ba81d2d5f2565efd61a00c9c9fba2a2c.ppt
- Количество слайдов: 35
 First and Second Trimester Screening Markers: Results of the FASTER Trial Jacob Canick, Ph. D on behalf of the FASTER Consortium 12 th International Conference on Prenatal Diagnosis and Therapy Budapest June 24 -27, 2004
First and Second Trimester Screening Markers: Results of the FASTER Trial Jacob Canick, Ph. D on behalf of the FASTER Consortium 12 th International Conference on Prenatal Diagnosis and Therapy Budapest June 24 -27, 2004
 The Faster Consortium • • Mary D’Alton overall PI Fergal Malone co-PI Nicholas Wald analysis Alicja Rudnicka analysis Allan Hackshaw analysis Jacob Canick laboratory Geralyn Messerlian lab Diana Bianchi fetal cells and fetal outcome • Kimberly Dukes data mgmt • • • Robert Ball Intermountain Utah David Nyberg Swedish Med Ctr Christine Comstock Beaumont Radek Bukowski UT-Galveston Richard Berkowitz Mount Sinai Susan Gross Albert Einstein Lorraine Dugoff Univ Colorado Sabrina Craigo Tufts NE Med Ctr Ilan Timor NYU Stephen Carr Women & Infants Honor Wolfe UNC Chapel Hill National Institute of Child Health and Human Development Grant RO 1 HD 38652
The Faster Consortium • • Mary D’Alton overall PI Fergal Malone co-PI Nicholas Wald analysis Alicja Rudnicka analysis Allan Hackshaw analysis Jacob Canick laboratory Geralyn Messerlian lab Diana Bianchi fetal cells and fetal outcome • Kimberly Dukes data mgmt • • • Robert Ball Intermountain Utah David Nyberg Swedish Med Ctr Christine Comstock Beaumont Radek Bukowski UT-Galveston Richard Berkowitz Mount Sinai Susan Gross Albert Einstein Lorraine Dugoff Univ Colorado Sabrina Craigo Tufts NE Med Ctr Ilan Timor NYU Stephen Carr Women & Infants Honor Wolfe UNC Chapel Hill National Institute of Child Health and Human Development Grant RO 1 HD 38652
 FASTER Components • Coordinating and educational center • Columbia University, New York • Enrollment centers • 15 prenatal diagnostic centers in the U. S. • Assays, reporting, and NT management • Women & Infants Hospital, Brown Medical School • Data management • DMStat, Inc. , Boston • Data analysis • Wolfson Institute for Preventive Medicine, London
FASTER Components • Coordinating and educational center • Columbia University, New York • Enrollment centers • 15 prenatal diagnostic centers in the U. S. • Assays, reporting, and NT management • Women & Infants Hospital, Brown Medical School • Data management • DMStat, Inc. , Boston • Data analysis • Wolfson Institute for Preventive Medicine, London
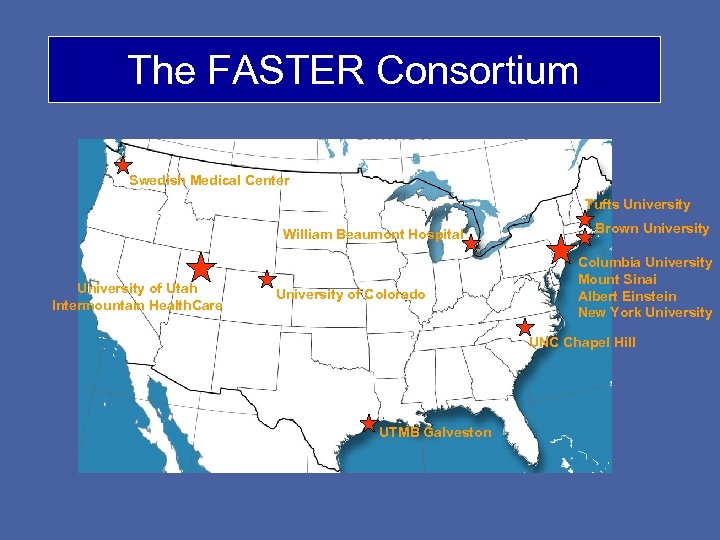 The FASTER Consortium Swedish Medical Center Tufts University William Beaumont Hospital University of Utah Intermountain Health. Care University of Colorado Brown University Columbia University Mount Sinai Albert Einstein New York University UNC Chapel Hill UTMB Galveston
The FASTER Consortium Swedish Medical Center Tufts University William Beaumont Hospital University of Utah Intermountain Health. Care University of Colorado Brown University Columbia University Mount Sinai Albert Einstein New York University UNC Chapel Hill UTMB Galveston
 OBJECTIVES • To define performance of first trimester combined ultrasound and serum screening. • To compare performance of first trimester combined screening and second trimester quad marker screening in the same women. • To describe optimal combinations of tests for population-based Down syndrome screening.
OBJECTIVES • To define performance of first trimester combined ultrasound and serum screening. • To compare performance of first trimester combined screening and second trimester quad marker screening in the same women. • To describe optimal combinations of tests for population-based Down syndrome screening.
