1f6ccf6c1b6f6657736c4c1d6af42ec4.ppt
- Количество слайдов: 79

FETAL AND NEONATAL ALLOIMMUNE THROMBOCYTOPENIA (FNAIT) Christine Wheeler, MD Transfusion Medicine Fellow

OVERVIEW Neonatal alloimmune thrombocytopenia (NAIT): most common etiology of severe thrombocytopenia and intracranial hemorrhage in term neonates Incidence: 1/1000 live births
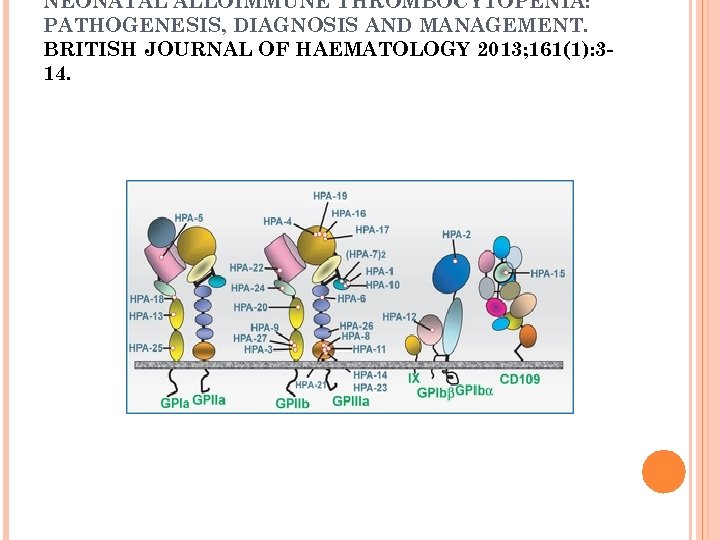
PATHOPHYSIOLOGY Incompatibility between mother and fetus for human platelet antigens (HPAs) Fetus inherits a platelet antigen from the father, which the mother does not possess and to which the mother becomes immunized Human platelet antigens (HPAs) are located on platelet membrane glycoproteins: GPIb-V-IX (von Willebrand receptor), GPIIb/IIIa (fibrinogen receptor), GPIa/IIa ( a collagen receptor) and CD 109
NEONATAL ALLOIMMUNE THROMBOCYTOPENIA: PATHOGENESIS, DIAGNOSIS AND MANAGEMENT. BRITISH JOURNAL OF HAEMATOLOGY 2013; 161(1): 314.
ALLOIMMUNIZATION TO HPA ANTIGENS Six bi-allelic platelet alloantigen systems are responsible for the majority of cases of NAIT due to platelet-specific alloantibodies: HPA-1, -2, -3, -4, -5 and -15 (a: high frequency allele, b: low frequency allele) Caucasians and Africans: 80 -85% of cases due to anti-HPA-1 a (2. 5% of mothers are HPA-1 a negative) Other platelet antigens more frequently implicated in NAIT: - HPA-5 b: 8 -15% - HPA-1 b: 1 -4% - HPA-3 a: 1 -2% - HPA-5 a: about 1% - HPA-15 b: 4%
ALLOIMMUNIZATION TO HPA-1 A Presence of the DRB 3*0101 allele increases immunization risk: this allele is important in the presentation of the HPA-1 a peptide in antibody production Women with the DRB 3*0101 allele generate more than 90% of HPA-1 a antibodies 10% of HPA-1 b 1 b mothers with an HPA-1 a fetus produce anti-HPA-1 a 30% of fetuses associated with HPA-1 a alloimmunized pregnancies have severe thrombocytopenia (<50, 000/mc. L)
ALLOIMMUNIZATION TO HPA ANTIGENS Low-frequency antigens (HPA-6 b to -14 b and HPA-16 b) may also be involved Asians: alloimmunization to HPA-4 b, -6 b and 21 b occurs more frequently than in Caucasians

ALLOIMMUNIZATION TO GLYCOPROTEIN IV Glycoprotein IV (CD 36, Nak) is a class B scavenger receptor protein Approximately 5% of Africans and Asians lack CD 36 expression and can become immunized if exposed to CD 36 by pregnancy or transfusion FNAIT can be caused by maternal alloimmunization to CD 36
ALLOIMMUNIZATION TO HLA ANTIGENS Class I HLA antigens are present on platelets Alloimmunization to class I HLA antigens is present in one third of multiparous women Infants born to these alloimmunized women usually have normal platelet counts One study did not demonstrate correlation between the presence or absence of HLA antibodies and neonatal platelet counts There are reports of apparent FNAIT possibly due to HLA antibodies It is possible that high titer HLA antibodies or antibodies directed against potent HLA antigens may cause FNAIT
CASE STUDY: NAIT DUE TO HLA ANTIBODIES? Term (39 weeks), small for gestational age, O positive male born of a 24 year old G 1 P 0 (now P 1) O positive female by normal spontaneous vaginal delivery: meconium stained amniotic fluid, Apgar scores 8/9 Baby was thrombocytopenic (platelet count <9000/mc. L, repeat 2 hours later without platelet transfusion was 46, 000/mc. L) No evidence of bleeding including no intracerebral bleed identified on imaging study 24 m. L of platelets transfused: platelet count 55, 000/mc. L (6 hours after issue); platelet count 87, 000/mc. L (20 hours later) with a subsequent fall to 64, 000/mc. L (12 hours later)
CASE STUDY: NAIT DUE TO HLA ANTIBODIES? Bonfils Blood Center was informed that NAIT was in the differential diagnosis and that maternal platelets might need to be collected by apheresis Requested blood sample from the mother to evaluate for the presence of anti-platelet antibodies

