available to calculate the structure factors… Extent of correlations responsible of the FSDP Rc Sαβ = 1 + 4πr ò r [g αβ (r) - 1] 2 Total S(k) for a given L= 15. 7 Å sinkr dr kr Real space (solid line) Sαβ = N α Nβ åij eik( R ia - R jβ ) Reciprocal space (dotted line): our approach The FSDP becomes clearly discernible for Rc in between 6 and 10 Å
Two ways are available to calculate the structure factors… Extent of correlations responsible of the FSDP Rc Sαβ = 1 + 4πr ò r [g αβ (r) - 1] 2 Total S(k) for a given L= 15. 7 Å sinkr dr kr Real space (solid line) Sαβ = N α Nβ åij eik( R ia - R jβ ) Reciprocal space (dotted line): our approach The FSDP becomes clearly discernible for Rc in between 6 and 10 Å
 Liquid Ge. Se 2: a prototype case FSDP I. T. Penfold and P. S. Salmon, PRL 67, 97(1991) FSDP Total S(k) FSDP in both total and Scc structure factors Scc/cge cse = 1+cgecse(Sgege-Sgese) +cgecse(Ssese-Sgese) For point charges (classical MD): no FSDP in SCC Scc/cgecse = SZZ charge-charge structure factor
Liquid Ge. Se 2: a prototype case FSDP I. T. Penfold and P. S. Salmon, PRL 67, 97(1991) FSDP Total S(k) FSDP in both total and Scc structure factors Scc/cge cse = 1+cgecse(Sgege-Sgese) +cgecse(Ssese-Sgese) For point charges (classical MD): no FSDP in SCC Scc/cgecse = SZZ charge-charge structure factor
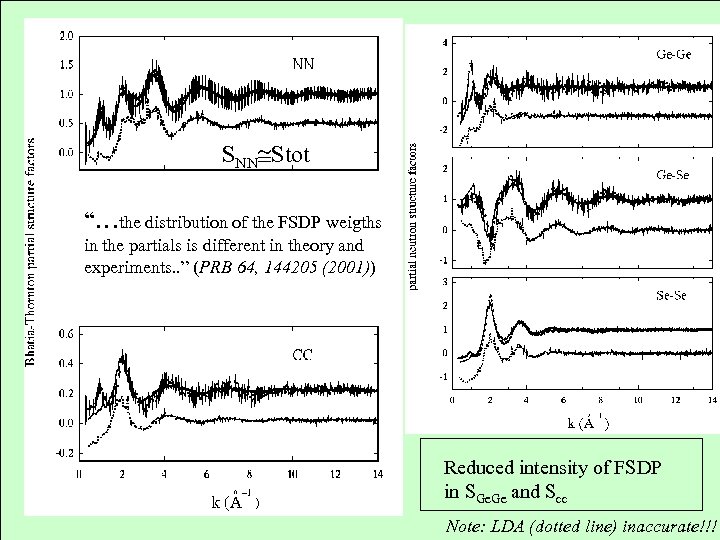 SNN Stot “…the distribution of the FSDP weigths in the partials is different in theory and experiments. . ” (PRB 64, 144205 (2001)) Reduced intensity of FSDP in SGe. Ge and Scc Note: LDA (dotted line) inaccurate!!!
SNN Stot “…the distribution of the FSDP weigths in the partials is different in theory and experiments. . ” (PRB 64, 144205 (2001)) Reduced intensity of FSDP in SGe. Ge and Scc Note: LDA (dotted line) inaccurate!!!