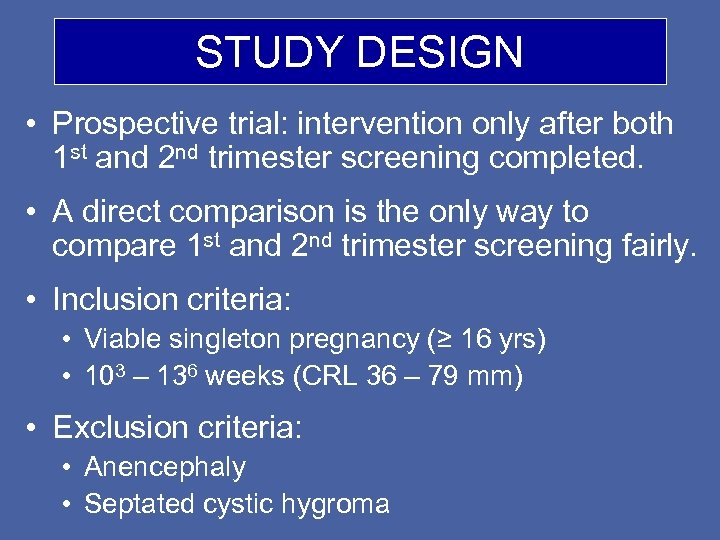 STUDY DESIGN • Prospective trial: intervention only after both 1 st and 2 nd trimester screening completed. • A direct comparison is the only way to compare 1 st and 2 nd trimester screening fairly. • Inclusion criteria: • Viable singleton pregnancy (≥ 16 yrs) • 103 – 136 weeks (CRL 36 – 79 mm) • Exclusion criteria: • Anencephaly • Septated cystic hygroma
STUDY DESIGN • Prospective trial: intervention only after both 1 st and 2 nd trimester screening completed. • A direct comparison is the only way to compare 1 st and 2 nd trimester screening fairly. • Inclusion criteria: • Viable singleton pregnancy (≥ 16 yrs) • 103 – 136 weeks (CRL 36 – 79 mm) • Exclusion criteria: • Anencephaly • Septated cystic hygroma
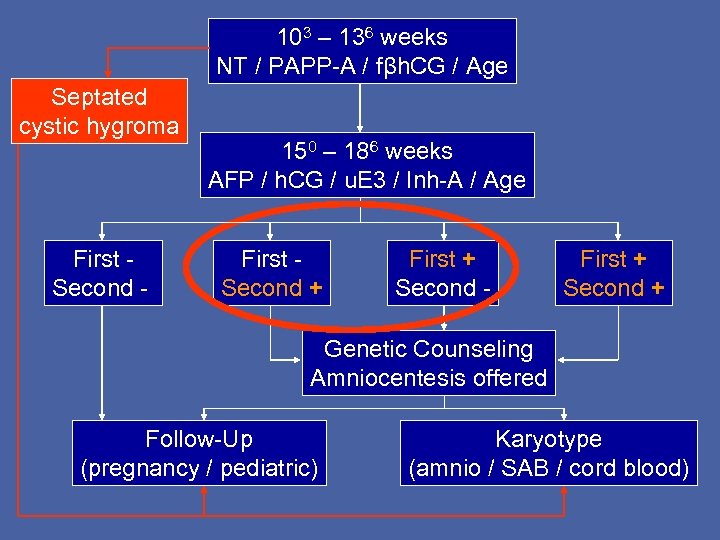 103 – 136 weeks NT / PAPP-A / fβh. CG / Age Septated cystic hygroma First Second - 150 – 186 weeks AFP / h. CG / u. E 3 / Inh-A / Age First Second + First + Second - First + Second + Genetic Counseling Amniocentesis offered Follow-Up (pregnancy / pediatric) Karyotype (amnio / SAB / cord blood)
103 – 136 weeks NT / PAPP-A / fβh. CG / Age Septated cystic hygroma First Second - 150 – 186 weeks AFP / h. CG / u. E 3 / Inh-A / Age First Second + First + Second - First + Second + Genetic Counseling Amniocentesis offered Follow-Up (pregnancy / pediatric) Karyotype (amnio / SAB / cord blood)
 Nuchal Translucency Sonography • 102 sonographers • Initial uniform practical training • Standard NT protocol • > 50 images each to confirm technique
Nuchal Translucency Sonography • 102 sonographers • Initial uniform practical training • Standard NT protocol • > 50 images each to confirm technique
 RESULTS • These results were reported at the Society for Maternal Fetal Medicine annual meeting, held in New Orleans in February 2004. • The results are from an interim analysis. • Final data analysis has now been completed, and the principal findings of the FASTER Trial are being prepared for publication. • The performance estimates will be slightly different in the final analysis.
