897adaba9a55f8da507f062645e8f408.ppt
- Количество слайдов: 38
 First-Line Treatment for MM Patients Myelomacenter. org run 9001@med. cornell. edu Ruben Niesvizky Department of Medicine, Division of Hematology/Oncology, Weill-Cornell Medical College / New York Presbyterian Hospital, New York, NY, USA
First-Line Treatment for MM Patients Myelomacenter. org run 9001@med. cornell. edu Ruben Niesvizky Department of Medicine, Division of Hematology/Oncology, Weill-Cornell Medical College / New York Presbyterian Hospital, New York, NY, USA
 Disclosures • Speaker’s bureau: Celgene, Millennium, Onyx
Disclosures • Speaker’s bureau: Celgene, Millennium, Onyx
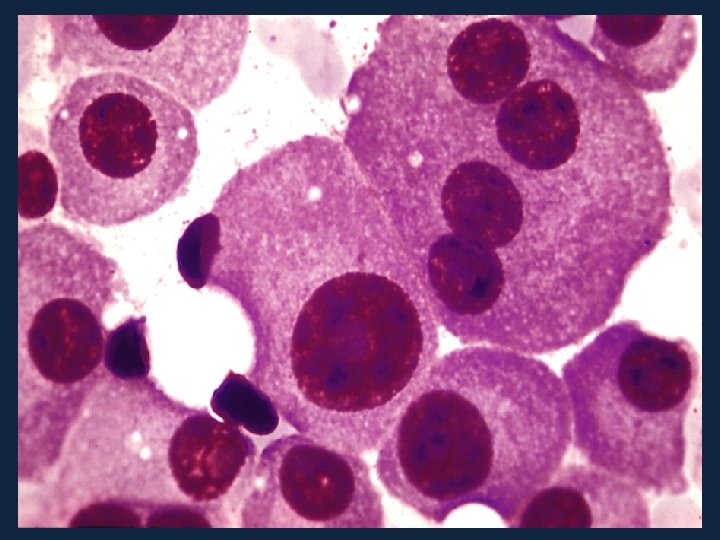


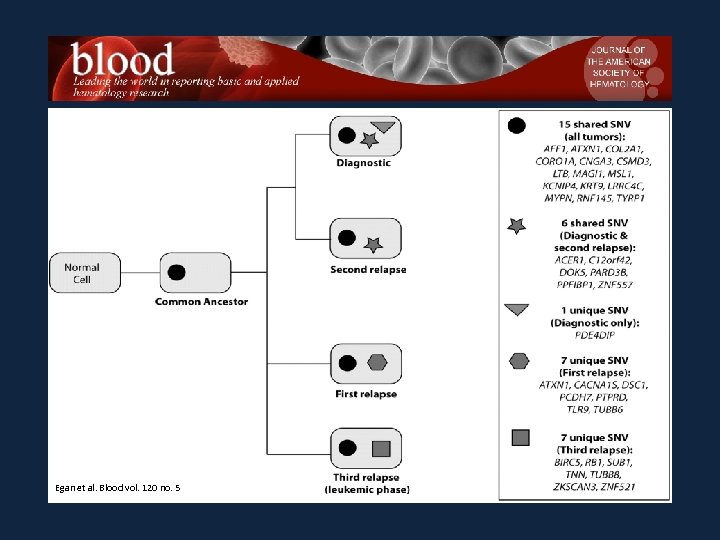 Egan et al. Blood vol. 120 no. 5
Egan et al. Blood vol. 120 no. 5
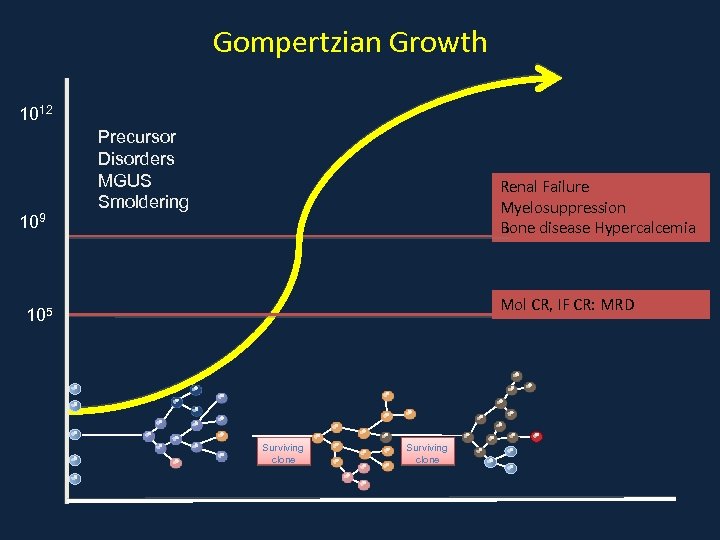 Gompertzian Growth 1012 109 Precursor Disorders MGUS Smoldering Renal Failure Myelosuppression Bone disease Hypercalcemia Mol CR, IF CR: MRD 105 Surviving clone
Gompertzian Growth 1012 109 Precursor Disorders MGUS Smoldering Renal Failure Myelosuppression Bone disease Hypercalcemia Mol CR, IF CR: MRD 105 Surviving clone
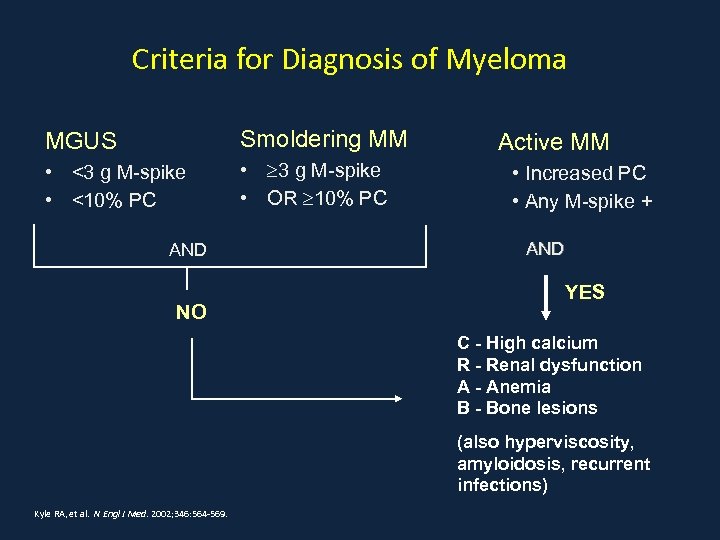 Criteria for Diagnosis of Myeloma MGUS Smoldering MM • <3 g M-spike • <10% PC • 3 g M-spike • OR 10% PC AND NO Active MM • Increased PC • Any M-spike + AND YES C - High calcium R - Renal dysfunction A - Anemia B - Bone lesions (also hyperviscosity, amyloidosis, recurrent infections) Kyle RA, et al. N Engl J Med. 2002; 346: 564 -569.