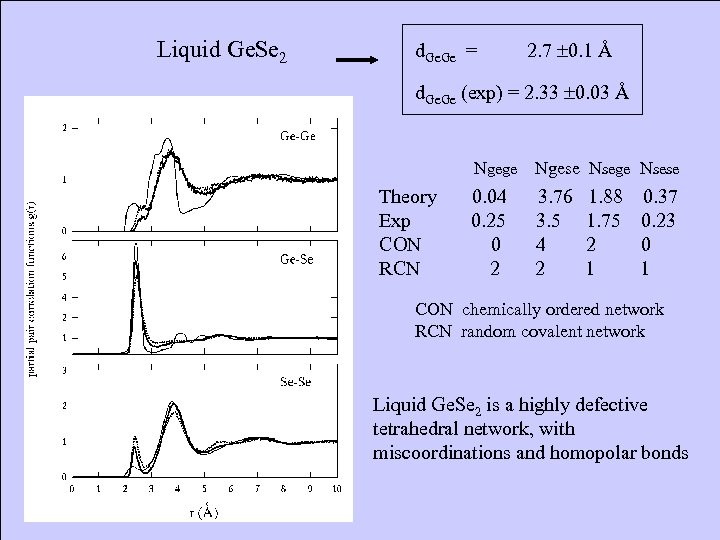 Liquid Ge. Se 2 d. Ge = 2. 7 0. 1 Å d. Ge (exp) = 2. 33 0. 03 Å Ngege Ngese Nsege Nsese Theory 0. 04 3. 76 1. 88 0. 37 Exp 0. 25 3. 5 1. 75 0. 23 CON 0 4 2 0 RCN 2 2 1 CON chemically ordered network RCN random covalent network Liquid Ge. Se 2 is a highly defective tetrahedral network, with miscoordinations and homopolar bonds
Liquid Ge. Se 2 d. Ge = 2. 7 0. 1 Å d. Ge (exp) = 2. 33 0. 03 Å Ngege Ngese Nsege Nsese Theory 0. 04 3. 76 1. 88 0. 37 Exp 0. 25 3. 5 1. 75 0. 23 CON 0 4 2 0 RCN 2 2 1 CON chemically ordered network RCN random covalent network Liquid Ge. Se 2 is a highly defective tetrahedral network, with miscoordinations and homopolar bonds

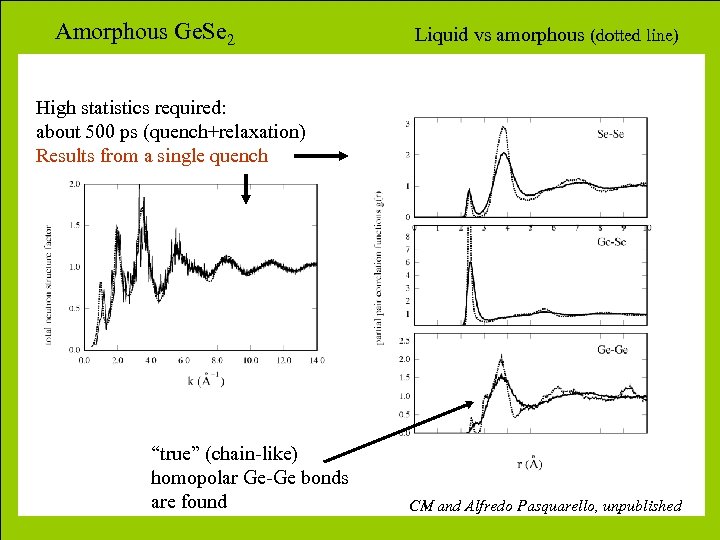 Amorphous Ge. Se 2 Liquid vs amorphous (dotted line) High statistics required: about 500 ps (quench+relaxation) Results from a single quench “true” (chain-like) homopolar Ge-Ge bonds are found CM and Alfredo Pasquarello, unpublished
Amorphous Ge. Se 2 Liquid vs amorphous (dotted line) High statistics required: about 500 ps (quench+relaxation) Results from a single quench “true” (chain-like) homopolar Ge-Ge bonds are found CM and Alfredo Pasquarello, unpublished