CASE STUDY: PAKPLUS RESULTS No antibodies to GPIIb/IIIa (HPA-1 a/1 a, HPA 3 a/3 a, HPA-4 a/, HPA-1 b/1 b, HPA-3 b/3 b), GPIa/IIa (HPA-5 a/5 a, HPA-5 b/5 b), GPIb/IX or GPIV detected Antibodies to Class I HLA antigens (HLA-A -B) detected: mother had history of VSD repair before age 10 years
CASE STUDY: HLA ANTIBODY SPECIFICITY Clinimmune Laboratory performed Luminex Single Ag HLA Class I test to determine HLA antibody specificity. Defined specificities: A 25, A 26, A 33, A 34, A 66, A 68, A 69, B 27, B 38, B 63, Cw 14 Tentative specificities: A 29, A 31, A 32, A 43, A 74, B 8, B 39, B 46, B 73, B 2708, Cw 9, Cw 10
CASE STUDY: NAIT DUE TO HLA ANTIBODIES? Follow-up information indicated thrombocytopenia not likely due to NAIT Hypoglycemia, leukopenia/neutropenia, and coagulopathy (decreased fibrinogen, elevated PT/INR/ PTT/D-dimers) present : cryoprecipitate and FFP transfused, vitamin K given; blood cultures negative at time of transfer Liver dysfunction with decreased albumin and hyperammonemia present: abdominal ultrasound and CT scan demonstrated multiple congenital extrahepatic portosystemic shunts with abnormal portal venous and hepatic venous connections Transferred to Colorado Children’s Hospital for further evaluation and treatment
ALLOIMMUNIZATION TO ABO ANTIGENS Usually A and B antigens are weakly expressed on platelets There is evidence that FNAIT can be caused by anti-A or anti-B if the baby has a high expression of the A or B antigen on his platelets and/or the mother has a high titer of Ig. G anti-A or anti-B ABO blood group incompatibility: babies less likely to have severe FNAIT if mother is blood group O compared to blood group A
PATHOPHYSIOLOGY It is likely that immunizing antigen is present in maternal blood at 10 -12 weeks gestation: early intrauterine intracranial hemorrhage (ICH) can occur HPAs are present on fetal platelets at 16 weeks gestation Fetal platelet exposure may not be sufficient to explain alloimmunization: GPIIIa, which carries HPA -1 a/1 b and is present on the surface of villous syncytiotrophoblast, may be responsible for alloimmunization Fc. Rn (neonatal Fc receptor binding Ig. G to fetal platelets), present on syncytiotrophoblast, transports antibodies into the fetal circulation

PATHOPHYSIOLOGY FNAIT can occur in first incompatible pregnancies and there is a high recurrence rate According to more recent data, most cases of maternal alloimmunization may result from exposure to fetal blood at or soon after birth More severe FNAIT tends to occur in subsequent pregnancies
DIFFERENTIAL DIAGNOSIS OF NEONATAL THROMBOCYTOPENIA Infection (such as toxoplasma, HIV, herpes virus, CMV, gram negative sepsis) Abnormal clotting (DIC, thrombosis) Perinatal hypoxic injury Maternal conditions (such as ITP, maternal medications, diabetes mellitus, placental insufficiency) Metabolic disease Trisomy 13, 18 or 21 Prematurity Vascular malformations (such as Kasabach-Merritt Syndrome) Bone marrow infiltration (such as leukemia) Familial thrombocytopenias
DIFFERENTIAL DIAGNOSIS NAIT favored over other causes of thrombocytopenia: platelet count < 50, 000/mc. L during first 24 hours after birth or thrombocytopenia of unknown cause present in a well neonate with a non-thrombocytopenic mother Neonates with NAIT can respond as well to random platelet transfusions as thrombocytopenic neonates without NAIT
LABORATORY DIAGNOSIS Maternal and paternal platelet antigen genotyping on blood samples in order to determine presence of incompatibility Fetal/neonatal platelet antigen genotyping is indicated if the father is heterozygous Detection of maternal platelet alloantibodies in blood samples (platelet-specific, class I HLA) Type paternal and maternal RBCs for ABO antigens
DIAGNOSIS: DETECTION OF PLATELET ANTIGENS DNA-based methods are currently used Allelic discrimination assays: 5’ fluorescently-labeled probes anneal to specific alleles PCR-sequence-specific primer amplification using electrophoresis and DNA band visualization PCR-RFLP (restriction fragment length polymorphism-PCR) Evaluate for the presence of HPA-1 through 6, -9 and -15
DETECTION OF ANTIBODIES BY NON-SPECIFIC TESTS: FLOW CYTOMETRY Detects platelet-specific, HLA and blood group antibodies Maternal serum is reacted against washed paternal and maternal platelets and platelets from group O donors with common HPA antigens Can detect labile and low density antigens Specific antibody target not identified Both Ig. G and Ig. M can be identified Sensitivity is high
DETECTION OF ANTIBODIES BY SPECIFIC SOLID PHASE ASSAYS Determine the specific glycoprotein to which the maternal alloantibody is directed: purified platelet glycoproteins are immobilized in these tests - specificity to a particular antigen can be identified (such as HPA-1 a) since serum is tested against a panel of typed platelets Types of assays: - MAIPA - MACE - ACE