Criteria for Diagnosis of Myeloma MGUS Smoldering MM • <3 g M-spike • <10% PC • 3 g M-spike • OR 10% PC AND NO Active MM • Increased PC • Any M-spike + AND YES C - High calcium R - Renal dysfunction A - Anemia B - Bone lesions (also hyperviscosity, amyloidosis, recurrent infections) Kyle RA, et al. N Engl J Med. 2002; 346: 564 -569.
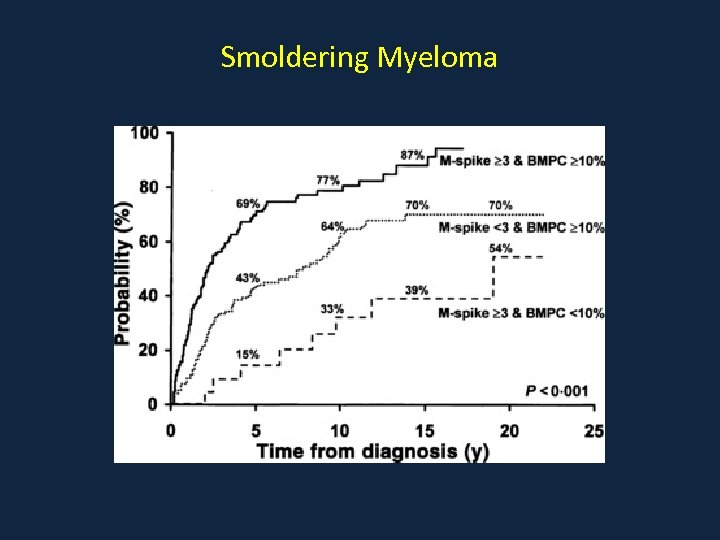 Smoldering Myeloma
Smoldering Myeloma
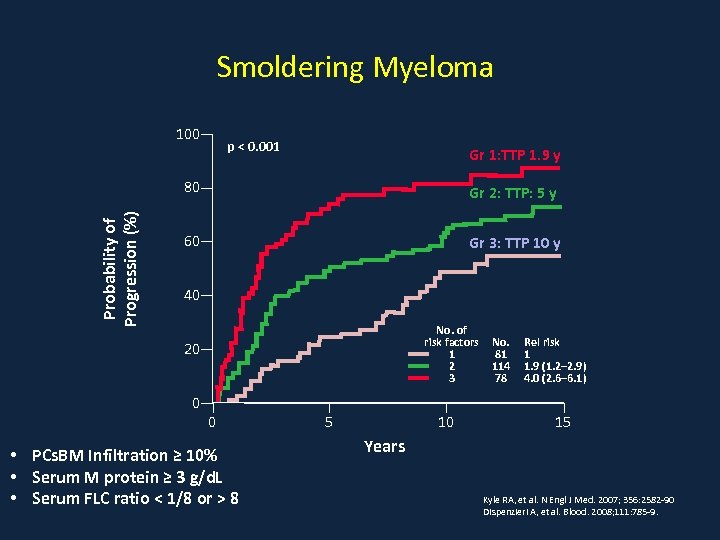 Smoldering Myeloma 100 p < 0. 001 Gr 1: TTP 1. 9 y Probability of Progression (%) 80 Gr 2: TTP: 5 y 60 Gr 3: TTP 10 y 40 No. of risk factors 1 2 3 20 0 0 • PCs. BM Infiltration ≥ 10% • Serum M protein ≥ 3 g/d. L • Serum FLC ratio < 1/8 or > 8 5 10 No. 81 114 78 Rel risk 1 1. 9 (1. 2– 2. 9) 4. 0 (2. 6– 6. 1) 15 Years Kyle RA, et al. N Engl J Med. 2007; 356: 2582 -90 Dispenzieri A, et al. Blood. 2008; 111: 785 -9.
Smoldering Myeloma 100 p < 0. 001 Gr 1: TTP 1. 9 y Probability of Progression (%) 80 Gr 2: TTP: 5 y 60 Gr 3: TTP 10 y 40 No. of risk factors 1 2 3 20 0 0 • PCs. BM Infiltration ≥ 10% • Serum M protein ≥ 3 g/d. L • Serum FLC ratio < 1/8 or > 8 5 10 No. 81 114 78 Rel risk 1 1. 9 (1. 2– 2. 9) 4. 0 (2. 6– 6. 1) 15 Years Kyle RA, et al. N Engl J Med. 2007; 356: 2582 -90 Dispenzieri A, et al. Blood. 2008; 111: 785 -9.
 ORIGINAL ARTICLE Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma María-Victoria Mateos et al. N Engl J Med. 2013; 369: 438 -447.
ORIGINAL ARTICLE Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma María-Victoria Mateos et al. N Engl J Med. 2013; 369: 438 -447.
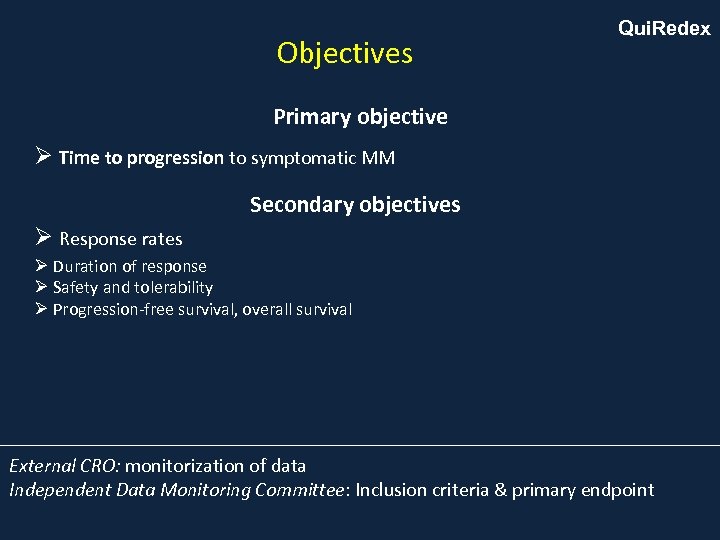 Objectives Qui. Redex Primary objective Ø Time to progression to symptomatic MM Secondary objectives Ø Response rates Ø Duration of response Ø Safety and tolerability Ø Progression-free survival, overall survival External CRO: monitorization of data Independent Data Monitoring Committee: Inclusion criteria & primary endpoint
Objectives Qui. Redex Primary objective Ø Time to progression to symptomatic MM Secondary objectives Ø Response rates Ø Duration of response Ø Safety and tolerability Ø Progression-free survival, overall survival External CRO: monitorization of data Independent Data Monitoring Committee: Inclusion criteria & primary endpoint