RESULTS • These results were reported at the Society for Maternal Fetal Medicine annual meeting, held in New Orleans in February 2004. • The results are from an interim analysis. • Final data analysis has now been completed, and the principal findings of the FASTER Trial are being prepared for publication. • The performance estimates will be slightly different in the final analysis.
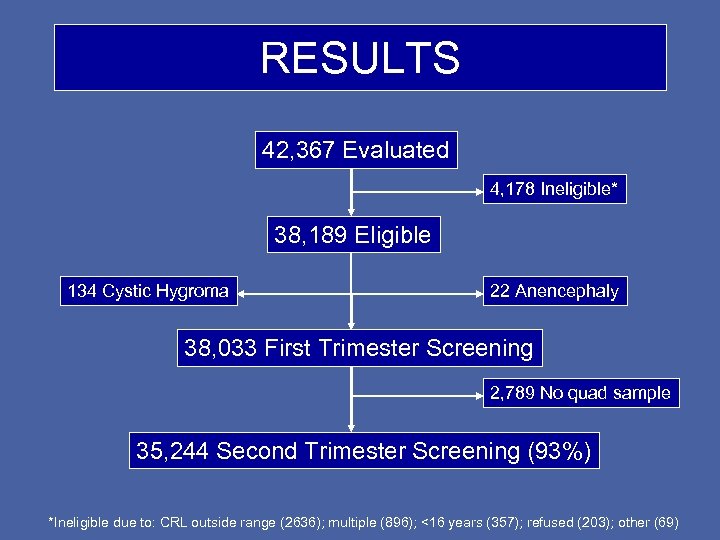 RESULTS 42, 367 Evaluated 4, 178 Ineligible* 38, 189 Eligible 134 Cystic Hygroma 22 Anencephaly 38, 033 First Trimester Screening 2, 789 No quad sample 35, 244 Second Trimester Screening (93%) *Ineligible due to: CRL outside range (2636); multiple (896); <16 years (357); refused (203); other (69)
RESULTS 42, 367 Evaluated 4, 178 Ineligible* 38, 189 Eligible 134 Cystic Hygroma 22 Anencephaly 38, 033 First Trimester Screening 2, 789 No quad sample 35, 244 Second Trimester Screening (93%) *Ineligible due to: CRL outside range (2636); multiple (896); <16 years (357); refused (203); other (69)
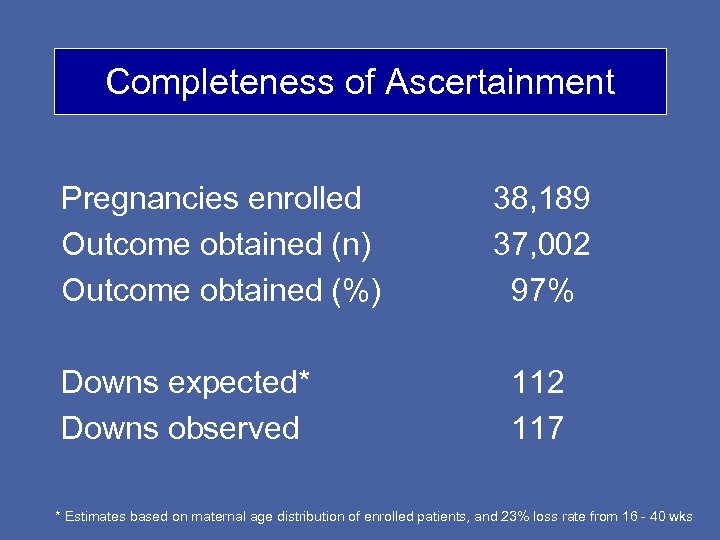 Completeness of Ascertainment Pregnancies enrolled Outcome obtained (n) Outcome obtained (%) Downs expected* Downs observed 38, 189 37, 002 97% 112 117 * Estimates based on maternal age distribution of enrolled patients, and 23% loss rate from 16 - 40 wks
Completeness of Ascertainment Pregnancies enrolled Outcome obtained (n) Outcome obtained (%) Downs expected* Downs observed 38, 189 37, 002 97% 112 117 * Estimates based on maternal age distribution of enrolled patients, and 23% loss rate from 16 - 40 wks
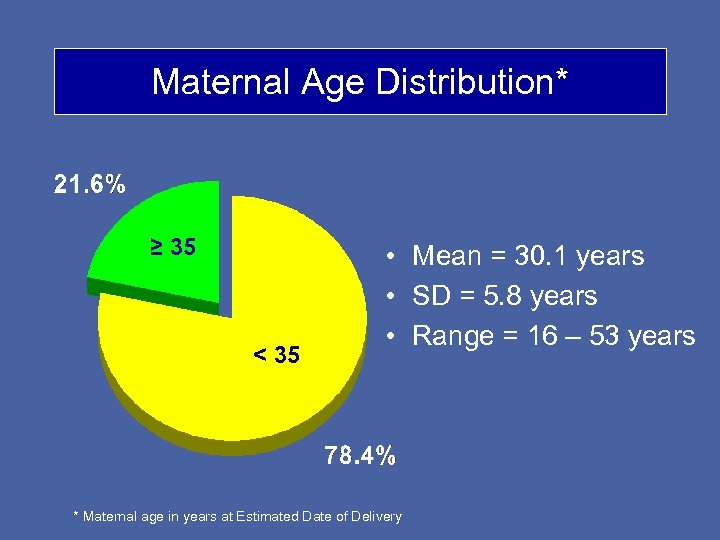 Maternal Age Distribution* ≥ 35 < 35 • Mean = 30. 1 years • SD = 5. 8 years • Range = 16 – 53 years * Maternal age in years at Estimated Date of Delivery
Maternal Age Distribution* ≥ 35 < 35 • Mean = 30. 1 years • SD = 5. 8 years • Range = 16 – 53 years * Maternal age in years at Estimated Date of Delivery