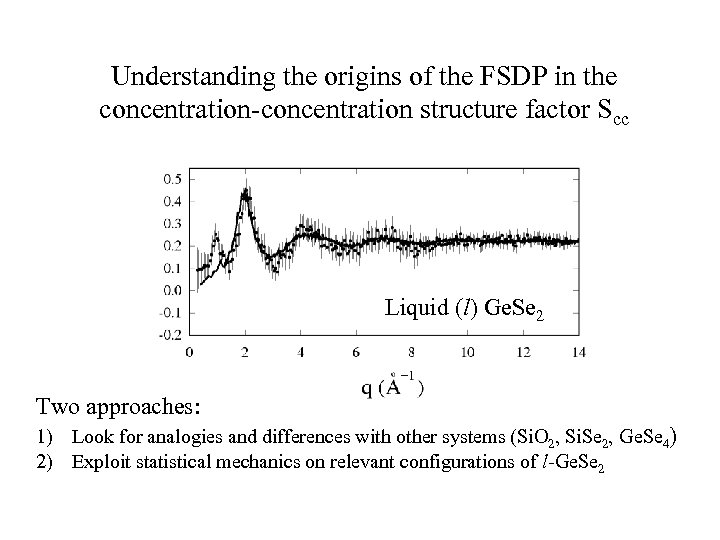 Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
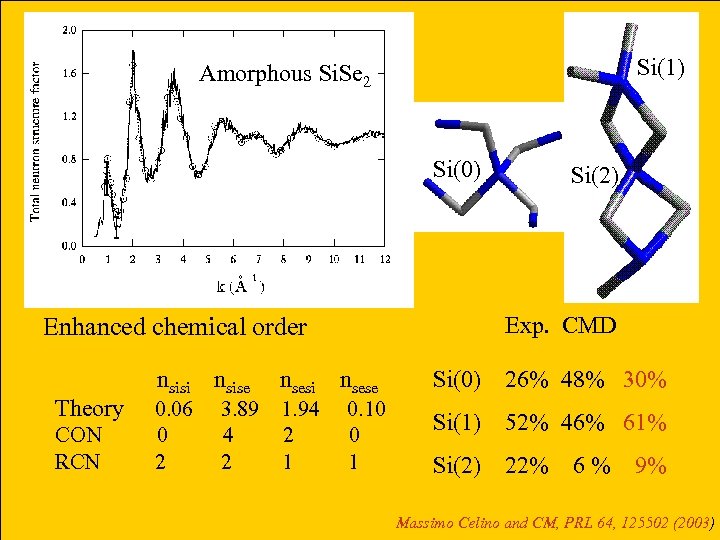 Si(1) Amorphous Si. Se 2 Si(0) Enhanced chemical order nsisi nsise nsesi nsese Theory 0. 06 3. 89 1. 94 0. 10 CON 0 4 2 0 RCN 2 1 Si(2) Exp. CMD Si(0) 26% 48% 30% Si(1) 52% 46% 61% Si(2) 22% 6 % 9% Massimo Celino and CM, PRL 64, 125502 (2003)
Si(1) Amorphous Si. Se 2 Si(0) Enhanced chemical order nsisi nsise nsesi nsese Theory 0. 06 3. 89 1. 94 0. 10 CON 0 4 2 0 RCN 2 1 Si(2) Exp. CMD Si(0) 26% 48% 30% Si(1) 52% 46% 61% Si(2) 22% 6 % 9% Massimo Celino and CM, PRL 64, 125502 (2003)
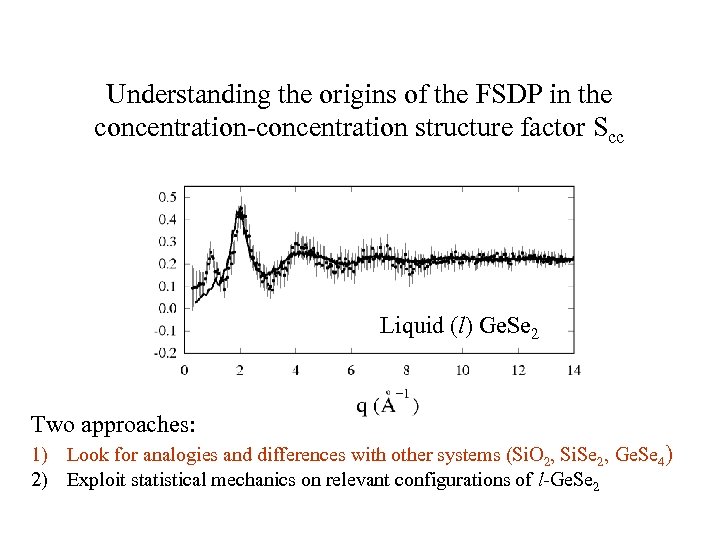 Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
 How to calculate Scc and Szz structure factors No FSDP in Szz (charge neutrality on IRO scales) Scc= cacx[1+cacx((SAA-SAX)+(SXX-SAX))] (CM and Alfredo Pasquarello PRB 68, 020201 (2003)) zi ionic charges (Ge= 4, Se =6) POINT-LIKE CHARGE (PLC) MODEL What is the behavior of Scc for systems having in common the FSDP in the total S(k). . ? ?