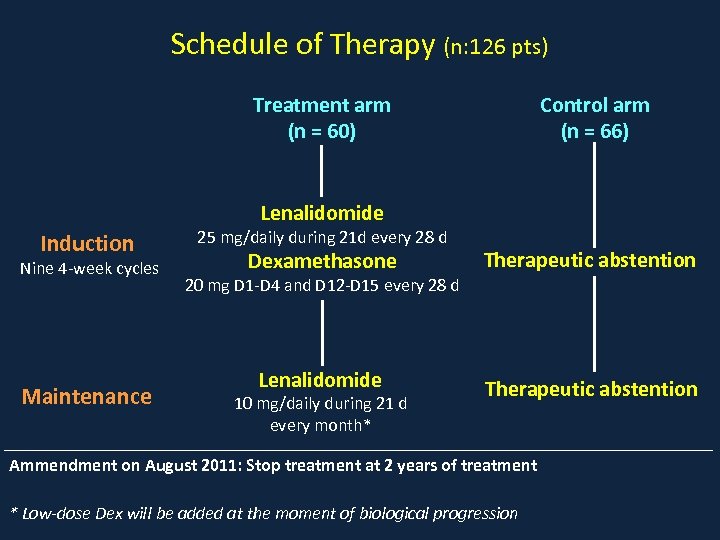 Schedule of Therapy (n: 126 pts) Treatment arm (n = 60) Control arm (n = 66) Lenalidomide Induction Nine 4 -week cycles Maintenance 25 mg/daily during 21 d every 28 d Dexamethasone Therapeutic abstention Lenalidomide Therapeutic abstention 20 mg D 1 -D 4 and D 12 -D 15 every 28 d 10 mg/daily during 21 d every month* Ammendment on August 2011: Stop treatment at 2 years of treatment * Low-dose Dex will be added at the moment of biological progression
Schedule of Therapy (n: 126 pts) Treatment arm (n = 60) Control arm (n = 66) Lenalidomide Induction Nine 4 -week cycles Maintenance 25 mg/daily during 21 d every 28 d Dexamethasone Therapeutic abstention Lenalidomide Therapeutic abstention 20 mg D 1 -D 4 and D 12 -D 15 every 28 d 10 mg/daily during 21 d every month* Ammendment on August 2011: Stop treatment at 2 years of treatment * Low-dose Dex will be added at the moment of biological progression
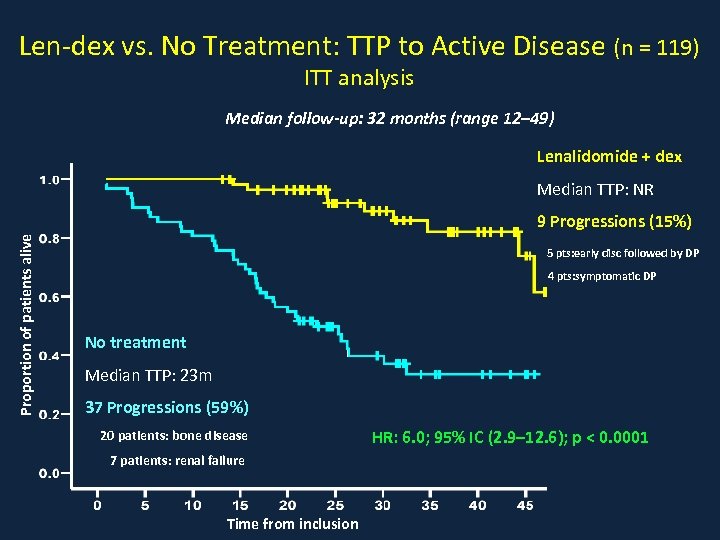 Len-dex vs. No Treatment: TTP to Active Disease (n = 119) ITT analysis Median follow-up: 32 months (range 12– 49) Lenalidomide + dex Median TTP: NR Proportion of patients alive 9 Progressions (15%) 5 pts: early disc followed by DP 4 pts: symptomatic DP No treatment Median TTP: 23 m 37 Progressions (59%) 20 patients: bone disease 7 patients: renal failure Time from inclusion HR: 6. 0; 95% IC (2. 9– 12. 6); p < 0. 0001
Len-dex vs. No Treatment: TTP to Active Disease (n = 119) ITT analysis Median follow-up: 32 months (range 12– 49) Lenalidomide + dex Median TTP: NR Proportion of patients alive 9 Progressions (15%) 5 pts: early disc followed by DP 4 pts: symptomatic DP No treatment Median TTP: 23 m 37 Progressions (59%) 20 patients: bone disease 7 patients: renal failure Time from inclusion HR: 6. 0; 95% IC (2. 9– 12. 6); p < 0. 0001
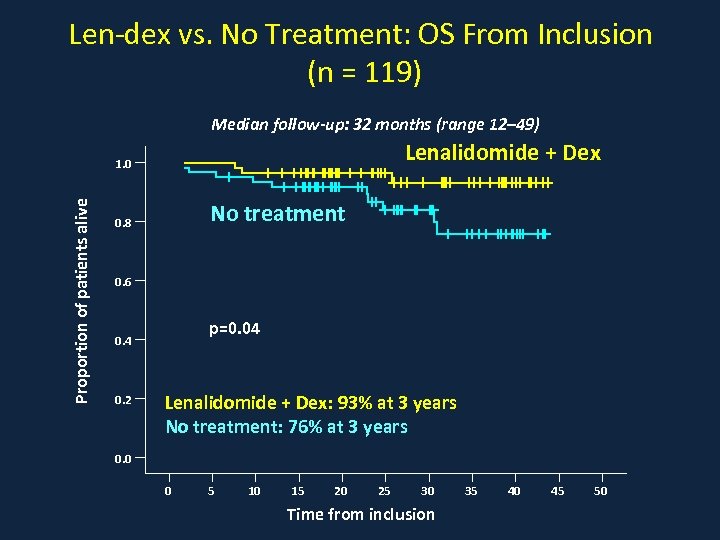 Len-dex vs. No Treatment: OS From Inclusion (n = 119) Median follow-up: 32 months (range 12– 49) Lenalidomide + Dex Proportion of patients alive 1. 0 No treatment 0. 8 0. 6 p=0. 04 0. 2 Lenalidomide + Dex: 93% at 3 years No treatment: 76% at 3 years 0. 0 0 5 10 15 20 25 30 Time from inclusion 35 40 45 50