SOLID PHASE ASSAYS: MAIPA MAIPA (monoclonal antibody immobilization of platelet antigens) performed in Europe and used to detect antibodies directed against HPA-15 a and -15 b (using fresh target platelets) Sensitize target platelets with patient serum Murine monoclonal antibody (MCA) attaches to target on platelet surface Platelets washed and solubilized Supernatant lysate, after centrifugation, is added to wells in a microtiter tray with immobilized goat antibody specific for mouse Ig. G Goat antibody attaches to MCA with bound human antibody Human antibody detected by enzyme labeled goat antihuman immunoglobulin

SOLID PHASE ASSAYS MACE (modified antigen capture enzymelinked immunosorbent assay): detects antibodies reactive with antigens on GPIIb/IIIa and GPIa/IIa ACE (antigen capture ELISA): detects antibodies reactive with antigens on GPIb/IX, the carrier for HPA 2 a/b and GPIV, as well as antibodies reactive with class I HLA antigens Pak. Plus: an example of an antigen capture ELISA
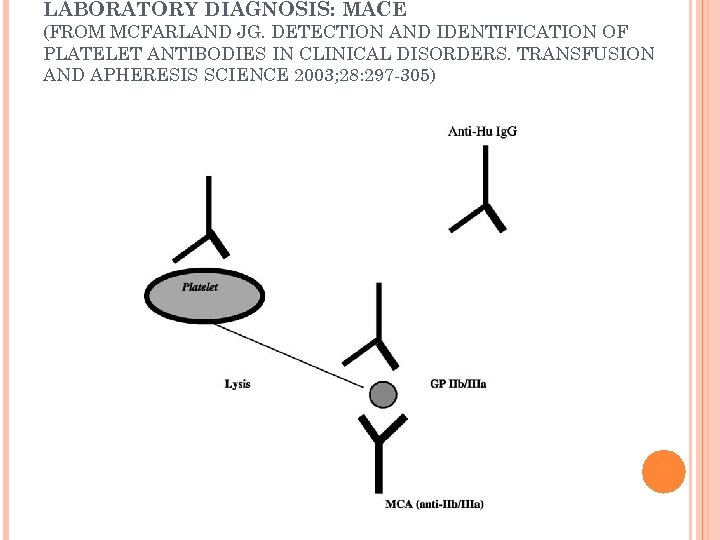
LABORATORY DIAGNOSIS: MACE (FROM MCFARLAND JG. DETECTION AND IDENTIFICATION OF PLATELET ANTIBODIES IN CLINICAL DISORDERS. TRANSFUSION AND APHERESIS SCIENCE 2003; 28: 297 -305)

SOLID PHASE ASSAYS: FLUORESCENTLY LABELED BEADBASED ASSAYS Platelet antigens attached to fluorescently labeled beads Can detect antibodies to low frequency antigens More sensitive, easier to perform and more expensive than ELISA

SOLID PHASE ASSAYS Advantages: specific for platelet glycoproteins or Class I HLA antigens Disadvantages: technically demanding, panels of typed platelets
DIAGNOSIS: INTERPRETATION OF TEST RESULTS HPA antibody may be present: maternal Ig. G reacts with paternal and normal panel platelets but not with maternal platelets; no HLA antibodies are detected Antibody directed against a low frequency HPA antigen or against A 1 or B may be present: maternal serum reacts with paternal platelets but not with platelets in the normal panel
DIAGNOSIS: INTERPRETATION OF TEST RESULTS DNA analysis can be used to identify low frequency antigens on paternal platelets Testing for alloimmunization due to A 1 or B when parents have incompatible blood types: - identify fathers who are high expressers of A 1 or B: test paternal platelets with monoclonal antibodies directed against A 1 or B and compare reaction strengths with normals - absorb maternal serum with A 1 or B red cells; if reactivity with paternal platelets is gone, ABO incompatibility is assumed

LABORATORY DIAGNOSIS: NO ANTIHPA ANTIBODY DETECTED Current assays may not detect alloantibodies directed against HPA-3 a and -3 b Low avidity HPA antibodies are not detected by standard assays in which the target antigen is washed - these low avidity antibodies can be detected by a technically demanding technique, surface plasmon resonance (SPR) - these low avidity antibodies are pathogenic: the antibodies destroy human platelets in a mouse model

LABORATORY DIAGNOSIS Parents incompatible for platelet antigens, no maternal anti-platelet antibodies detected: - FNAIT may still be present if there is clinical suspicion - repeat test for maternal anti-platelet antibodies at 6 -9 weeks after delivery

CLINICAL PRESENTATION: NEONATE Typically the first newborn affected with NAIT in a family presents with bleeding and thrombocytopenia or severe unexplained thrombocytopenia at or soon after birth ICH can occur 24 -48 hours after birth