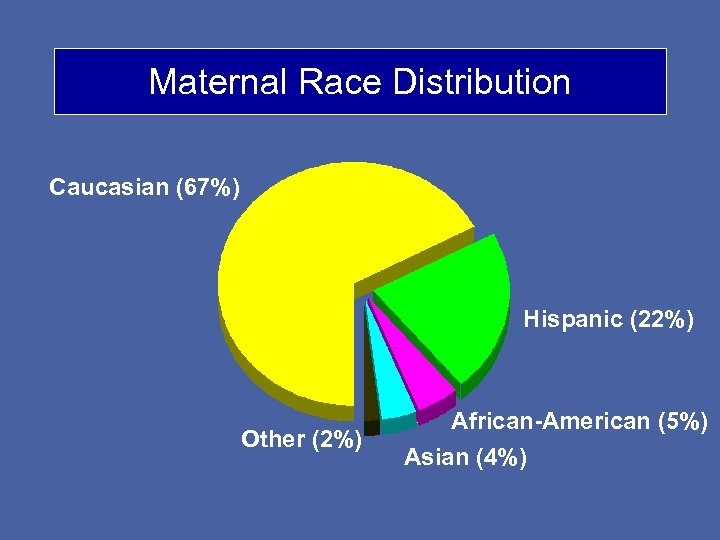 Maternal Race Distribution Caucasian (67%) Hispanic (22%) Other (2%) African-American (5%) Asian (4%)
Maternal Race Distribution Caucasian (67%) Hispanic (22%) Other (2%) African-American (5%) Asian (4%)
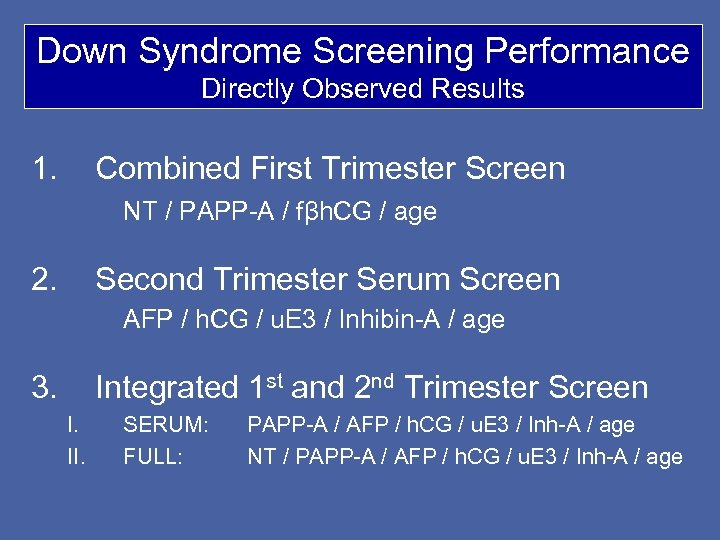 Down Syndrome Screening Performance Directly Observed Results 1. Combined First Trimester Screen NT / PAPP-A / fβh. CG / age 2. Second Trimester Serum Screen AFP / h. CG / u. E 3 / Inhibin-A / age 3. Integrated 1 st and 2 nd Trimester Screen I. II. SERUM: FULL: PAPP-A / AFP / h. CG / u. E 3 / Inh-A / age NT / PAPP-A / AFP / h. CG / u. E 3 / Inh-A / age
Down Syndrome Screening Performance Directly Observed Results 1. Combined First Trimester Screen NT / PAPP-A / fβh. CG / age 2. Second Trimester Serum Screen AFP / h. CG / u. E 3 / Inhibin-A / age 3. Integrated 1 st and 2 nd Trimester Screen I. II. SERUM: FULL: PAPP-A / AFP / h. CG / u. E 3 / Inh-A / age NT / PAPP-A / AFP / h. CG / u. E 3 / Inh-A / age
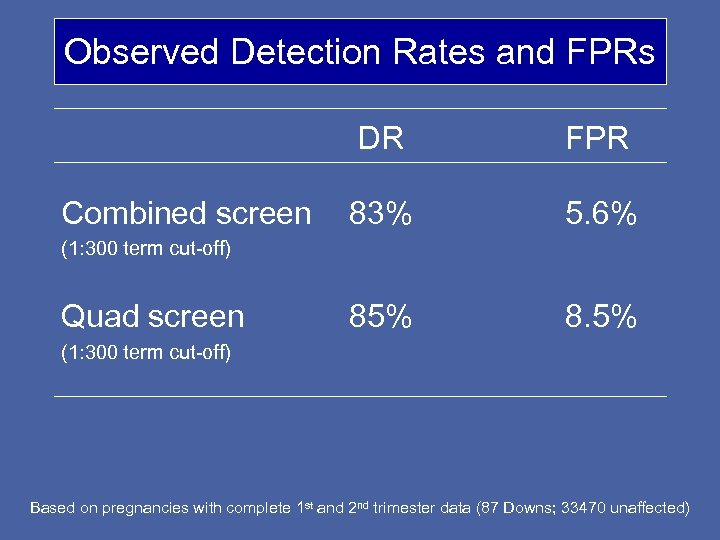 Observed Detection Rates and FPRs DR Combined screen FPR 83% 5. 6% 85% 8. 5% (1: 300 term cut-off) Quad screen (1: 300 term cut-off) Based on pregnancies with complete 1 st and 2 nd trimester data (87 Downs; 33470 unaffected)
Observed Detection Rates and FPRs DR Combined screen FPR 83% 5. 6% 85% 8. 5% (1: 300 term cut-off) Quad screen (1: 300 term cut-off) Based on pregnancies with complete 1 st and 2 nd trimester data (87 Downs; 33470 unaffected)
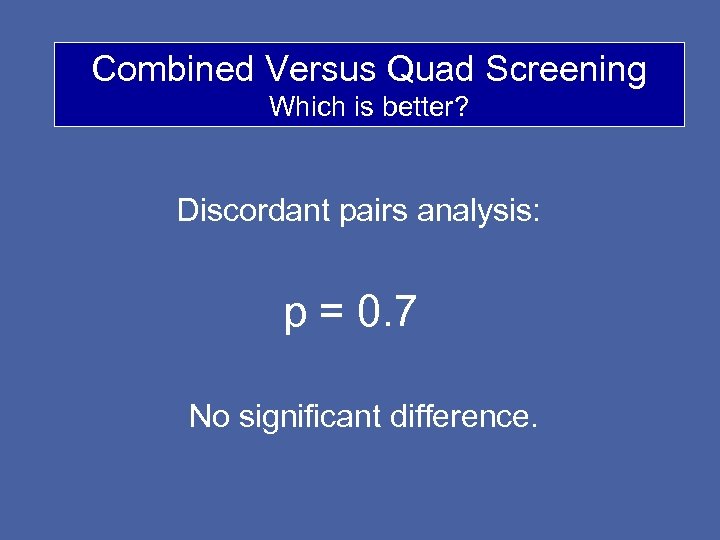 Combined Versus Quad Screening Which is better? Discordant pairs analysis: p = 0. 7 No significant difference.
Combined Versus Quad Screening Which is better? Discordant pairs analysis: p = 0. 7 No significant difference.
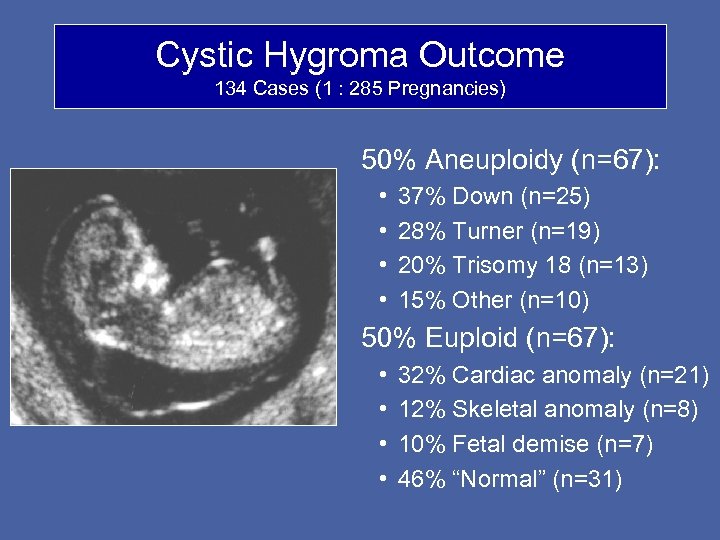 Cystic Hygroma Outcome 134 Cases (1 : 285 Pregnancies) 50% Aneuploidy (n=67): • • 37% Down (n=25) 28% Turner (n=19) 20% Trisomy 18 (n=13) 15% Other (n=10) 50% Euploid (n=67): • • 32% Cardiac anomaly (n=21) 12% Skeletal anomaly (n=8) 10% Fetal demise (n=7) 46% “Normal” (n=31)