Len-dex vs. No Treatment: OS From Inclusion (n = 119) Median follow-up: 32 months (range 12– 49) Lenalidomide + Dex Proportion of patients alive 1. 0 No treatment 0. 8 0. 6 p=0. 04 0. 2 Lenalidomide + Dex: 93% at 3 years No treatment: 76% at 3 years 0. 0 0 5 10 15 20 25 30 Time from inclusion 35 40 45 50
 • Carfilzomib, Lenalidomide, and Dexamethasone for Smoldering Multiple Myeloma • Biomarker Study of Elotuzumab in High Risk Smoldering Myeloma
• Carfilzomib, Lenalidomide, and Dexamethasone for Smoldering Multiple Myeloma • Biomarker Study of Elotuzumab in High Risk Smoldering Myeloma
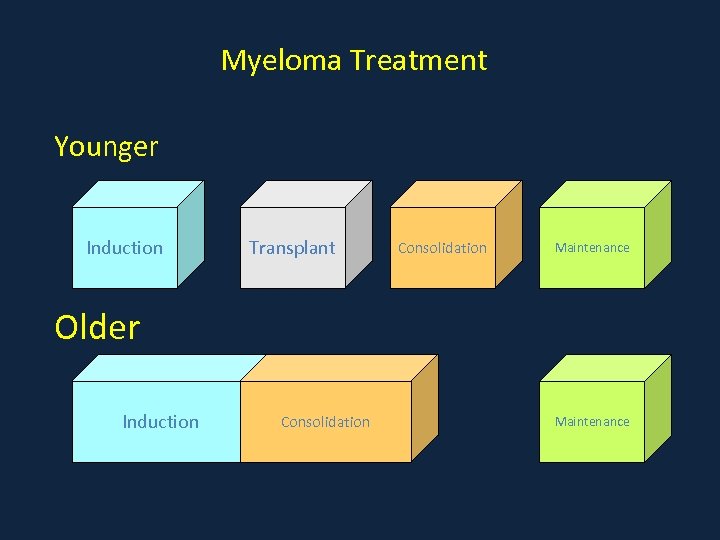 Myeloma Treatment Younger Induction Transplant Consolidation Maintenance Older Induction Consolidation Maintenance
Myeloma Treatment Younger Induction Transplant Consolidation Maintenance Older Induction Consolidation Maintenance
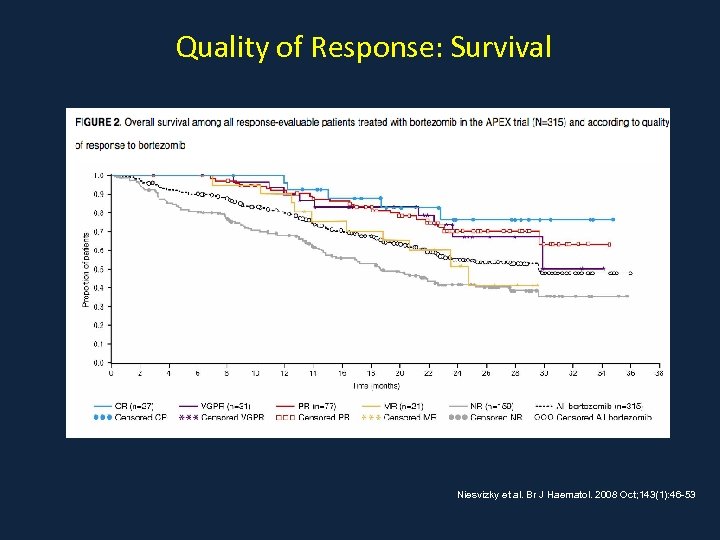 Quality of Response: Survival Niesvizky et al. Br J Haematol. 2008 Oct; 143(1): 46 -53
Quality of Response: Survival Niesvizky et al. Br J Haematol. 2008 Oct; 143(1): 46 -53
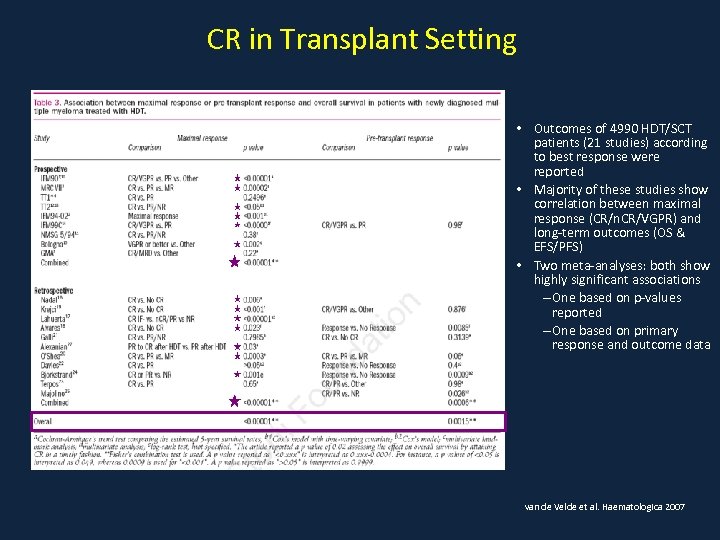 CR in Transplant Setting • Outcomes of 4990 HDT/SCT patients (21 studies) according to best response were reported • Majority of these studies show correlation between maximal response (CR/n. CR/VGPR) and long-term outcomes (OS & EFS/PFS) • Two meta-analyses: both show highly significant associations – One based on p-values reported – One based on primary response and outcome data van de Velde et al. Haematologica 2007
CR in Transplant Setting • Outcomes of 4990 HDT/SCT patients (21 studies) according to best response were reported • Majority of these studies show correlation between maximal response (CR/n. CR/VGPR) and long-term outcomes (OS & EFS/PFS) • Two meta-analyses: both show highly significant associations – One based on p-values reported – One based on primary response and outcome data van de Velde et al. Haematologica 2007
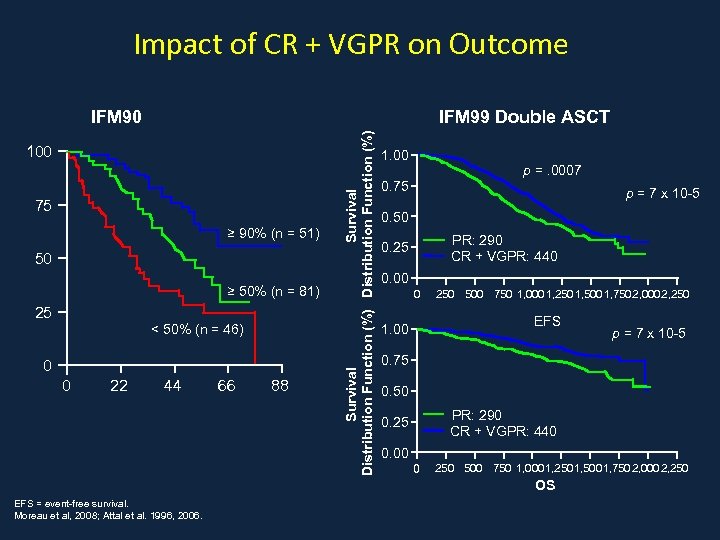 Impact of CR + VGPR on Outcome IFM 99 Double ASCT 100 75 ≥ 90% (n = 51) 50 ≥ 50% (n = 81) 25 < 50% (n = 46) 0 0 22 44 66 88 Survival Distribution Function (%) IFM 90 1. 00 p =. 0007 0. 75 p = 7 x 10 -5 0. 50 PR: 290 CR + VGPR: 440 0. 25 0. 00 0 250 500 750 1, 000 1, 250 1, 500 1, 750 2, 000 2, 250 EFS 1. 00 0. 75 0. 50 PR: 290 CR + VGPR: 440 0. 25 0. 00 0 250 500 750 1, 000 1, 250 1, 500 1, 750 2, 000 2, 250 OS EFS = event-free survival. Moreau et al, 2008; Attal et al. 1996, 2006. p = 7 x 10 -5