CLINICAL PRESENTATION No bleeding Petechiae Hematomas Gastrointestinal bleeding Hemoptysis Hematuria Retinal hemorrhage Intracranial hemorrhage (ICH)
CLINICAL PRESENTATION: INTRACRANIAL HEMORRHAGE Neonatal: 15% of neonates with platelet counts less than 50, 000/mc. L with HPA-1 a NAIT develop ICH with an associated mortality of 10%-15% Antenatal (up to 80% of cases): can occur as early as 16 -24 weeks; 42% occur prior to 30 weeks 79% risk of ICH in a subsequent pregnancy if previous pregnancy had ICH or intrauterine fetal demise Significant neurologic sequelae of ICH: hydrocephalus, seizures, blindness, brainstem atrophy, poor neurologic development
CLINICAL PRESENTATION: ANTIBODY SPECIFICITIES AND BLEEDING Anti-HPA-5 b (compared with anti-HPA-1 a) - milder bleeding - nadir platelet counts < 30, 000/mc. L are less frequent - bleeding occurs at higher platelet counts HPA-1 a is expressed on endothelial cells and anti-HPA-1 a may disrupt intercellular connections with consequent bleeding Antibodies against GP IIb/IIIa and GP Ia/IIa may cause platelet dysfunction

CLINICAL PRESENTATION: NEONATE Without treatment, thrombocytopenia usually resolves in the first two weeks after delivery Thrombocytopenia may persist for several months
MANAGEMENT OF NAIT IN NEONATES: TRANSFUSION TRIGGER No bleeding, platelet count > 50, 000/mc. L: observe Transfusion trigger for well term neonate varied among Canadian neonatologists : 20, 000 to 50, 000/mc. L Other expert opinion is to maintain platelet count as follows: - well term neonates: >30, 000/mc. L - bleeding risk factors: > 50, 000/mc. L - ICH/other bleeding: > 100, 000/mc. L
MANAGEMENT OF NAIT IN NEONATES: PLATELET TRANSFUSIONS If HPA-typed platelets are unavailable, random donor platelets (ABO compatible, volume reduced if needed, irradiated, cytomegalovirus negative) can lead to an increase in platelet counts to > 40, 000/mc. L and can reduce the risk of bleeding In one study, transfusion with random donor platelets led to lower mean platelet increments and shorter platelet survival compared with HPA -typed platelets

MANAGEMENT OF NAIT IN NEONATES: HPA-COMPATIBLE PLATELET TRANSFUSIONS Some blood centers can provide HPA-1 b/1 b platelets Mother can provided antigen-negative platelets by apheresis - remove antibody-containing plasma by volume reduction and resuspend platelets in normal saline or wash platelets in normal saline or AB plasma - irradiate platelets to prevent TAGVHD

MANAGEMENT OF NAIT IN NEONATES: IVIG British Committee for Standards in Haematology: keep platelet count > 30, 000/mc. L by transfusion with HPA-compatible platelets (10 -20 m. L/kg) and add high-dose IVIG Other expert opinion: use IVIG adjunctively with platelet transfusions to increase platelet survival
MANAGEMENT OF NAIT IN NEONATES: IVIG Platelet count increases > 30, 000/mc. L occur at rates of 70 -80% with the use of IVIG in some studies Maximal effect of IVIG is reached at 24 -72 hours Doses of IVIG: - platelet count < 30, 000/mc. L: 0. 4 -1. 0 g/kg/d for 2 -5 days - platelet count > 30, 000/mc. L and < 50, 000/mc. L without bleeding: total dose of 2 g/kg over 2 -5 days
MANAGEMENT OF ANTENATAL FNAIT Maternal alloantibody previously detected Determine fetal compatibility: - determine paternal platelet genotype - father homozygous for incompatible antigen: all fetuses incompatible with maternal alloantibody and at risk for FNAIT - father heterozygous for incompatible antigen: fetus has 50% chance of possessing incompatible antigen and need to genotype fetal platelets

MANAGEMANT OF ANTENATAL FNAIT: GENOTYPING OF FETAL PLATELETS Invasive methods: - Amniotic fluid: 18 -20 weeks - Chorionic villus tissue: 8 -10 wks Adverse effects of invasive methods: premature labor, death in utero
MANAGEMENT OF ANTENATAL FNAIT: GENOTYPING OF FETAL PLATELETS Non-invasive method: - Cell-free fetal DNA extracted from maternal blood: two assays based on real-time polymerase chain reaction and screening for single nucleotide polymorphisms provide reliable results as early as 15 weeks gestation - Assays available for HPA-1 aa and HPA-1 bb women and are being developed for HPA-5 and HPA-3 Limitations of non-invasive method: - possible false-negative results due to low quantity of fetal DNA extracted from maternal blood - a fetal marker independent of fetal sex is needed
MANAGEMENT OF ANTENATAL FNAIT Approximation of degree of thrombocytopenia in incompatible fetuses helps determine risk of ICH Invasive approach of PUBS (percutaneous umbilical blood sampling) to determine fetal platelet count and perform in utero platelet transfusions - fetal loss of 1. 3%/procedure and 58%/pregnancy
MANAGEMENT OF ANTENATAL FNAIT Correlation between strength of maternal alloantibody and degree of fetal thrombocytopenia is uncertain: - some studies identified an approximately inverse relationship between maternal antibody strength and neonatal platelet count - in one study, an anti-HPA-1 a antibody level >3. 0 IU/m. L at 22 or 34 weeks had a positive predictive value of 54% and a negative predictive value of 95% for severe thrombocytopenia Maternal alloantibody levels decrease throughout gestation and highest antibody levels occur up to 6 weeks after delivery