How to calculate Scc and Szz structure factors No FSDP in Szz (charge neutrality on IRO scales) Scc= cacx[1+cacx((SAA-SAX)+(SXX-SAX))] (CM and Alfredo Pasquarello PRB 68, 020201 (2003)) zi ionic charges (Ge= 4, Se =6) POINT-LIKE CHARGE (PLC) MODEL What is the behavior of Scc for systems having in common the FSDP in the total S(k). . ? ?
 Correlation between FSDP in Scc and chemical disorder Case I: perfect chemical order: l-Si. O 2 (theory) Case II: small deviations from chemical order: a-Si. Se 2 (theory) Case III: large deviations from chemical order: l-Ge. Se 2 (theory)
Correlation between FSDP in Scc and chemical disorder Case I: perfect chemical order: l-Si. O 2 (theory) Case II: small deviations from chemical order: a-Si. Se 2 (theory) Case III: large deviations from chemical order: l-Ge. Se 2 (theory)
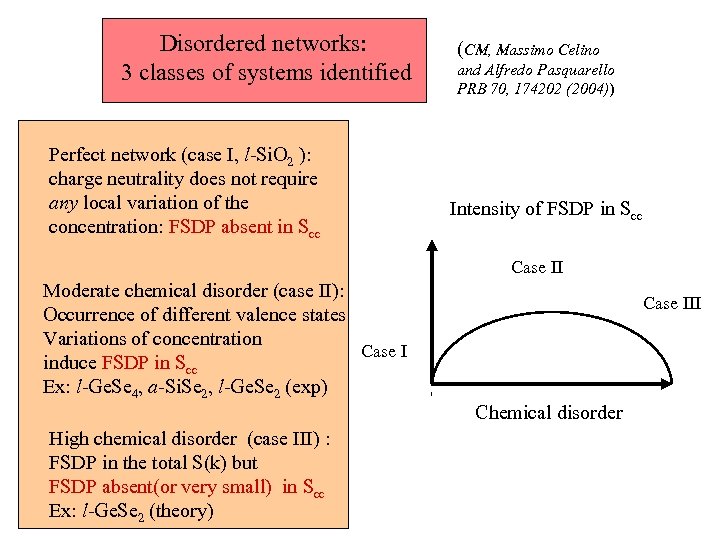 Disordered networks: 3 classes of systems identified Perfect network (case I, l-Si. O 2 ): charge neutrality does not require any local variation of the concentration: FSDP absent in Scc (CM, Massimo Celino and Alfredo Pasquarello PRB 70, 174202 (2004)) Intensity of FSDP in Scc Case II Moderate chemical disorder (case II): Occurrence of different valence states Variations of concentration Case I induce FSDP in Scc Ex: l-Ge. Se 4, a-Si. Se 2, l-Ge. Se 2 (exp) High chemical disorder (case III) : FSDP in the total S(k) but FSDP absent(or very small) in Scc Ex: l-Ge. Se 2 (theory) Case III Chemical disorder
