016be4a3de3595a90f15a7054d3ab61b.ppt
- Количество слайдов: 112
 Ferrous and Non-ferrous materials Dr. Siddhalingeshwar I. G.
Ferrous and Non-ferrous materials Dr. Siddhalingeshwar I. G.
 Engineers are often involved in materials selection decisions, which necessitates that they have some familiarity with the general characteristics of a wide variety of materials. In addition, access to data bases containing property values for a large number of materials may be required. Hence this chapter presents an opportunity for the learners to introduce them to knowledge on ferrous and nonferrous materials.
Engineers are often involved in materials selection decisions, which necessitates that they have some familiarity with the general characteristics of a wide variety of materials. In addition, access to data bases containing property values for a large number of materials may be required. Hence this chapter presents an opportunity for the learners to introduce them to knowledge on ferrous and nonferrous materials.
 Chapter 7: Lesson schedule 01. Introduction to ferrous and non-ferrous alloys. 02. Types of Cast iron properties and applications. 03. AISI and BIS designation of steels 04. Non ferrous alloys – composition, properties and applications. 05. Non ferrous alloys – composition, properties and applications. 06. Application areas (Tool materials, etc) 3
Chapter 7: Lesson schedule 01. Introduction to ferrous and non-ferrous alloys. 02. Types of Cast iron properties and applications. 03. AISI and BIS designation of steels 04. Non ferrous alloys – composition, properties and applications. 05. Non ferrous alloys – composition, properties and applications. 06. Application areas (Tool materials, etc) 3
 Learning Outcomes Identify the physical properties of different steel alloys, aluminum alloys based on the chemical composition. Analyze the suitability of ferrous / non-ferrous materials as candidate material to be used to design a particular component.
Learning Outcomes Identify the physical properties of different steel alloys, aluminum alloys based on the chemical composition. Analyze the suitability of ferrous / non-ferrous materials as candidate material to be used to design a particular component.
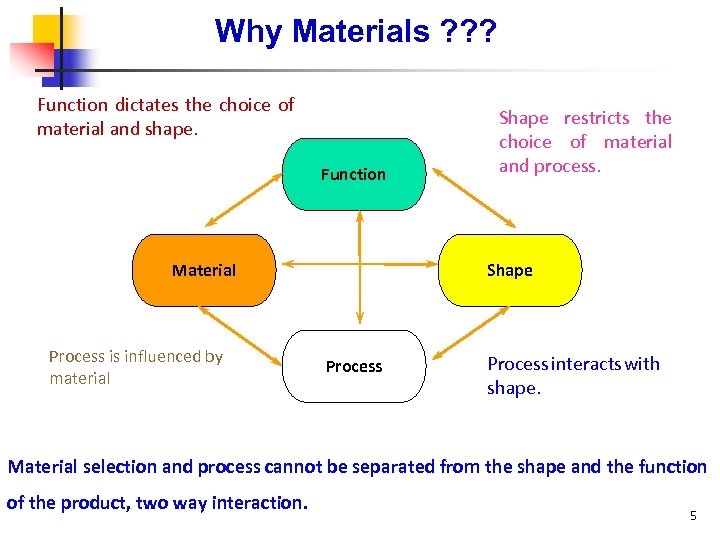 Why Materials ? ? ? Function dictates the choice of material and shape. Function Material Process is influenced by material Shape restricts the choice of material and process. Shape Process interacts with shape. Material selection and process cannot be separated from the shape and the function of the product, two way interaction. 5