MANAGEMENT OF ANTENATAL FNAIT Antenatal management is controversial Currently a more conservative approach without invasive procedures is favored: maternal IVIG and/or corticosteroids Degree of fetal thrombocytopenia and risk of ICH can be reduced by the use of high dose IVIG (with or without corticosteroids) Studies indicate a tendency to higher neonatal platelet counts with a combination of IVIG and corticosteroids but with no significant decrease in ICH risk

MANAGEMENT OF ANTENATAL FNAIT: COCHRANE REVIEW 2011 (RAYMANT R, ET AL) Most evidence for antenatal therapy in FNAIT is from observational studies Objective of review: “determine the optimal antenatal treatment of fetomaternal alloimmune thrombocytopenia to prevent fetal and neonatal haemorrhage and death” Evaluated randomized clinical trials comparing an intervention with no therapy or comparing two interventions: four studies met criteria with one trial comparing IVIG with corticosteroid and three trials comparing IVIG and corticosteroid with only IVIG

MANAGEMENT OF ANTENATAL FNAIT: COCHRANE REVIEW (RAYMANT R, ET AL) Conclusion: best antenatal treatment for FNAIT unclear; more studies needed Stratified treatment based on sibling history appears to be safe IVIG or prednisone (optimal doses unknown): firstline therapy when pre-treatment fetal platelet count > 20, 000/mc. L and previously affected sibling had no peripartum hemorrhage IVIG plus prednisone superior to IVIG alone in raising platelet count when pre-treatment fetal platelet count < 20, 000/mc. L or sibling had peripartum hemorrhage (optimal doses and timing unclear)
MANAGEMENT OF ANTENATAL FNAIT: TREATMENT ALGORITHM FNAIT severity in current pregnancy dependent on presence and time of occurrence of antenatal ICH in previous pregnancy: ICH occurring in early pregnancy predicts severe disease in the next pregnancy Pacheco et al have developed a treatment algorithm using IVIG and prednisone based on risk (for ICH) stratification with four strata: no fetal blood sampling performed One study demonstrated that the use of IVIG in subsequent pregnancies reduced the risk of recurrence of ICH from 79% to 11%
FNAIT TREATMENT ALGORITHM: STRATUM 1 Thrombocytopenia or ICH of unknown cause in prior fetus/neonate No HPA antibody identified: paternal incompatibility for HPAs may have been determined Unknown risk for ICH in current pregnancy Treatment: serial monitoring for presence of maternal anti-HPA antibodies during gestation; no treatment unless an HPA antibody is found or there is high clinical suspicion for FNAIT without detectable antibody
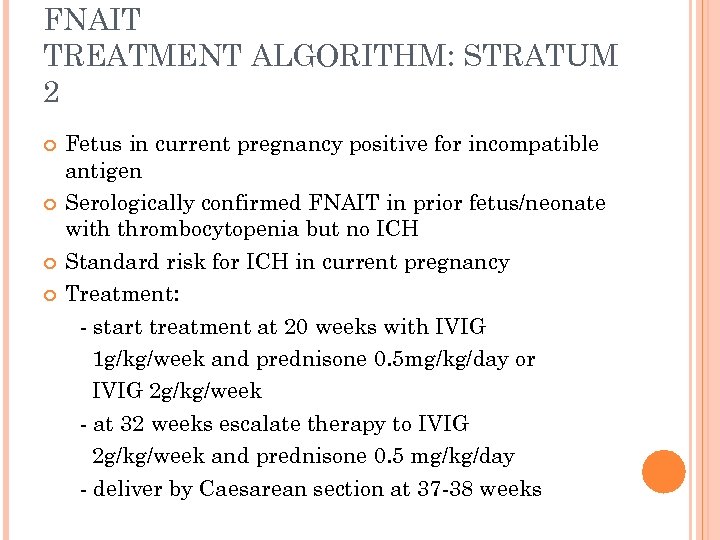
FNAIT TREATMENT ALGORITHM: STRATUM 2 Fetus in current pregnancy positive for incompatible antigen Serologically confirmed FNAIT in prior fetus/neonate with thrombocytopenia but no ICH Standard risk for ICH in current pregnancy Treatment: - start treatment at 20 weeks with IVIG 1 g/kg/week and prednisone 0. 5 mg/kg/day or IVIG 2 g/kg/week - at 32 weeks escalate therapy to IVIG 2 g/kg/week and prednisone 0. 5 mg/kg/day - deliver by Caesarean section at 37 -38 weeks
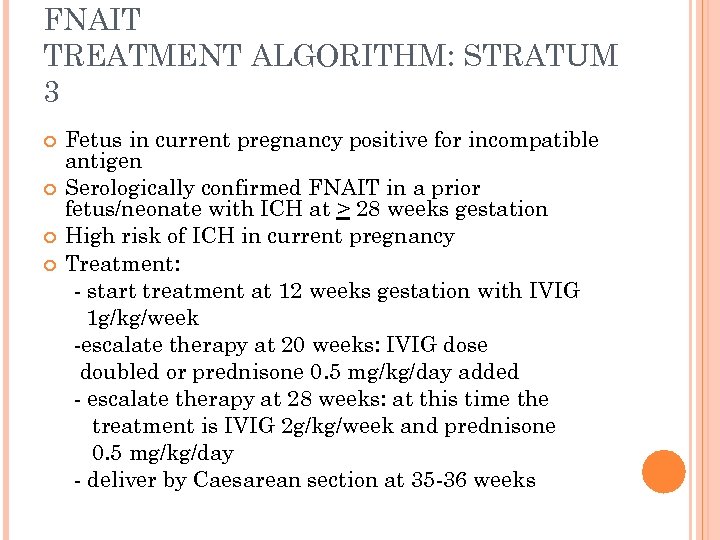
FNAIT TREATMENT ALGORITHM: STRATUM 3 Fetus in current pregnancy positive for incompatible antigen Serologically confirmed FNAIT in a prior fetus/neonate with ICH at > 28 weeks gestation High risk of ICH in current pregnancy Treatment: - start treatment at 12 weeks gestation with IVIG 1 g/kg/week -escalate therapy at 20 weeks: IVIG dose doubled or prednisone 0. 5 mg/kg/day added - escalate therapy at 28 weeks: at this time the treatment is IVIG 2 g/kg/week and prednisone 0. 5 mg/kg/day - deliver by Caesarean section at 35 -36 weeks
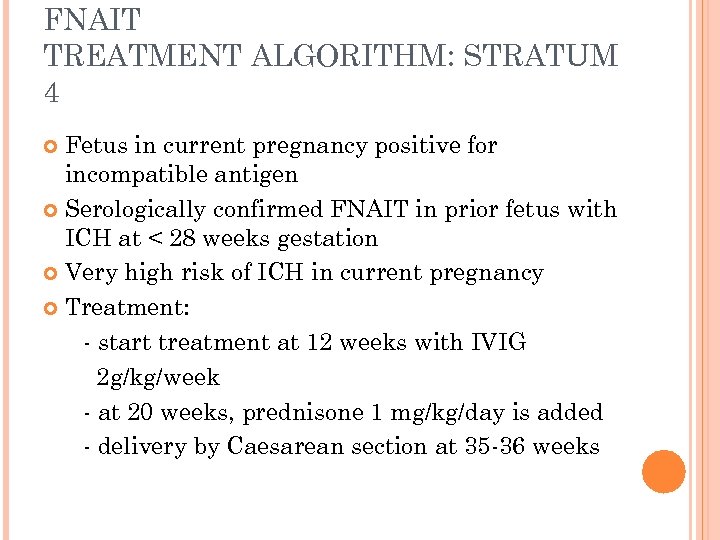
FNAIT TREATMENT ALGORITHM: STRATUM 4 Fetus in current pregnancy positive for incompatible antigen Serologically confirmed FNAIT in prior fetus with ICH at < 28 weeks gestation Very high risk of ICH in current pregnancy Treatment: - start treatment at 12 weeks with IVIG 2 g/kg/week - at 20 weeks, prednisone 1 mg/kg/day is added - delivery by Caesarean section at 35 -36 weeks