Disordered networks: 3 classes of systems identified Perfect network (case I, l-Si. O 2 ): charge neutrality does not require any local variation of the concentration: FSDP absent in Scc (CM, Massimo Celino and Alfredo Pasquarello PRB 70, 174202 (2004)) Intensity of FSDP in Scc Case II Moderate chemical disorder (case II): Occurrence of different valence states Variations of concentration Case I induce FSDP in Scc Ex: l-Ge. Se 4, a-Si. Se 2, l-Ge. Se 2 (exp) High chemical disorder (case III) : FSDP in the total S(k) but FSDP absent(or very small) in Scc Ex: l-Ge. Se 2 (theory) Case III Chemical disorder
 Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
Understanding the origins of the FSDP in the concentration-concentration structure factor Scc Liquid (l) Ge. Se 2 Two approaches: 1) Look for analogies and differences with other systems (Si. O 2, Si. Se 2, Ge. Se 4) 2) Exploit statistical mechanics on relevant configurations of l-Ge. Se 2
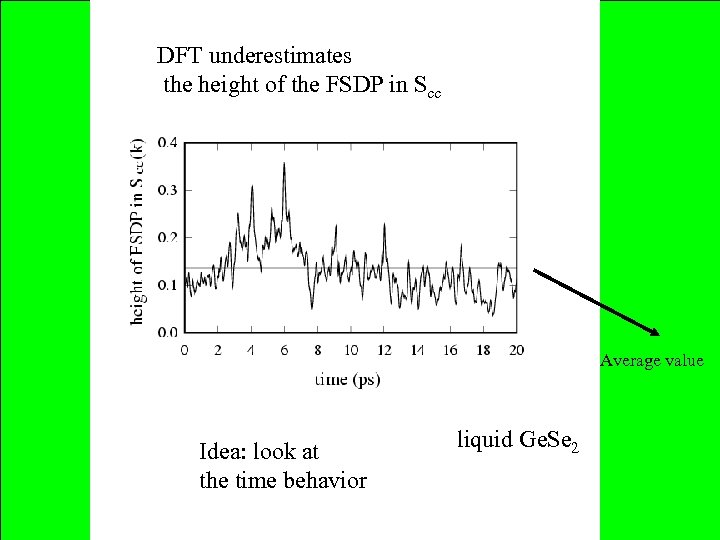 DFT underestimates the height of the FSDP in Scc Average value Idea: look at the time behavior liquid Ge. Se 2
DFT underestimates the height of the FSDP in Scc Average value Idea: look at the time behavior liquid Ge. Se 2
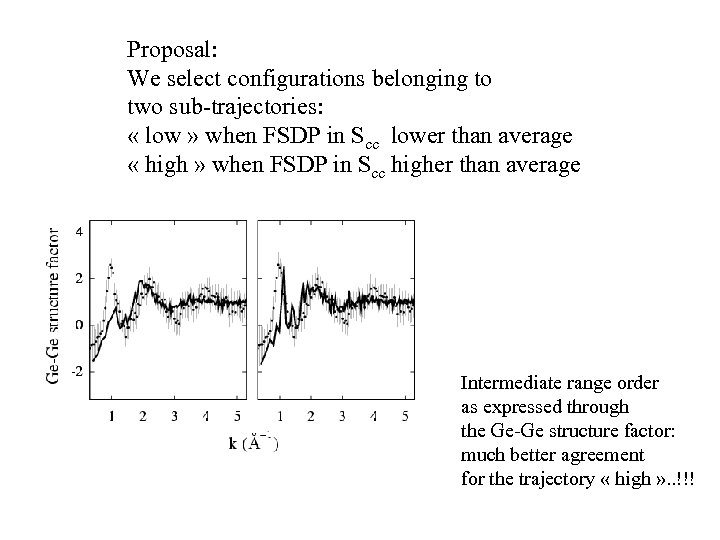 Proposal: We select configurations belonging to two sub-trajectories: « low » when FSDP in Scc lower than average « high » when FSDP in Scc higher than average Intermediate range order as expressed through the Ge-Ge structure factor: much better agreement for the trajectory « high » . . !!!
Proposal: We select configurations belonging to two sub-trajectories: « low » when FSDP in Scc lower than average « high » when FSDP in Scc higher than average Intermediate range order as expressed through the Ge-Ge structure factor: much better agreement for the trajectory « high » . . !!!