Why Materials ? ? ? Function dictates the choice of material and shape. Function Material Process is influenced by material Shape restricts the choice of material and process. Shape Process interacts with shape. Material selection and process cannot be separated from the shape and the function of the product, two way interaction. 5
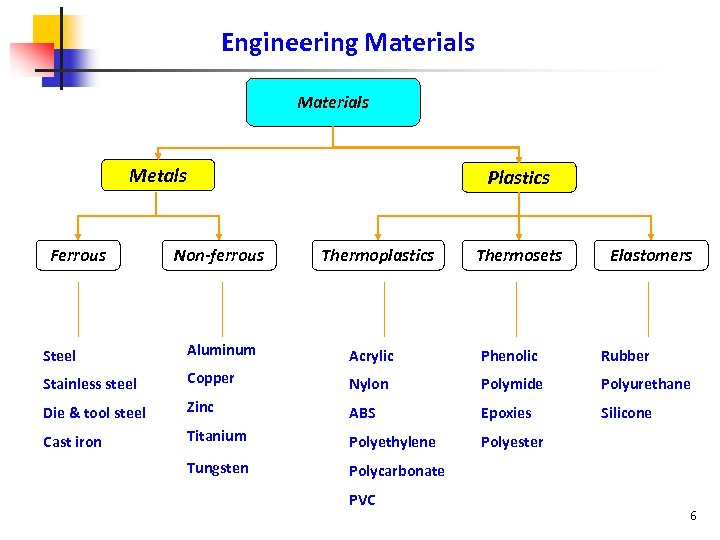 Engineering Materials Metals Ferrous Plastics Non-ferrous Thermoplastics Thermosets Elastomers Steel Aluminum Acrylic Phenolic Rubber Stainless steel Copper Nylon Polymide Polyurethane Die & tool steel Zinc ABS Epoxies Silicone Cast iron Titanium Polyethylene Polyester Tungsten Polycarbonate PVC 6
Engineering Materials Metals Ferrous Plastics Non-ferrous Thermoplastics Thermosets Elastomers Steel Aluminum Acrylic Phenolic Rubber Stainless steel Copper Nylon Polymide Polyurethane Die & tool steel Zinc ABS Epoxies Silicone Cast iron Titanium Polyethylene Polyester Tungsten Polycarbonate PVC 6
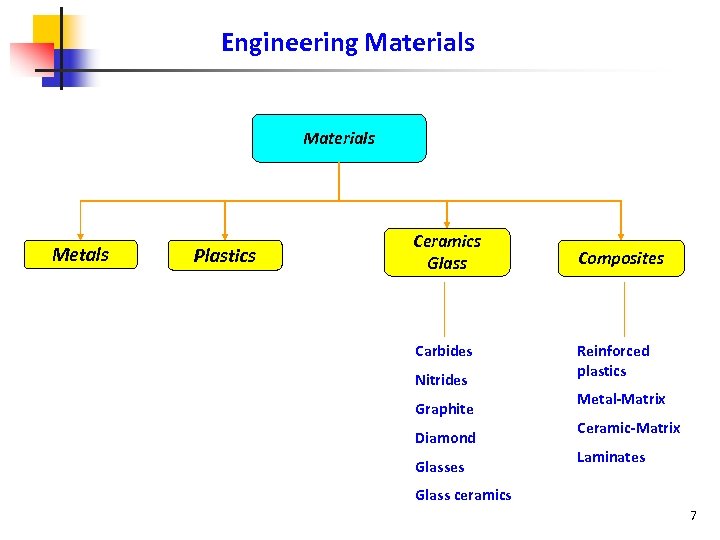 Engineering Materials Metals Plastics Ceramics Glass Carbides Nitrides Graphite Diamond Glasses Composites Reinforced plastics Metal-Matrix Ceramic-Matrix Laminates Glass ceramics 7
Engineering Materials Metals Plastics Ceramics Glass Carbides Nitrides Graphite Diamond Glasses Composites Reinforced plastics Metal-Matrix Ceramic-Matrix Laminates Glass ceramics 7
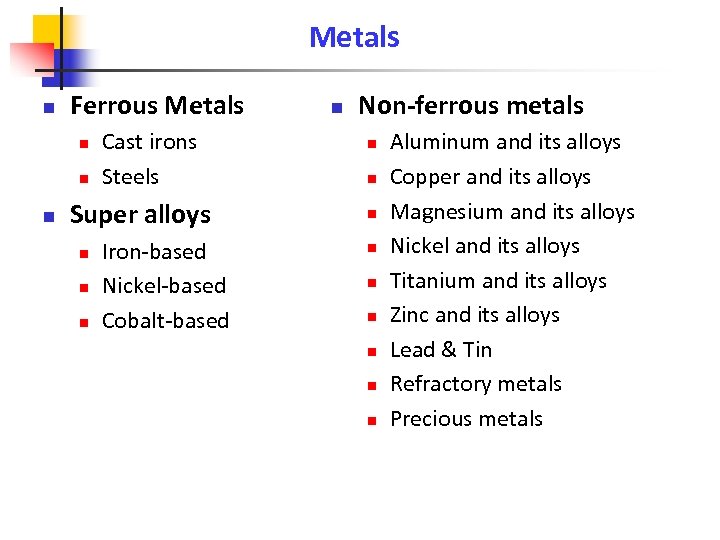 Metals n Ferrous Metals n n n Cast irons Steels Super alloys n n n Iron-based Nickel-based Cobalt-based n Non-ferrous metals n n n n n Aluminum and its alloys Copper and its alloys Magnesium and its alloys Nickel and its alloys Titanium and its alloys Zinc and its alloys Lead & Tin Refractory metals Precious metals
Metals n Ferrous Metals n n n Cast irons Steels Super alloys n n n Iron-based Nickel-based Cobalt-based n Non-ferrous metals n n n n n Aluminum and its alloys Copper and its alloys Magnesium and its alloys Nickel and its alloys Titanium and its alloys Zinc and its alloys Lead & Tin Refractory metals Precious metals
 Where do metals come from? • Metals form part of the earth’s crust as metal ore. • To obtain useful metals, the metal ore is mined and washed to remove other minerals and unwanted materials. • Iron ore is the basis for most steels. • To extract pure iron the iron ore is heated in a furnace in a process known as smelting.