MANAGEMENT OF ANTENATAL FNAIT: INVASIVE PROCEDURES Fetal blood sampling (FBS) is performed to evaluate the fetal platelet count, transfuse platelets and monitor therapy Intrauterine platelet transfusions for platelet counts < 50, 000/mc. L can be performed in babies unresponsive to IVIG and steroids

MANAGEMENT OF ANTENATAL FNAIT: INVASIVE PROCEDURES Adverse effects: - bleeding - fetal cardiac arrhythmia - contamination of amniotic fluid - premature delivery - fetal mortality - maternal sensitization to incompatible antigen may occur
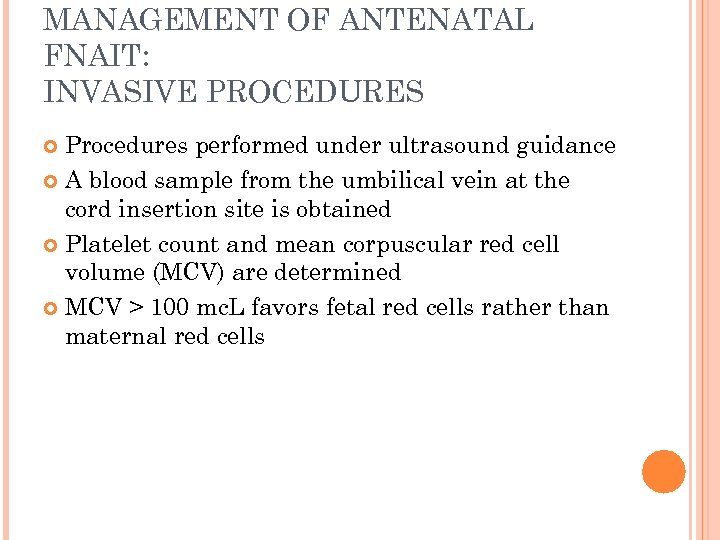
MANAGEMENT OF ANTENATAL FNAIT: INVASIVE PROCEDURES Procedures performed under ultrasound guidance A blood sample from the umbilical vein at the cord insertion site is obtained Platelet count and mean corpuscular red cell volume (MCV) are determined MCV > 100 mc. L favors fetal red cells rather than maternal red cells
MANAGEMENT OF ANTENATAL FNAIT: PLATELET TRANSFUSIONS Platelets to be transfused should be antigennegative, irradiated and CMV safe. HPA-1 a-negative platelets can be provided by some blood suppliers Platelets from the mother, obtained by apheresis 48 -72 hours before transfusion, can be used. Incompatible platelets can be used in urgent situations.
MANAGEMENT OF ANTENATAL FNAIT: PLATELET TRANSFUSIONS A blood sample is obtained at the end of transfusion in order to evaluate the platelet count. Platelet transfusions are performed weekly since the platelet life span is 8 -10 days. Delivery at 32 weeks gestation may be best if the baby is undergoing serial transfusions. Antigen-negative platelets should be available at delivery.