Cystic Hygroma Outcome 134 Cases (1 : 285 Pregnancies) 50% Aneuploidy (n=67): • • 37% Down (n=25) 28% Turner (n=19) 20% Trisomy 18 (n=13) 15% Other (n=10) 50% Euploid (n=67): • • 32% Cardiac anomaly (n=21) 12% Skeletal anomaly (n=8) 10% Fetal demise (n=7) 46% “Normal” (n=31)
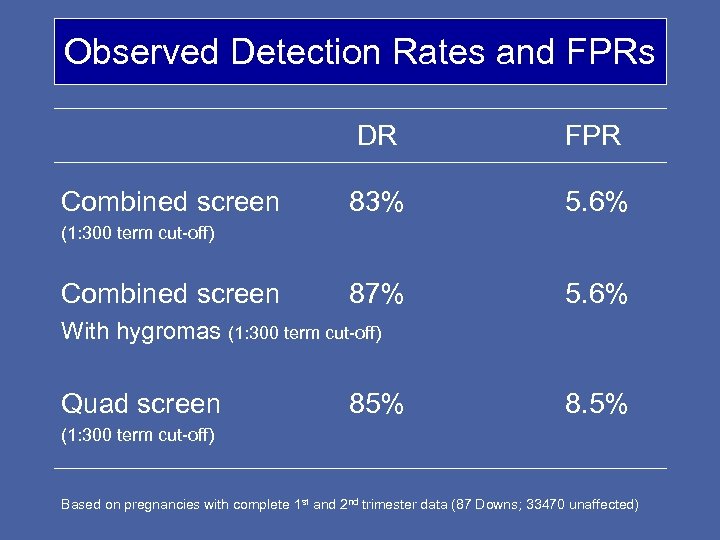 Observed Detection Rates and FPRs DR Combined screen FPR 83% 5. 6% 87% 5. 6% (1: 300 term cut-off) Combined screen With hygromas (1: 300 term cut-off) Quad screen 85% 8. 5% (1: 300 term cut-off) Based on pregnancies with complete 1 st and 2 nd trimester data (87 Downs; 33470 unaffected)
Observed Detection Rates and FPRs DR Combined screen FPR 83% 5. 6% 87% 5. 6% (1: 300 term cut-off) Combined screen With hygromas (1: 300 term cut-off) Quad screen 85% 8. 5% (1: 300 term cut-off) Based on pregnancies with complete 1 st and 2 nd trimester data (87 Downs; 33470 unaffected)
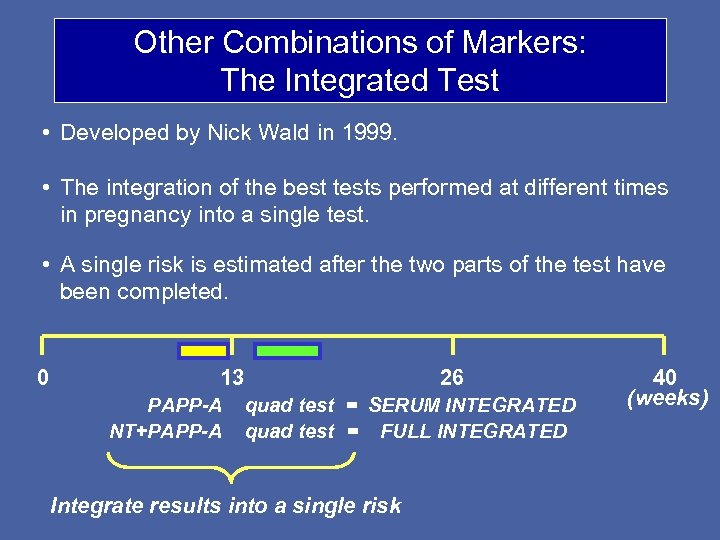 Other Combinations of Markers: The Integrated Test • Developed by Nick Wald in 1999. • The integration of the best tests performed at different times in pregnancy into a single test. • A single risk is estimated after the two parts of the test have been completed. 0 13 PAPP-A NT+PAPP-A 26 quad test = SERUM INTEGRATED quad test = FULL INTEGRATED Integrate results into a single risk 40 (weeks)
Other Combinations of Markers: The Integrated Test • Developed by Nick Wald in 1999. • The integration of the best tests performed at different times in pregnancy into a single test. • A single risk is estimated after the two parts of the test have been completed. 0 13 PAPP-A NT+PAPP-A 26 quad test = SERUM INTEGRATED quad test = FULL INTEGRATED Integrate results into a single risk 40 (weeks)
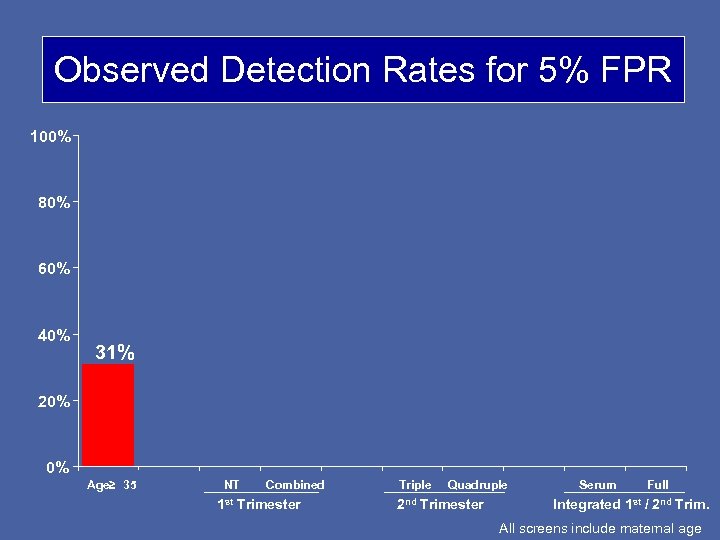 Observed Detection Rates for 5% FPR 100% 80% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