Where do metals come from? • Metals form part of the earth’s crust as metal ore. • To obtain useful metals, the metal ore is mined and washed to remove other minerals and unwanted materials. • Iron ore is the basis for most steels. • To extract pure iron the iron ore is heated in a furnace in a process known as smelting.
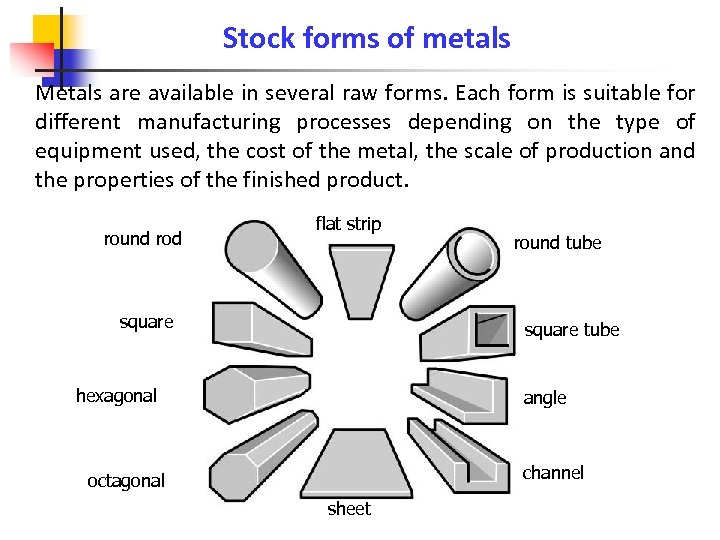 Stock forms of metals Metals are available in several raw forms. Each form is suitable for different manufacturing processes depending on the type of equipment used, the cost of the metal, the scale of production and the properties of the finished product. round rod flat strip square round tube square tube hexagonal angle channel octagonal sheet
Stock forms of metals Metals are available in several raw forms. Each form is suitable for different manufacturing processes depending on the type of equipment used, the cost of the metal, the scale of production and the properties of the finished product. round rod flat strip square round tube square tube hexagonal angle channel octagonal sheet
 Ferrous metals are obtained from iron ore. You might recognize the letters ‘Fe’ from the periodic table, where they represent iron. Ferrous facts Iron replaced bronze as the principal metal by 1000 BC. Early pots and pans made from iron poisoned the users! Early steels were made by adding carbon to iron as it was melted over a charcoal fire. Ferrous metals: contain iron will corrode unless protected are attracted by a magnet are strong, rigid and cheap.
Ferrous metals are obtained from iron ore. You might recognize the letters ‘Fe’ from the periodic table, where they represent iron. Ferrous facts Iron replaced bronze as the principal metal by 1000 BC. Early pots and pans made from iron poisoned the users! Early steels were made by adding carbon to iron as it was melted over a charcoal fire. Ferrous metals: contain iron will corrode unless protected are attracted by a magnet are strong, rigid and cheap.