MANAGEMENT OF ANTENATAL FNAIT: MATERNAL PLATELETS Infectious disease testing of the mother should occur several days before the platelet donation Since IVIG may cause falsely positive infectious disease testing results, performing ID testing before initiation of IVIG will help to recognize false positive results Platelets should be washed to remove antibody.
MANAGEMENT OF ANTENATAL FNAIT: VOLUME OF PLATELET TRANSFUSION Volume transfused (m. L) = Fetoplacental volume (m. L) x (target final-initial platelet count) x 2 Platelet count of transfused concentrate Fetoplacental volume = ultrasound fetal weight (g) x 0. 14 Factor of 2 in equation: sequestration of platelets in spleen or liver Target final platelet count: 300, 000 -500, 000/mc. L, which enables a nadir platelet count of 30, 00050, 000/mc. L just before the next transfusion

ADVERSE EFFECTS OF PREDNISONE Hypertension Fluid retention Hyperglycemia Diabetes Immunosuppression Mood swings Acne Osteoporosis

INTRAVENOUS IMMUNOGLOBULIN G (IVIG) Different products (Gamunex, Privigen, Gammagard, Flebogamma, Octagam) Usual infusion rate: 1 g/kg over 4 -5 hours Infusion rate of first infusion should be slower First infusion should be in a hospital setting

IVIG Adverse effects: headache, flushing, myalgias, low back pain, nausea, hypotension, wheezing, fever, chills; rare: aseptic meningitis, kidney injury, thrombosis, hemolytic anemia, stroke Premedication: corticosteroids, acetaminophen, diphenhydramine; prednisone combined with acetaminophen may also help manage headaches Hydration with intravenous fluids and slowing the infusion rate also help ameliorate reactions Contraindications: Ig. A deficiency; anaphylactic or severe systemic reactions to human immunoglobulin/blood-derived products
SCREENING PROGRAMS TO DETECT HPA-1 A ALLOIMMUNIZATION Identify HPA-1 a-alloimmunized women (0. 10. 2%) or women who are HPA-1 a negative (>2%), and possibly DRB 3*0101 positive (about 0. 7% of pregnancies) Serial screening of women at risk of antibody development during gestation Disadvantages of screening programs: cost of IVIG, adverse effects of prednisone used to treat the side effects of IVIG

PROPHYLACTIC ANTI-HPA-1 A ANTIBODY TREATMENT OF FNAIT About 75% of women are immunized at the time of delivery Results from a mouse study support the use of anti-HPA-1 a given to non-immunized HPA-1 anegative women after delivery of an HPA-1 apositive child in the prevention of HPA-1 a alloimmunization and FNAIT
PROPHYLACTIC ANTI-HPA-1 A ANTIBODY TREATMENT OF FNAIT: SOURCE Engineered monoclonal or recombinant antibodies: - such antibodies may not be effective: efforts to produce engineered anti-D antibodies to prevent HDFN have not been successful Polyclonal antibodies purified from immunized subjects: - use pooled plasma from HPA-1 a immunized individuals - FNAIT is rare with few immunized individuals available: international efforts required

PROPHYLACTIC ANTI-HPA-1 A ANTIBODY TREATMENT OF FNAIT: PROFNAIT PROJECT “Human platelet antigen-1 a immunoglobulin” is an orphan drug designated by the European Commission in October, 2011 The drug will be developed by Prophylix Pharma AS Four stages: - collect 2000 L of plasma from HPA-1 a-immunized women - produce drug - phase I/II trial using healthy HPA-1 a-negative males - phase III clinical trial treating nonimmunized HPA 1 a-negative women
REFERENCES Arinsburg, SA, Shaz BH, Westhoff C, et al. Determination of human platelet antigen typing by molecular methods: importance in diagnosis and early treatment of neonatal alloimmune thrombocytopenia. Am J Hematol 2012; 87: 525 -28. Bertrand G, Drame M, Martageix C, et al. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood 2011; 117(11): 3209 -13. Bussel JB, Berkowitz RL, Hung C, et al. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. Am J Obstet Gynecol 2010; 203: 135. e 1 -14.