Observed Detection Rates for 5% FPR 100% 80% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
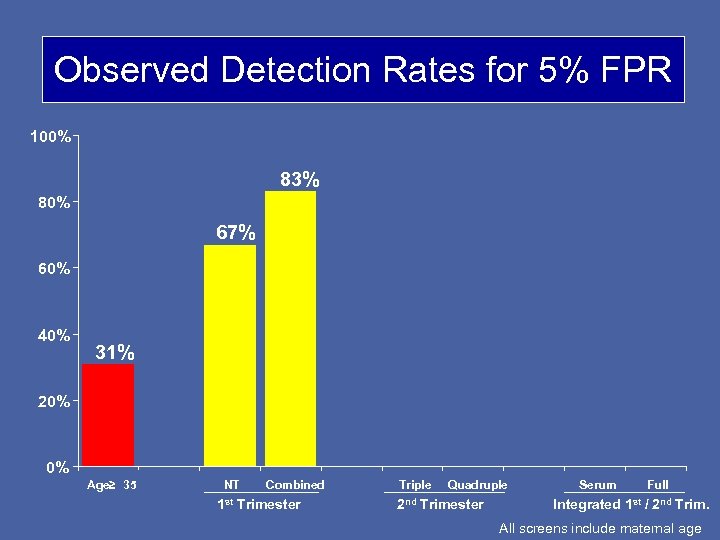 Observed Detection Rates for 5% FPR 100% 83% 80% 67% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
Observed Detection Rates for 5% FPR 100% 83% 80% 67% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
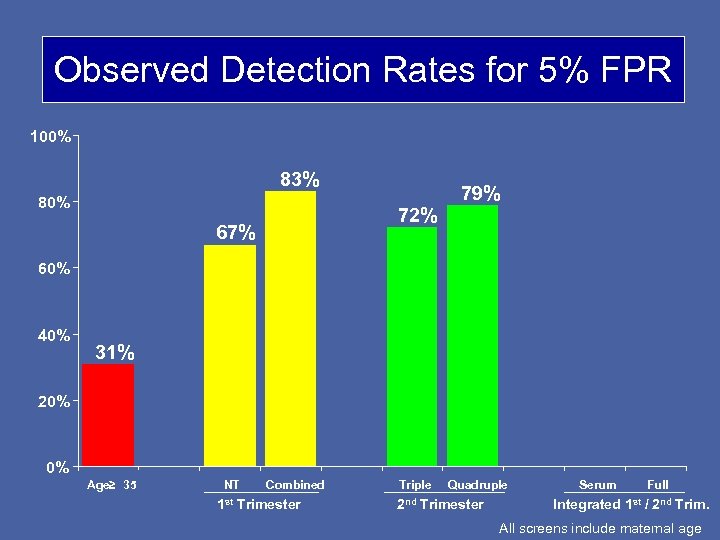 Observed Detection Rates for 5% FPR 100% 83% 80% 72% 67% 79% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
Observed Detection Rates for 5% FPR 100% 83% 80% 72% 67% 79% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
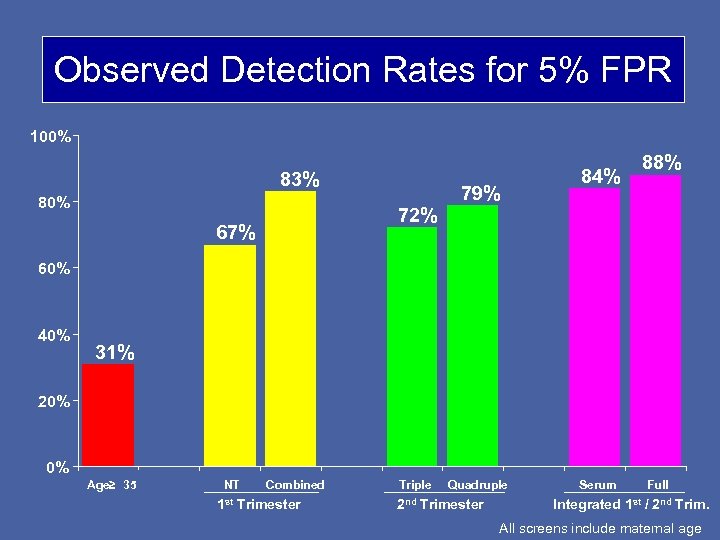 Observed Detection Rates for 5% FPR 100% 83% 80% 72% 67% 79% 84% 88% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
Observed Detection Rates for 5% FPR 100% 83% 80% 72% 67% 79% 84% 88% 60% 40% 31% 20% 0% Age≥ 35 NT Combined 1 st Trimester Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim. All screens include maternal age
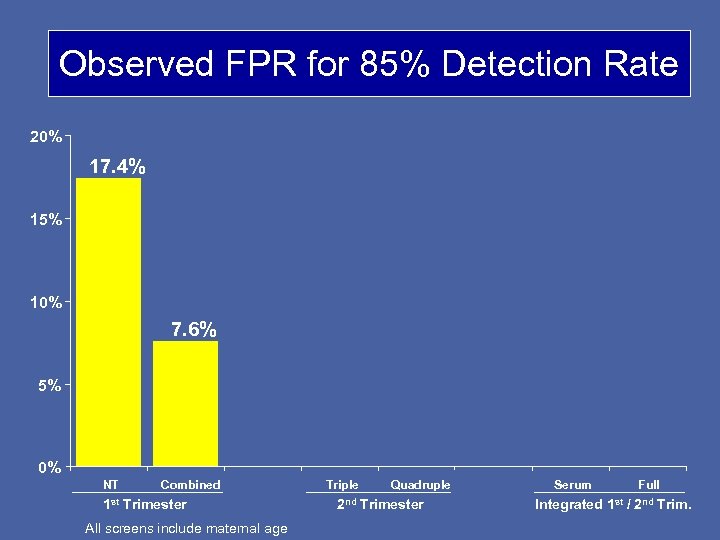 Observed FPR for 85% Detection Rate 20% 17. 4% 15% 10% 7. 6% 5% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
Observed FPR for 85% Detection Rate 20% 17. 4% 15% 10% 7. 6% 5% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
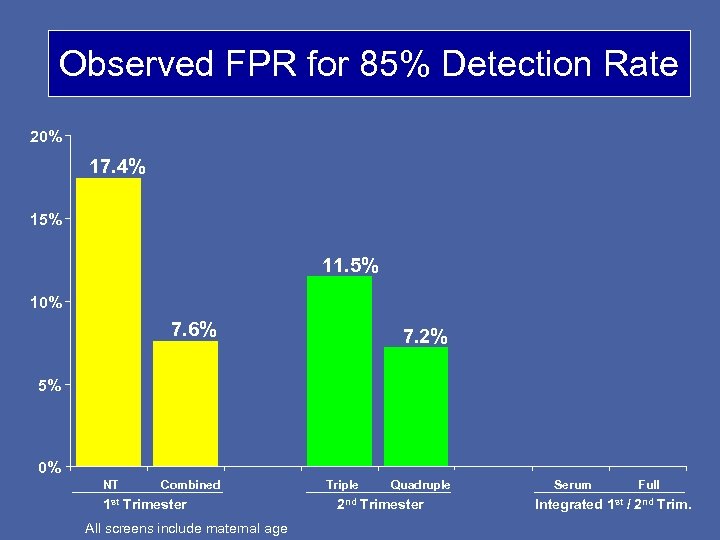 Observed FPR for 85% Detection Rate 20% 17. 4% 15% 11. 5% 10% 7. 6% 7. 2% 5% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
Observed FPR for 85% Detection Rate 20% 17. 4% 15% 11. 5% 10% 7. 6% 7. 2% 5% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
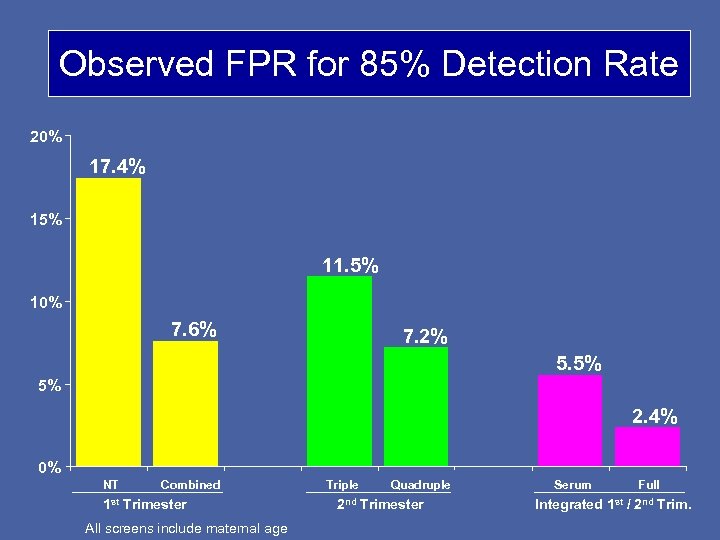 Observed FPR for 85% Detection Rate 20% 17. 4% 15% 11. 5% 10% 7. 6% 7. 2% 5. 5% 5% 2. 4% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
Observed FPR for 85% Detection Rate 20% 17. 4% 15% 11. 5% 10% 7. 6% 7. 2% 5. 5% 5% 2. 4% 0% NT Combined 1 st Trimester All screens include maternal age Triple Quadruple 2 nd Trimester Serum Full Integrated 1 st / 2 nd Trim.