 « low » By using the same scheme, we compare the two sets of Bhatia-Thornton structure factors Note : the NNs are very close: both sets yield very good total structure factors « high »
« low » By using the same scheme, we compare the two sets of Bhatia-Thornton structure factors Note : the NNs are very close: both sets yield very good total structure factors « high »
 Comparing an extensive list of structural properties (coordination numbers, rings topology, g (r) ) the results on the two trajectories are very close… Where does the difference come from. . ? Number of Ge in two fourfold rings (chains of edge-sharing tetrahedra): Ge* units Warning: 1 and 2 form a Ge*, 2 and 3 do not…!!! The trajectory « high » has a higher number of Ge* subunits
Comparing an extensive list of structural properties (coordination numbers, rings topology, g (r) ) the results on the two trajectories are very close… Where does the difference come from. . ? Number of Ge in two fourfold rings (chains of edge-sharing tetrahedra): Ge* units Warning: 1 and 2 form a Ge*, 2 and 3 do not…!!! The trajectory « high » has a higher number of Ge* subunits
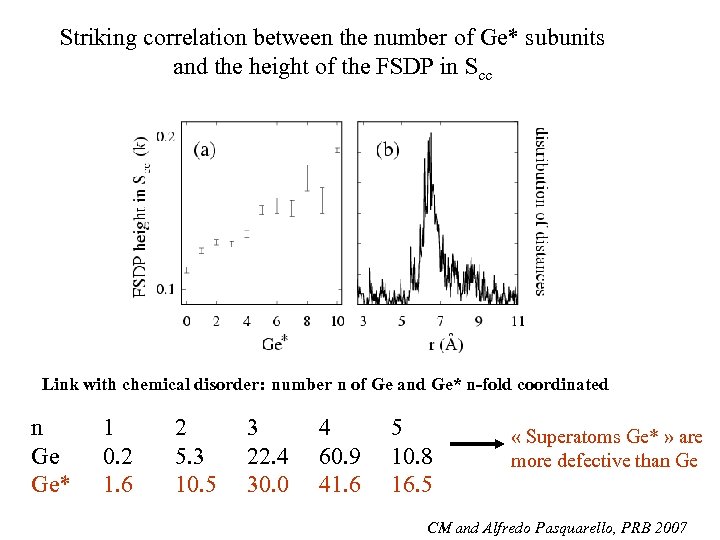 Striking correlation between the number of Ge* subunits and the height of the FSDP in Scc Link with chemical disorder: number n of Ge and Ge* n-fold coordinated n Ge Ge* 1 0. 2 1. 6 2 5. 3 10. 5 3 22. 4 30. 0 4 60. 9 41. 6 5 10. 8 16. 5 « Superatoms Ge* » are more defective than Ge CM and Alfredo Pasquarello, PRB 2007
Striking correlation between the number of Ge* subunits and the height of the FSDP in Scc Link with chemical disorder: number n of Ge and Ge* n-fold coordinated n Ge Ge* 1 0. 2 1. 6 2 5. 3 10. 5 3 22. 4 30. 0 4 60. 9 41. 6 5 10. 8 16. 5 « Superatoms Ge* » are more defective than Ge CM and Alfredo Pasquarello, PRB 2007
 Structural studies of disordered network-forming materials: quantitative AND predictive power of first-principles molecular dynamics at short and intermediate range-order distances Ge-Se based systems: challenging and stimulating for DFT approaches Many thanks to IPCMS CNRS IDRIS (France), CINES (France) CSCS (Switzerland) Computer centers Work in collaboration with Alfredo Pasquarello (early stages: Roberto Car) on Si-Se systems Massimo Celino
Structural studies of disordered network-forming materials: quantitative AND predictive power of first-principles molecular dynamics at short and intermediate range-order distances Ge-Se based systems: challenging and stimulating for DFT approaches Many thanks to IPCMS CNRS IDRIS (France), CINES (France) CSCS (Switzerland) Computer centers Work in collaboration with Alfredo Pasquarello (early stages: Roberto Car) on Si-Se systems Massimo Celino