REFERENCES Curtis BR, Fick A, Lochowicz AJ, et al. Neonatal alloimmune thrombocytopenia associated with maternalfetal incompatibility for blood group B. Transfusion 2008; 48: 358 -64. Curtis BR, Mc. Farland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology 2009; 25(3): 125 -35. Espinoza JP, Caradeux J, Norwitz ER, et al. Fetal and neonatal alloimmune thrombocytopenia. Rev Obstet Gynecol 2013; 6(1): e 15 -21. Giers G, Riethmacher R, Lorenz H, et al. Repeated intrauterine Ig. G infusions in foetal alloimmune thrombocytopenia do not increase foetal platelet counts. Vox Sanguinis 2010; 99: 348 -53.
REFERENCES Heikal NM, Smock KJ. Laboratory testing for platelet antibodies. Am J Hematol 2013; 88(9): 818 -21. Kamphuis MM, Paridaans N, Porcelijn L, et al. Screening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic review. BJOG 2010; 117: 1335 -43. Kato S, Sugiura T, Ueda H, et al. Massive intracranial hemorrhage caused by neonatal alloimmune thrombocytopenia associated with anti-group A antibody. Journal of Perinatology 2013; 33: 79 -82. Killie MK, Husebekk A, Kjeldsen-Kragh J, et al. A prospective study of maternal anti-HPA 1 a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica 2008; 93(6): 870 -7.
REFERENCES King KE, Kao KJ, Bray PF, et al. The role of HLA antibodies in neonatal thrombocytopenia: a prospective study. Tissue Antigens 1996; 47(3): 20611. Kjeldsen-Kragh J, Killie MK, Tomter G, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood 2007; 110: 833 -9. Kjeldsen-Kragh J, Ni H, Skogen B. Towards a prophylactic treatment of HPA-related foetal and neonatal alloimmune thrombocytopenia. Curr Opin Hematol 2012; 19: 469 -74.

REFERENCES Kumpel BM. Would it be possible to prevent HPA-1 a alloimmunization to reduce the incidence of fetal and neonatal alloimmune thrombocytopenia? Transfusion 2012; 52: 1393 -7. Le Toriellec E, Chenet C, Kaplan C. Safe fetal platelet genotyping: new developments. Transfusion 2013; 53: 175562. LIFECODES Pak. Plus assay. Gen-Probe Incorporated, Waukesha, WI 53186. Mc. Farland JG. Detection and identification of platelet antibodies in clinical disorders. Transfusion and Apheresis Science 2003; 28: 297 -305. Mechoulan A, Kaplan C, Muller JY, et al. Fetal alloimmune thrombocytopenia: is less invasive antenatal management safe? The Journal of Maternal-Fetal and Neonatal Medicine 2011; 24(4): 564 -7.

REFERENCES Pacheco LD, Berkowitz RL, Moise KJ, et al. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet Gynecol 2011; 118: 1157 -63. Peterson JA, Kanack A, Nayak D, et al. Prevalence and clinical significance of low-avidity HPA-1 a antibodies in women exposed to HPA-1 a during pregnancy. Transfusion 2013; 53(6): 1309 -18. Peterson JA, Mc. Farland JG, Curtis BR, et al. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. British Journal of Haematology 2013; 161: 314. Rayment R, Brunskill SJ, Soothill PW, et al. Antenatal interventions for fetomaternal alloimmune thrombocytopenia. Cochrane Database of Systematic Reviews 2011; Issue 5: 1 -52.

REFERENCES Risson DC, Davies MW, Williams BA. Review of neonatal alloimmune thrombocytopenia. Journal of Paediatrics and Child Health 2012; 48: 816 -22. Roback JD, Grossman BJ, Harris T, et al, eds. Technical manual. 17 th ed. Bethesda, MD: AABB, 2011: 639 -41. Sachs UJ. Fetal/neonatal alloimmune thrombocytopenia. Thrombosis Research 2013; 131, Suppl. 1: S 42 -6. Sainio S, Javela K, Tuimala J, et al. Usefulness of maternal anti-HPA-1 a antibody quantitation in predicting severity of foetomaternal alloimmune thrombocytopenia. Transfusion Medicine 2013; 23(2): 114 -20.

REFERENCES Saito S, Ota M, Komatsu Y, et al. Serologic analysis of three cases of neonatal alloimmune thrombocytopenia associated with HLA antibodies. Transfusion 2003; 43: 90817. Salomon O, Rosenberg N. Predicting risk severity and response of fetal neonatal alloimmune thrombocytopenia. British Journal of Haematology 2013; 162: 304 -12. Strong NK, Eddleman KA. Diagnosis and management of neonatal alloimmune thrombocytopenia in pregnancy. Clin Lab Med 2013; 33: 311 -25. Symington A, Paes B. Fetal and neonatal alloimmune thrombocytopenia: harvesting the evidence to develop a clinical approach to management. Am J Perinatol 2011; 28: 137 -44.
REFERENCES Thude H, Schorner U, Helfricht C, et al. Neonatal alloimmune thrombocytopenia caused by human leucocyte antigen-B 27 antibody. Transfusion Medicine 2006; 16: 143 -9. Tiller H, Kamphuis MM, Flodmark O, et al. Fetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: an observational cohort study of 43 cases from an international multicentre registry. BMJ Open 2013; 3: e 002490. doi: 10. 1136/bmjopen-2012 -002490. Tiller H, Killie MK, Chen P, et al. Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model. Transfusion 2012; 52: 1446 -57.
REFERENCES Turner ML, Bessos H, Fagge T, et al. Prospective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1 a. Transfusion 2005; 45: 1945 -56. Vinograd CA, Bussel JB. Antenatal treatment of fetal alloimmune thrombocytopenia: a current perspective. Haematologica 2010; 95(11): 1807 -11.
1f6ccf6c1b6f6657736c4c1d6af42ec4.ppt