Impact of CR + VGPR on Outcome IFM 99 Double ASCT 100 75 ≥ 90% (n = 51) 50 ≥ 50% (n = 81) 25 < 50% (n = 46) 0 0 22 44 66 88 Survival Distribution Function (%) IFM 90 1. 00 p =. 0007 0. 75 p = 7 x 10 -5 0. 50 PR: 290 CR + VGPR: 440 0. 25 0. 00 0 250 500 750 1, 000 1, 250 1, 500 1, 750 2, 000 2, 250 EFS 1. 00 0. 75 0. 50 PR: 290 CR + VGPR: 440 0. 25 0. 00 0 250 500 750 1, 000 1, 250 1, 500 1, 750 2, 000 2, 250 OS EFS = event-free survival. Moreau et al, 2008; Attal et al. 1996, 2006. p = 7 x 10 -5
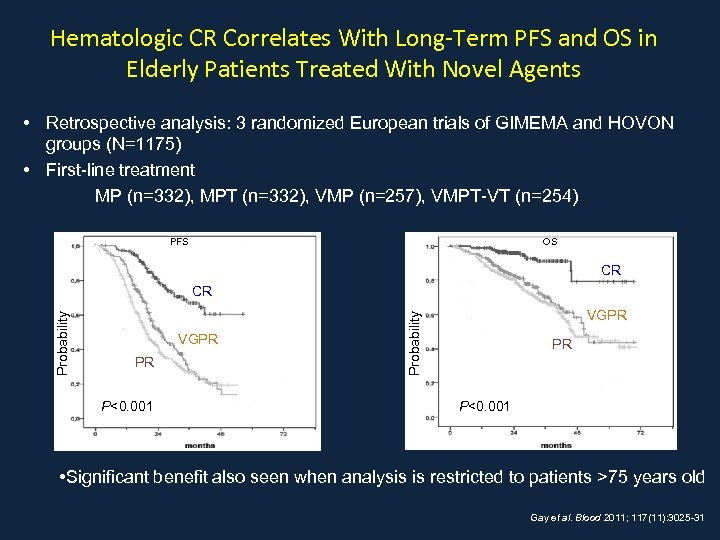 Hematologic CR Correlates With Long-Term PFS and OS in Elderly Patients Treated With Novel Agents • Retrospective analysis: 3 randomized European trials of GIMEMA and HOVON groups (N=1175) • First-line treatment MP (n=332), MPT (n=332), VMP (n=257), VMPT-VT (n=254) OS PFS CR VGPR PR P<0. 001 VGPR Probability CR PR P<0. 001 • Significant benefit also seen when analysis is restricted to patients >75 years old Gay et al. Blood 2011; 117(11): 3025 -31
Hematologic CR Correlates With Long-Term PFS and OS in Elderly Patients Treated With Novel Agents • Retrospective analysis: 3 randomized European trials of GIMEMA and HOVON groups (N=1175) • First-line treatment MP (n=332), MPT (n=332), VMP (n=257), VMPT-VT (n=254) OS PFS CR VGPR PR P<0. 001 VGPR Probability CR PR P<0. 001 • Significant benefit also seen when analysis is restricted to patients >75 years old Gay et al. Blood 2011; 117(11): 3025 -31
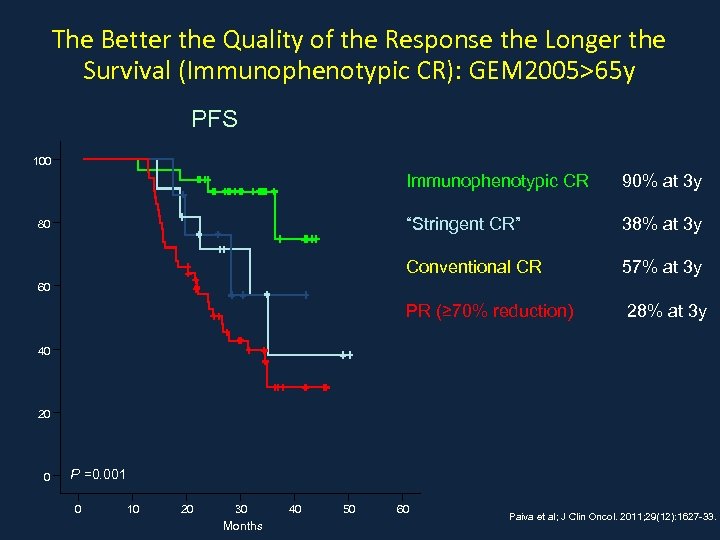 The Better the Quality of the Response the Longer the Survival (Immunophenotypic CR): GEM 2005>65 y PFS 100 Immunophenotypic CR “Stringent CR” 38% at 3 y Conventional CR 57% at 3 y PR (≥ 70% reduction) 80 90% at 3 y 28% at 3 y 60 40 20 0 P =0. 001 0 10 20 30 Months 40 50 60 Paiva et al; J Clin Oncol. 2011; 29(12): 1627 -33.
The Better the Quality of the Response the Longer the Survival (Immunophenotypic CR): GEM 2005>65 y PFS 100 Immunophenotypic CR “Stringent CR” 38% at 3 y Conventional CR 57% at 3 y PR (≥ 70% reduction) 80 90% at 3 y 28% at 3 y 60 40 20 0 P =0. 001 0 10 20 30 Months 40 50 60 Paiva et al; J Clin Oncol. 2011; 29(12): 1627 -33.