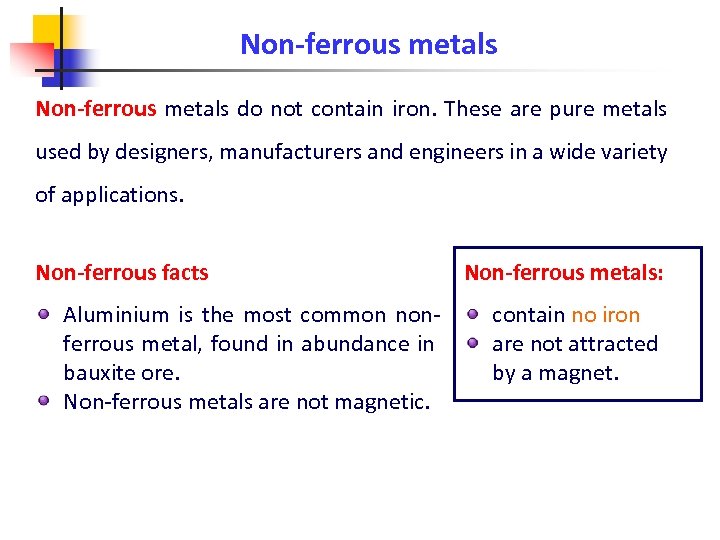 Non-ferrous metals do not contain iron. These are pure metals used by designers, manufacturers and engineers in a wide variety of applications. Non-ferrous facts Aluminium is the most common nonferrous metal, found in abundance in bauxite ore. Non-ferrous metals are not magnetic. Non-ferrous metals: contain no iron are not attracted by a magnet.
Non-ferrous metals do not contain iron. These are pure metals used by designers, manufacturers and engineers in a wide variety of applications. Non-ferrous facts Aluminium is the most common nonferrous metal, found in abundance in bauxite ore. Non-ferrous metals are not magnetic. Non-ferrous metals: contain no iron are not attracted by a magnet.
 Alloys Sometimes ferrous and non-ferrous metals require different properties in order to function better in specific situations. Alloying metals involves mixing two or more metals and other elements to improve their properties. Alloying metals can: Alloying lower the melting point alter thermal and electrical properties make a material harder for cutting purposes improve resistance to corrosion help metal to flow better into a cast.
Alloys Sometimes ferrous and non-ferrous metals require different properties in order to function better in specific situations. Alloying metals involves mixing two or more metals and other elements to improve their properties. Alloying metals can: Alloying lower the melting point alter thermal and electrical properties make a material harder for cutting purposes improve resistance to corrosion help metal to flow better into a cast.
 Ferrous Metals
Ferrous Metals
 Ferrous Metals Ferrous is an adjective used to indicate the presence of iron. The word is derived from the Latin word ferrum ("iron"). Ferrous metals include steel and pig iron (with a carbon content of a few percent) and alloys of iron with other metals (such as stainless steel). Manipulation of atom-to-atom relationships between iron, carbon, and various alloying elements establishes the specific properties of ferrous metals.
Ferrous Metals Ferrous is an adjective used to indicate the presence of iron. The word is derived from the Latin word ferrum ("iron"). Ferrous metals include steel and pig iron (with a carbon content of a few percent) and alloys of iron with other metals (such as stainless steel). Manipulation of atom-to-atom relationships between iron, carbon, and various alloying elements establishes the specific properties of ferrous metals.