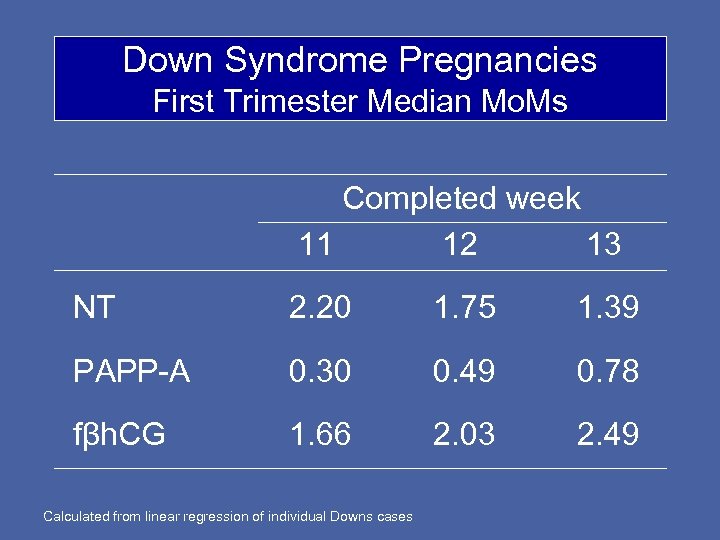 Down Syndrome Pregnancies First Trimester Median Mo. Ms Completed week 11 12 13 NT 2. 20 1. 75 1. 39 PAPP-A 0. 30 0. 49 0. 78 fβh. CG 1. 66 2. 03 2. 49 Calculated from linear regression of individual Downs cases
Down Syndrome Pregnancies First Trimester Median Mo. Ms Completed week 11 12 13 NT 2. 20 1. 75 1. 39 PAPP-A 0. 30 0. 49 0. 78 fβh. CG 1. 66 2. 03 2. 49 Calculated from linear regression of individual Downs cases
 Down Syndrome Screening Performance Using these data and applying them to the U. S. population of pregnancies, the following results were obtained:
Down Syndrome Screening Performance Using these data and applying them to the U. S. population of pregnancies, the following results were obtained:
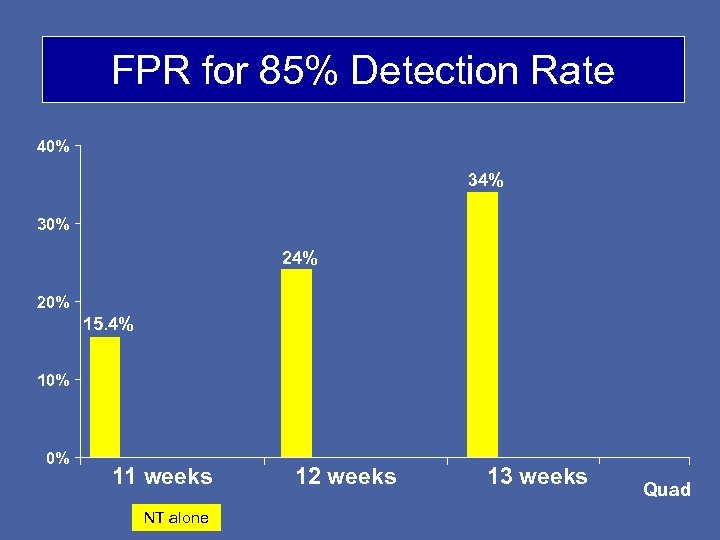 FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 11 weeks NT alone 12 weeks 13 weeks Quad
FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 11 weeks NT alone 12 weeks 13 weeks Quad
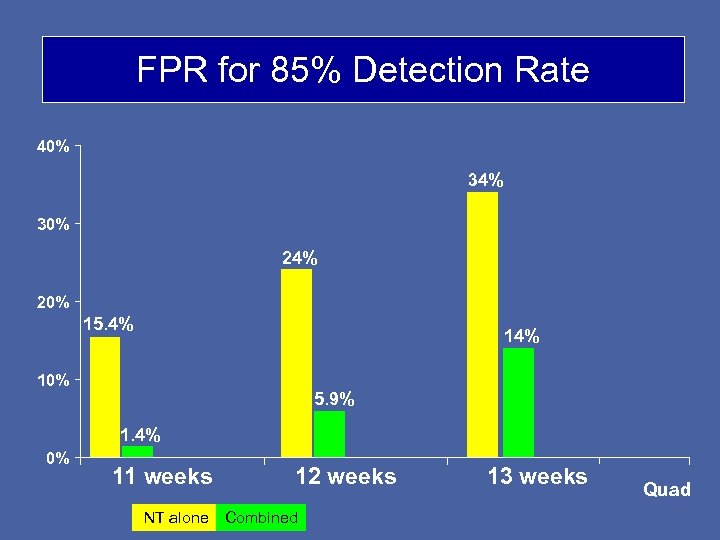 FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 5. 9% 1. 4% 0% 11 weeks 12 weeks NT alone Combined 13 weeks Quad
FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 5. 9% 1. 4% 0% 11 weeks 12 weeks NT alone Combined 13 weeks Quad
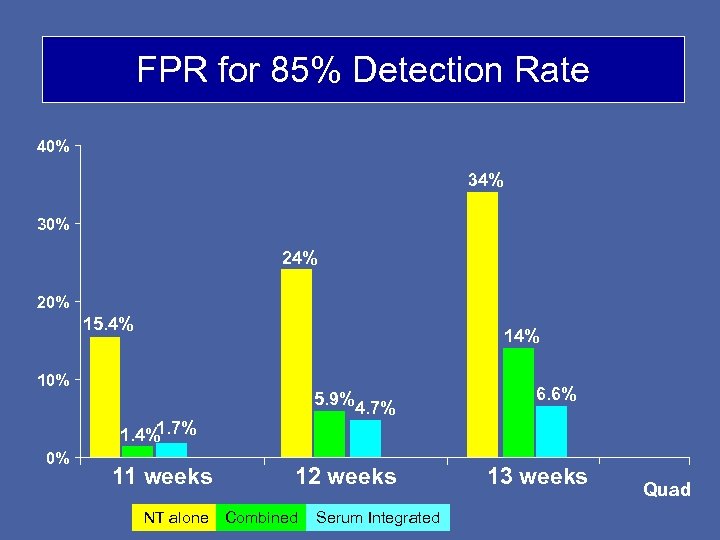 FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 5. 9% 1. 7% 1. 4% 0% 11 weeks 4. 7% 12 weeks NT alone Combined Serum Integrated 6. 6% 13 weeks Quad
FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 5. 9% 1. 7% 1. 4% 0% 11 weeks 4. 7% 12 weeks NT alone Combined Serum Integrated 6. 6% 13 weeks Quad
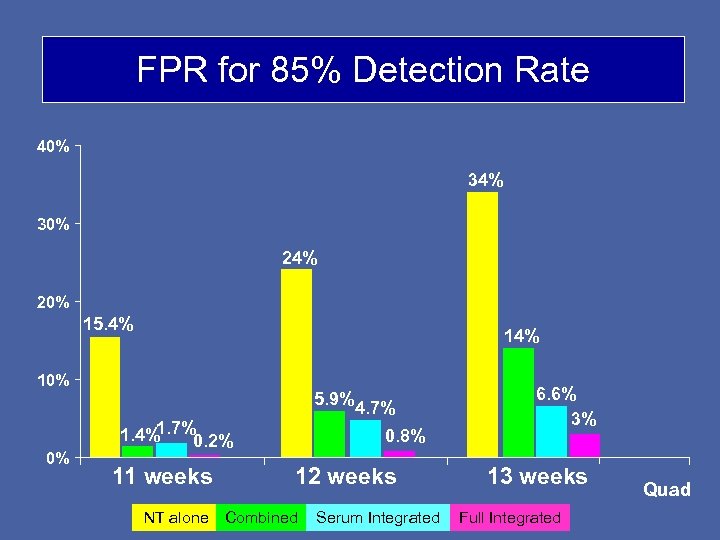 FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 5. 9% 1. 7% 1. 4% 0. 2% 11 weeks 4. 7% 0. 8% 12 weeks NT alone Combined Serum Integrated 6. 6% 3% 13 weeks Full Integrated Quad
FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 5. 9% 1. 7% 1. 4% 0. 2% 11 weeks 4. 7% 0. 8% 12 weeks NT alone Combined Serum Integrated 6. 6% 3% 13 weeks Full Integrated Quad