 Abstract Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
Abstract Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
 Abstract Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
Abstract Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
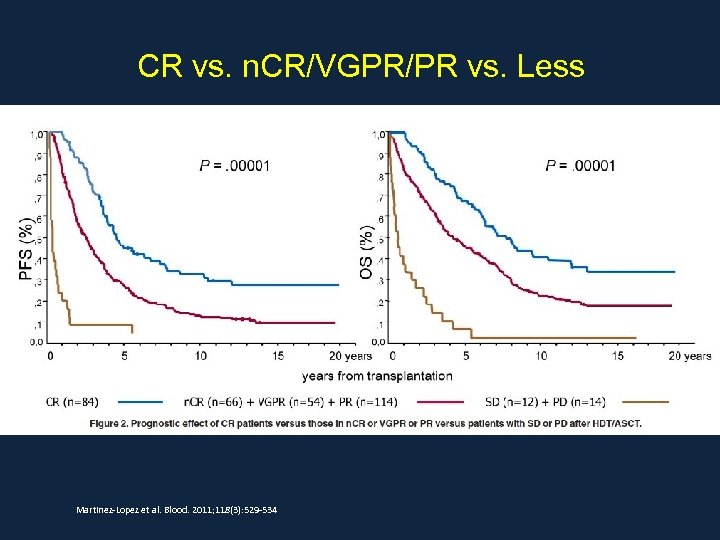 CR vs. n. CR/VGPR/PR vs. Less Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
CR vs. n. CR/VGPR/PR vs. Less Martinez-Lopez et al. Blood. 2011; 118(3): 529 -534
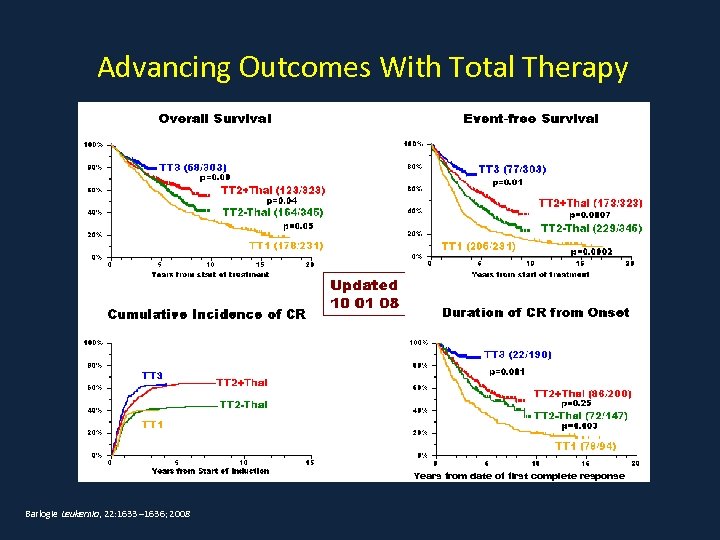 Advancing Outcomes With Total Therapy Years from date of first complete response Barlogie Leukemia, 22: 1633 – 1636; 2008
Advancing Outcomes With Total Therapy Years from date of first complete response Barlogie Leukemia, 22: 1633 – 1636; 2008
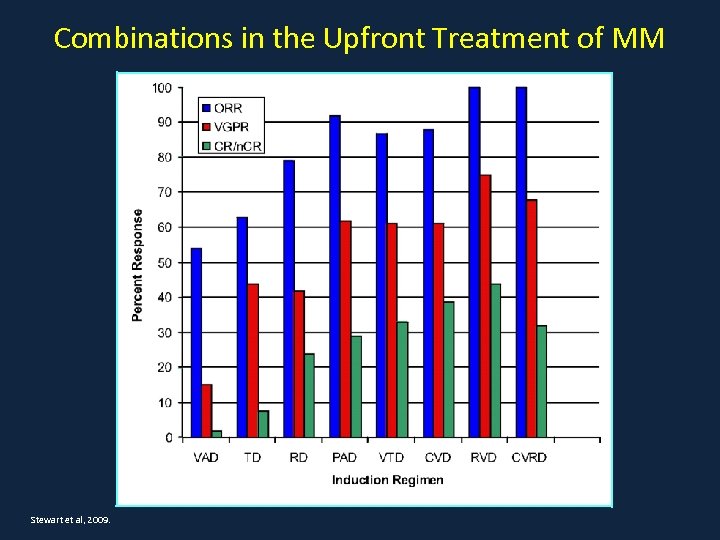 Combinations in the Upfront Treatment of MM Stewart et al, 2009.
Combinations in the Upfront Treatment of MM Stewart et al, 2009.
 Bi. RD (Clarithromycin/lenalidomide/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. 2008; 111: 1101 -1109 Prepublished online Nov 7, 2007; doi: 10. 1182/blood-2007 -05 -090258 Ruben Niesvizky, David S. Jayabalan, Paul J. Christos, Jessica R. Furst, Tara Naib, Scott Ely, Jessica Jalbrzikowski, Roger N. Pearse, Faiza Zafar, Karen Pekle, April La. Row, Richard Lent, Tomer Mark, Hearn J. Cho, Tsiporah Shore, Jeffrey Tepler, John Harpel, Michael W. Schuster, Susan Mathew, John P. Leonard, Madhu Mazumdar, Selina Chen-Kiang and Morton Coleman
Bi. RD (Clarithromycin/lenalidomide/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. 2008; 111: 1101 -1109 Prepublished online Nov 7, 2007; doi: 10. 1182/blood-2007 -05 -090258 Ruben Niesvizky, David S. Jayabalan, Paul J. Christos, Jessica R. Furst, Tara Naib, Scott Ely, Jessica Jalbrzikowski, Roger N. Pearse, Faiza Zafar, Karen Pekle, April La. Row, Richard Lent, Tomer Mark, Hearn J. Cho, Tsiporah Shore, Jeffrey Tepler, John Harpel, Michael W. Schuster, Susan Mathew, John P. Leonard, Madhu Mazumdar, Selina Chen-Kiang and Morton Coleman
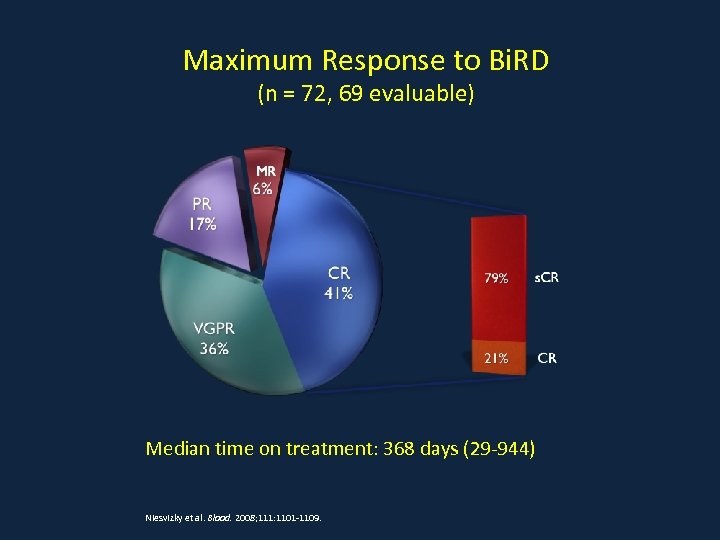 Maximum Response to Bi. RD (n = 72, 69 evaluable) Median time on treatment: 368 days (29 -944) Niesvizky et al. Blood. 2008; 111: 1101 -1109.
Maximum Response to Bi. RD (n = 72, 69 evaluable) Median time on treatment: 368 days (29 -944) Niesvizky et al. Blood. 2008; 111: 1101 -1109.