 Overview of cast iron n n Iron with 1. 7 to 4. 5% carbon and 0. 5 to 3% silicon Lower melting point and more fluid than steel (better castability) Low cost material usually produced by sand casting A wide range of properties, depending on composition & cooling rate n Strength n Hardness n Ductility n Thermal conductivity n Damping capacity
Overview of cast iron n n Iron with 1. 7 to 4. 5% carbon and 0. 5 to 3% silicon Lower melting point and more fluid than steel (better castability) Low cost material usually produced by sand casting A wide range of properties, depending on composition & cooling rate n Strength n Hardness n Ductility n Thermal conductivity n Damping capacity
 Cast Irons v Cast iron - Ferrous alloys containing sufficient carbon so that the eutectic reaction occurs during solidification. v Types of cast irons: v Gray cast iron v White cast iron v Malleable cast iron v Ductile or nodular, cast iron v Compacted graphite cast iron 17
Cast Irons v Cast iron - Ferrous alloys containing sufficient carbon so that the eutectic reaction occurs during solidification. v Types of cast irons: v Gray cast iron v White cast iron v Malleable cast iron v Ductile or nodular, cast iron v Compacted graphite cast iron 17
 Production of cast iron n Pig iron, scrap steel, limestone and carbon (coke) n Cupola n Electric arc furnace n Electric induction furnace n Usually sand cast, but can be gravity die cast in reusable graphite moulds n Not formed, finished by machining
Production of cast iron n Pig iron, scrap steel, limestone and carbon (coke) n Cupola n Electric arc furnace n Electric induction furnace n Usually sand cast, but can be gravity die cast in reusable graphite moulds n Not formed, finished by machining
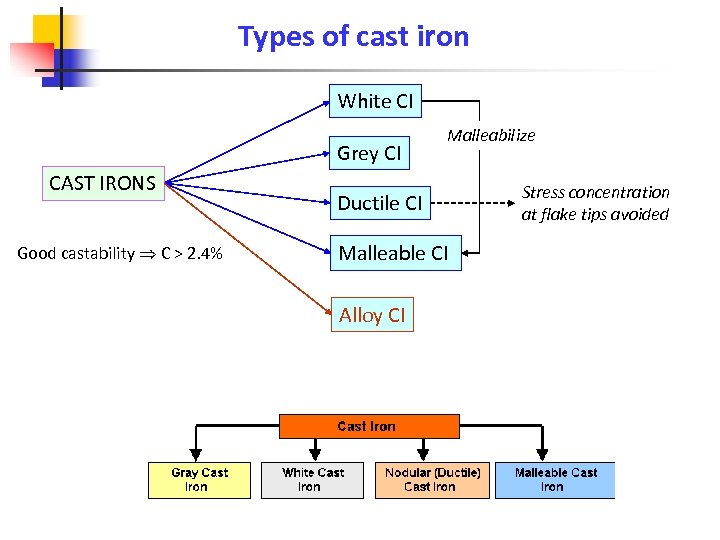 Types of cast iron White CI Grey CI CAST IRONS Good castability C > 2. 4% Malleabilize Ductile CI Malleable CI Alloy CI Stress concentration at flake tips avoided
Types of cast iron White CI Grey CI CAST IRONS Good castability C > 2. 4% Malleabilize Ductile CI Malleable CI Alloy CI Stress concentration at flake tips avoided