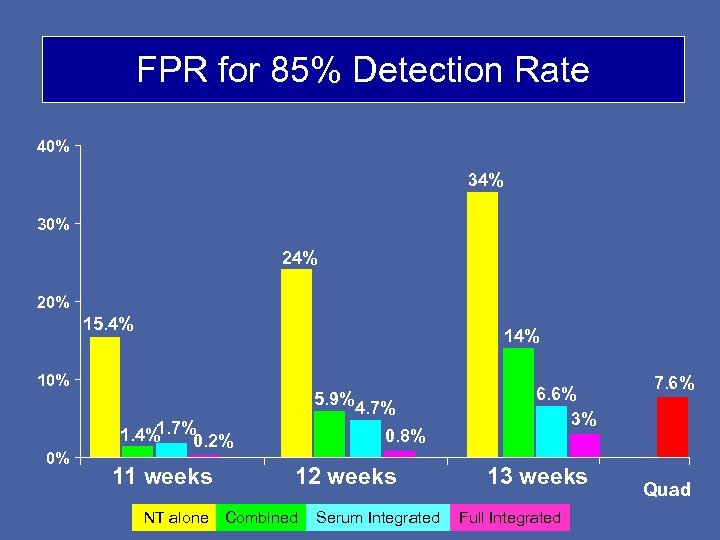 FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 5. 9% 1. 7% 1. 4% 0. 2% 11 weeks 4. 7% 0. 8% 12 weeks NT alone Combined Serum Integrated 6. 6% 3% 13 weeks Full Integrated 7. 6% Quad
FPR for 85% Detection Rate 40% 34% 30% 24% 20% 15. 4% 10% 0% 5. 9% 1. 7% 1. 4% 0. 2% 11 weeks 4. 7% 0. 8% 12 weeks NT alone Combined Serum Integrated 6. 6% 3% 13 weeks Full Integrated 7. 6% Quad
 CONCLUSIONS • 1 st trimester combined and 2 nd trimester quad screening are similarly effective. • The Integrated test performs better than either the 1 st or 2 nd trimester screening methods. • 1 st trimester markers vary by gestational age. Algorithms should account for these gestational age effects.
CONCLUSIONS • 1 st trimester combined and 2 nd trimester quad screening are similarly effective. • The Integrated test performs better than either the 1 st or 2 nd trimester screening methods. • 1 st trimester markers vary by gestational age. Algorithms should account for these gestational age effects.
 ACKNOWLEDGMENTS • • • • Columbia: K. Welch, R. Denchy, K. Berentsen Univ Utah: F. Porter, L. Cannon, K. Nelson, C. Loucks, A. Yoshimura Swedish: D. Luthy, S. Coe Beaumont: D. Schmidt, J. Esler UTMB: G. Saade, G. Hankins, J. Lee Mount Sinai: K. Eddleman, Y. Kharbutli Montefiore: I. Merkatz, S. Carter U Colorado: J. Hobbins, L. Schultz Tufts U: B. Isquith, B. Berlin NYU: M. Paidas, J. Borsuk Brown U: C. Duquette UNC: R. Baughman DM-STAT: D. Emig, T. Tripp, J. Vidaver, L. Sullivan, N. Tibbetts, P. Folan NICHD: J. Hanson, D. Alexander, F. de la Cruz …. . and all 102 sonographers who participated
ACKNOWLEDGMENTS • • • • Columbia: K. Welch, R. Denchy, K. Berentsen Univ Utah: F. Porter, L. Cannon, K. Nelson, C. Loucks, A. Yoshimura Swedish: D. Luthy, S. Coe Beaumont: D. Schmidt, J. Esler UTMB: G. Saade, G. Hankins, J. Lee Mount Sinai: K. Eddleman, Y. Kharbutli Montefiore: I. Merkatz, S. Carter U Colorado: J. Hobbins, L. Schultz Tufts U: B. Isquith, B. Berlin NYU: M. Paidas, J. Borsuk Brown U: C. Duquette UNC: R. Baughman DM-STAT: D. Emig, T. Tripp, J. Vidaver, L. Sullivan, N. Tibbetts, P. Folan NICHD: J. Hanson, D. Alexander, F. de la Cruz …. . and all 102 sonographers who participated