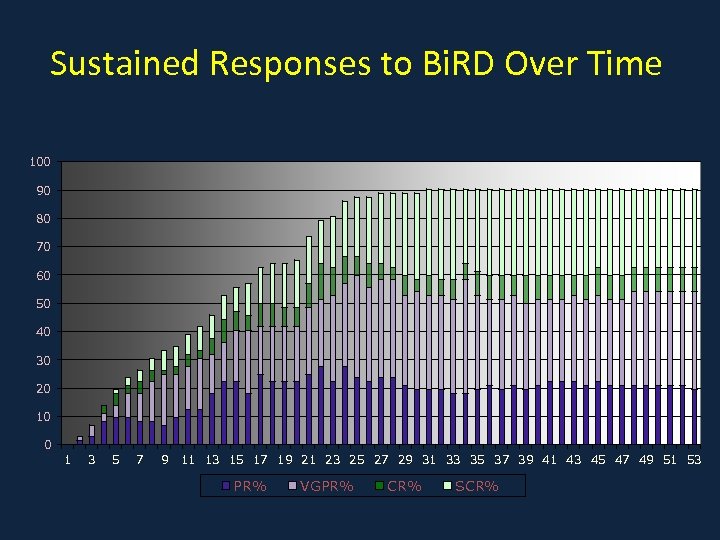 Sustained Responses to Bi. RD Over Time 100 90 80 70 60 50 40 30 20 10 0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 PR% VGPR% CR% SCR%
Sustained Responses to Bi. RD Over Time 100 90 80 70 60 50 40 30 20 10 0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 PR% VGPR% CR% SCR%
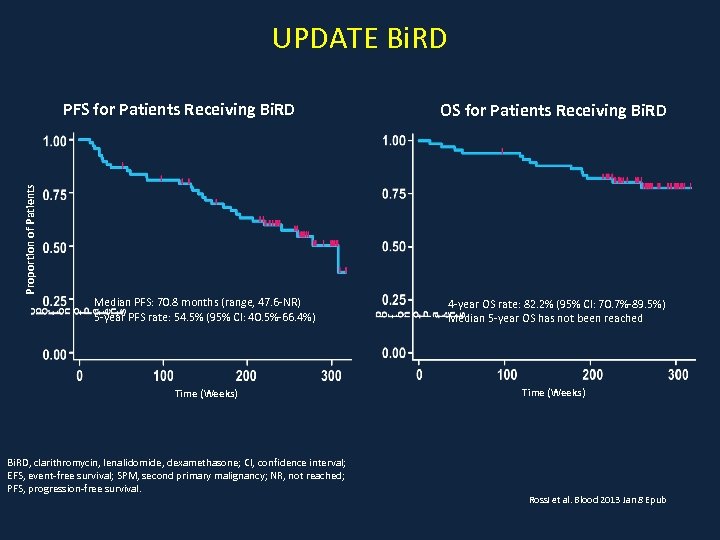 UPDATE Bi. RD OS for Patients Receiving Bi. RD Proportion of Patients PFS for Patients Receiving Bi. RD Median PFS: 70. 8 months (range, 47. 6 -NR) 5 -year PFS rate: 54. 5% (95% CI: 40. 5%-66. 4%) 4 -year OS rate: 82. 2% (95% CI: 70. 7%-89. 5%) Median 5 -year OS has not been reached Time (Weeks) Bi. RD, clarithromycin, lenalidomide, dexamethasone; CI, confidence interval; EFS, event-free survival; SPM, second primary malignancy; NR, not reached; PFS, progression-free survival. Rossi et al. Blood 2013 Jan 8 Epub
UPDATE Bi. RD OS for Patients Receiving Bi. RD Proportion of Patients PFS for Patients Receiving Bi. RD Median PFS: 70. 8 months (range, 47. 6 -NR) 5 -year PFS rate: 54. 5% (95% CI: 40. 5%-66. 4%) 4 -year OS rate: 82. 2% (95% CI: 70. 7%-89. 5%) Median 5 -year OS has not been reached Time (Weeks) Bi. RD, clarithromycin, lenalidomide, dexamethasone; CI, confidence interval; EFS, event-free survival; SPM, second primary malignancy; NR, not reached; PFS, progression-free survival. Rossi et al. Blood 2013 Jan 8 Epub
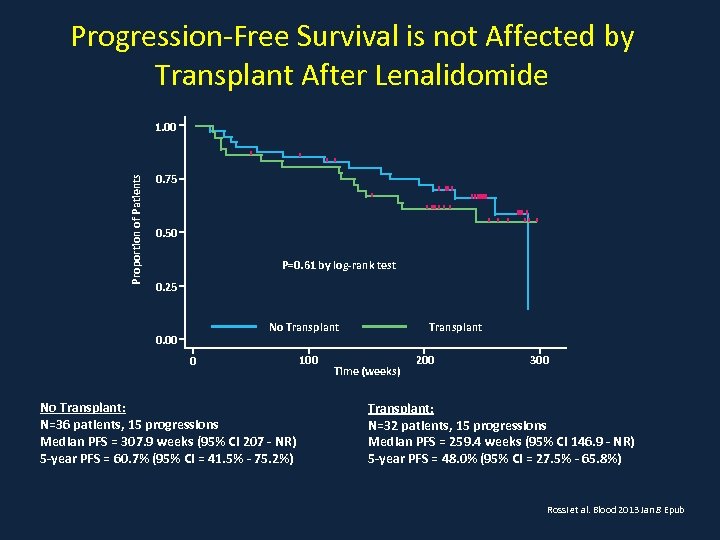 Progression-Free Survival is not Affected by Transplant After Lenalidomide Proportion of Patients 1. 00 0. 75 0. 50 P=0. 61 by log-rank test 0. 25 No Transplant 0. 00 0 No Transplant: N=36 patients, 15 progressions Median PFS = 307. 9 weeks (95% CI 207 - NR) 5 -year PFS = 60. 7% (95% CI = 41. 5% - 75. 2%) 100 Transplant Time (weeks) 200 300 Transplant: N=32 patients, 15 progressions Median PFS = 259. 4 weeks (95% CI 146. 9 - NR) 5 -year PFS = 48. 0% (95% CI = 27. 5% - 65. 8%) Rossi et al. Blood 2013 Jan 8 Epub
Progression-Free Survival is not Affected by Transplant After Lenalidomide Proportion of Patients 1. 00 0. 75 0. 50 P=0. 61 by log-rank test 0. 25 No Transplant 0. 00 0 No Transplant: N=36 patients, 15 progressions Median PFS = 307. 9 weeks (95% CI 207 - NR) 5 -year PFS = 60. 7% (95% CI = 41. 5% - 75. 2%) 100 Transplant Time (weeks) 200 300 Transplant: N=32 patients, 15 progressions Median PFS = 259. 4 weeks (95% CI 146. 9 - NR) 5 -year PFS = 48. 0% (95% CI = 27. 5% - 65. 8%) Rossi et al. Blood 2013 Jan 8 Epub
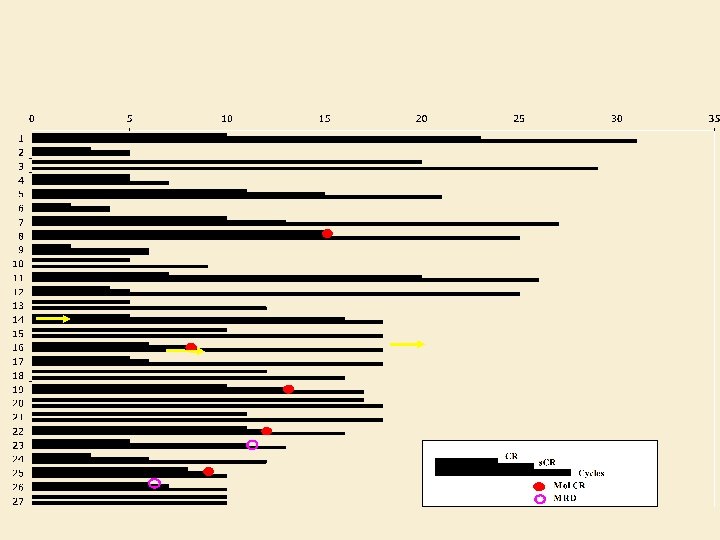 PCR Analysis Pt. #15 Pt. #40 Pt. #43 Pt. #59 pre pre post pre No new clones were identified and the primary clone was undetectable in 5/7 tested pairs post