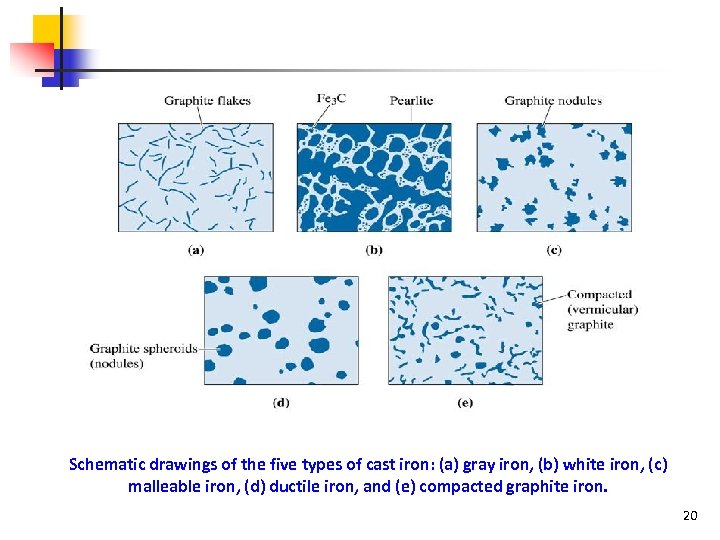 Schematic drawings of the five types of cast iron: (a) gray iron, (b) white iron, (c) malleable iron, (d) ductile iron, and (e) compacted graphite iron. 20
Schematic drawings of the five types of cast iron: (a) gray iron, (b) white iron, (c) malleable iron, (d) ductile iron, and (e) compacted graphite iron. 20
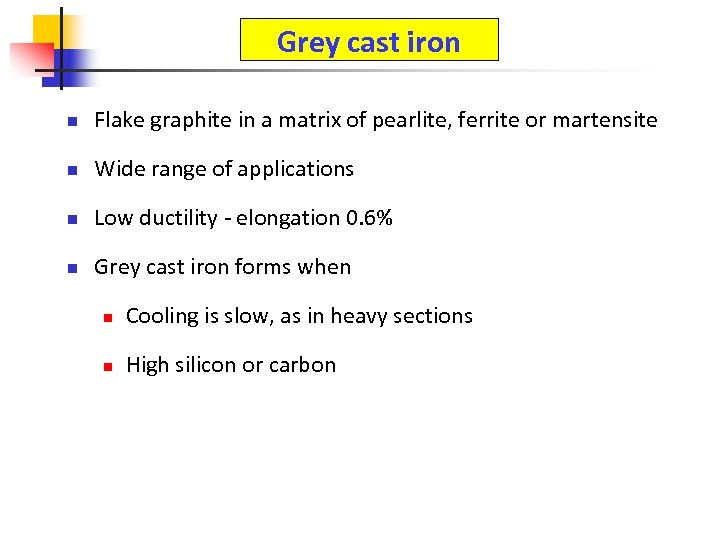 Grey cast iron n Flake graphite in a matrix of pearlite, ferrite or martensite n Wide range of applications n Low ductility - elongation 0. 6% n Grey cast iron forms when n Cooling is slow, as in heavy sections n High silicon or carbon
Grey cast iron n Flake graphite in a matrix of pearlite, ferrite or martensite n Wide range of applications n Low ductility - elongation 0. 6% n Grey cast iron forms when n Cooling is slow, as in heavy sections n High silicon or carbon
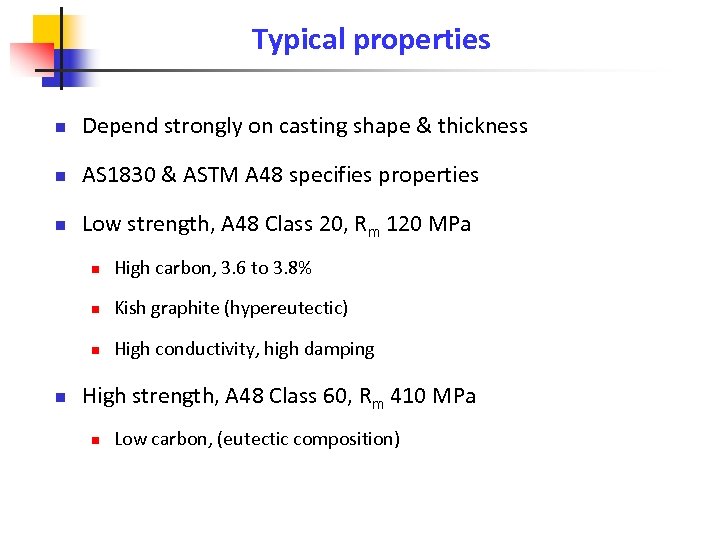 Typical properties n Depend strongly on casting shape & thickness n AS 1830 & ASTM A 48 specifies properties n Low strength, A 48 Class 20, Rm 120 MPa n n Kish graphite (hypereutectic) n n High carbon, 3. 6 to 3. 8% High conductivity, high damping High strength, A 48 Class 60, Rm 410 MPa n Low carbon, (eutectic composition)
Typical properties n Depend strongly on casting shape & thickness n AS 1830 & ASTM A 48 specifies properties n Low strength, A 48 Class 20, Rm 120 MPa n n Kish graphite (hypereutectic) n n High carbon, 3. 6 to 3. 8% High conductivity, high damping High strength, A 48 Class 60, Rm 410 MPa n Low carbon, (eutectic composition)
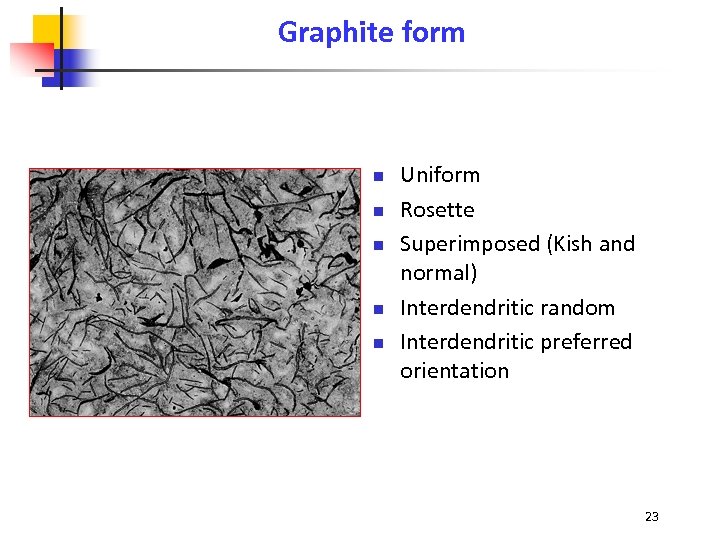 Graphite form n n n Uniform Rosette Superimposed (Kish and normal) Interdendritic random Interdendritic preferred orientation 23
Graphite form n n n Uniform Rosette Superimposed (Kish and normal) Interdendritic random Interdendritic preferred orientation 23
 Matrix structure n Pearlite or ferrite n Transformation is to ferrite when n Cooling rate is slow n High silicon content n High carbon equivalence n Presence of fine undercooled graphite 24
Matrix structure n Pearlite or ferrite n Transformation is to ferrite when n Cooling rate is slow n High silicon content n High carbon equivalence n Presence of fine undercooled graphite 24