PCR Analysis Pt. #15 Pt. #40 Pt. #43 Pt. #59 pre pre post pre No new clones were identified and the primary clone was undetectable in 5/7 tested pairs post
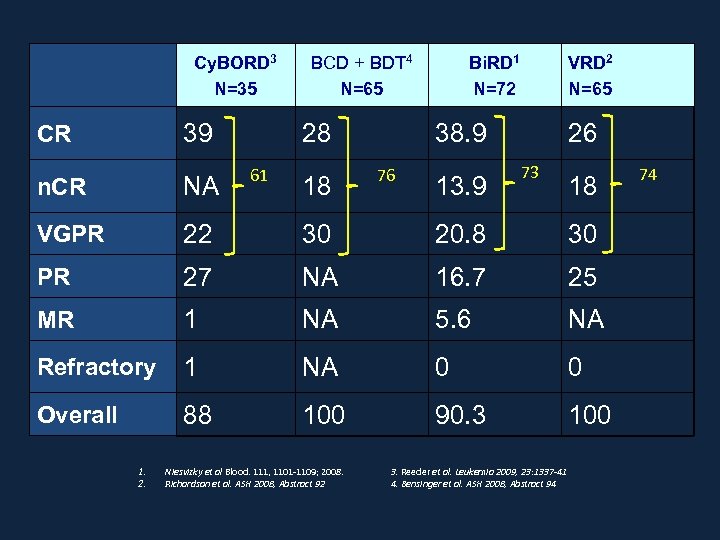 Cy. BORD 3 N=35 39 CR BCD + BDT 4 N=65 28 61 VRD 2 N=65 38. 9 76 73 NA VGPR 22 30 20. 8 30 PR 27 NA 16. 7 25 MR 1 NA 5. 6 NA Refractory 1 NA 0 0 Overall 88 100 90. 3 100 Niesvizky et al Blood. 111, 1101 -1109; 2008. Richardson et al. ASH 2008, Abstract 92 13. 9 26 n. CR 1. 2. 18 Bi. RD 1 N=72 3. Reeder et al. Leukemia 2009, 23: 1337 -41 4. Bensinger et al. ASH 2008, Abstract 94 18 74
Cy. BORD 3 N=35 39 CR BCD + BDT 4 N=65 28 61 VRD 2 N=65 38. 9 76 73 NA VGPR 22 30 20. 8 30 PR 27 NA 16. 7 25 MR 1 NA 5. 6 NA Refractory 1 NA 0 0 Overall 88 100 90. 3 100 Niesvizky et al Blood. 111, 1101 -1109; 2008. Richardson et al. ASH 2008, Abstract 92 13. 9 26 n. CR 1. 2. 18 Bi. RD 1 N=72 3. Reeder et al. Leukemia 2009, 23: 1337 -41 4. Bensinger et al. ASH 2008, Abstract 94 18 74
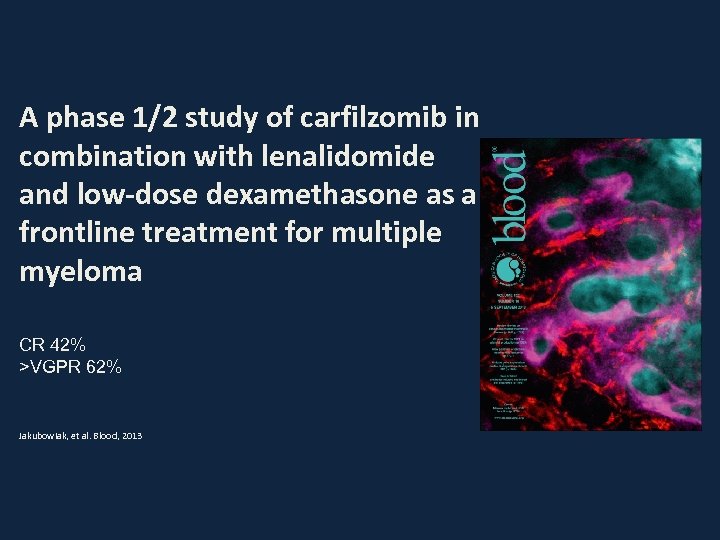 A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma CR 42% >VGPR 62% Jakubowiak, et al. Blood, 2013
A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma CR 42% >VGPR 62% Jakubowiak, et al. Blood, 2013
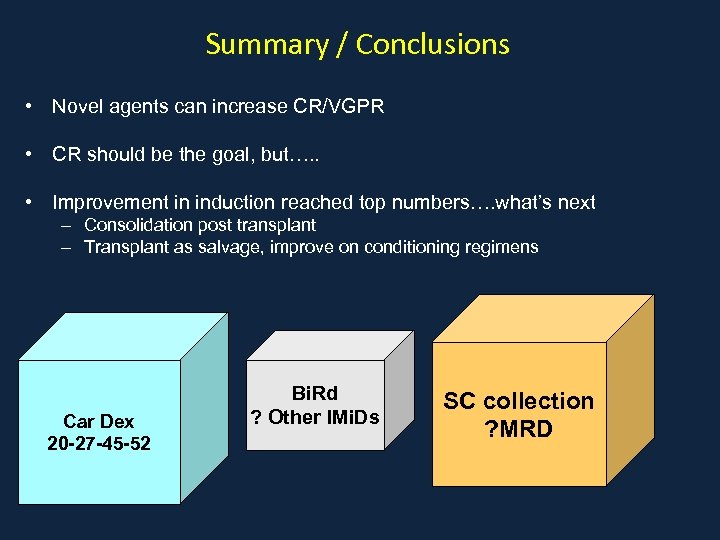 Summary / Conclusions • Novel agents can increase CR/VGPR • CR should be the goal, but…. . • Improvement in induction reached top numbers…. what’s next – Consolidation post transplant – Transplant as salvage, improve on conditioning regimens Car Dex 20 -27 -45 -52 Bi. Rd ? Other IMi. Ds SC collection ? MRD
Summary / Conclusions • Novel agents can increase CR/VGPR • CR should be the goal, but…. . • Improvement in induction reached top numbers…. what’s next – Consolidation post transplant – Transplant as salvage, improve on conditioning regimens Car Dex 20 -27 -45 -52 Bi. Rd ? Other IMi. Ds SC collection ? MRD
 Myeloma. Center. org Morton Coleman Faiza Zafar Roger N Pearse Tomer Mark Adriana C Rossi Karen Pekle Arthur Perry Tsiporah Shore Koen Van Besien Linda Tangenstam Kathleen Pogonowski NCI K 23 Award: CA 109260 -01 Selina Chen-Kiang Monica Guzman Scott Ely Yashpal Agrawal David S. Jayabalan Stanley Goldsmith Maureen Lane Paul Christos Susan Mathew
Myeloma. Center. org Morton Coleman Faiza Zafar Roger N Pearse Tomer Mark Adriana C Rossi Karen Pekle Arthur Perry Tsiporah Shore Koen Van Besien Linda Tangenstam Kathleen Pogonowski NCI K 23 Award: CA 109260 -01 Selina Chen-Kiang Monica Guzman Scott Ely Yashpal Agrawal David S. Jayabalan Stanley Goldsmith Maureen Lane Paul Christos Susan Mathew