 Properties of grey cast iron n n Machinability is excellent Ductility is low (0. 6%), impact resistance low Damping capacity high Thermal conductivity high Dry and normal wear properties excellent Applications n n n Engines n Cylinder blocks, liners, Brake drums, clutch plates Pressure pipe fittings (AS 2544) Machinery beds Furnace parts, ingot and glass moulds
Properties of grey cast iron n n Machinability is excellent Ductility is low (0. 6%), impact resistance low Damping capacity high Thermal conductivity high Dry and normal wear properties excellent Applications n n n Engines n Cylinder blocks, liners, Brake drums, clutch plates Pressure pipe fittings (AS 2544) Machinery beds Furnace parts, ingot and glass moulds
 Ductile iron n Inoculation with Ce or Mg or both causes graphite to form as spherulites, rather than flakes n Also known as spheroidal graphite (SG), and nodular graphite iron n Far better ductility than grey cast iron
Ductile iron n Inoculation with Ce or Mg or both causes graphite to form as spherulites, rather than flakes n Also known as spheroidal graphite (SG), and nodular graphite iron n Far better ductility than grey cast iron
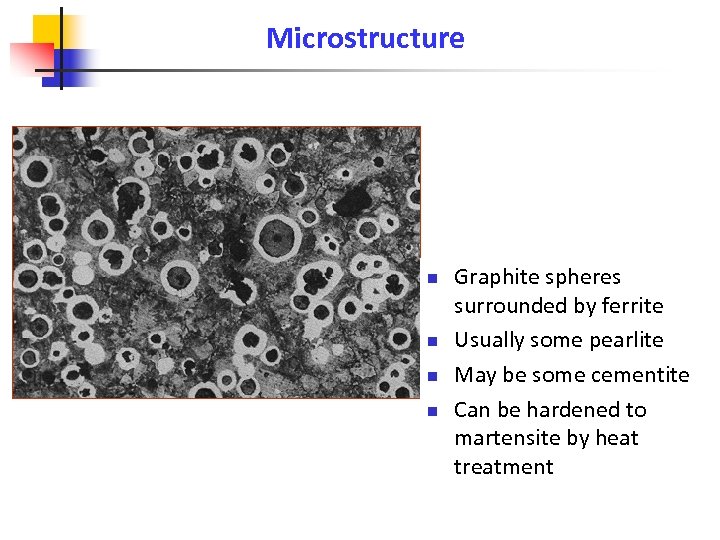 Microstructure n n Graphite spheres surrounded by ferrite Usually some pearlite May be some cementite Can be hardened to martensite by heat treatment
Microstructure n n Graphite spheres surrounded by ferrite Usually some pearlite May be some cementite Can be hardened to martensite by heat treatment
 Production n Composition similar to grey cast iron except for higher purity. n Melt is added to inoculant in ladle. n Magnesium as wire, ingots or pellets is added to ladle before adding hot iron. n Mg vapour rises through melt, removing sulphur.
Production n Composition similar to grey cast iron except for higher purity. n Melt is added to inoculant in ladle. n Magnesium as wire, ingots or pellets is added to ladle before adding hot iron. n Mg vapour rises through melt, removing sulphur.
 Properties n Strength higher than grey cast iron n Ductility up to 6% as cast or 20% annealed n Low cost n n Simple manufacturing process makes complex shapes Machinability better than steel
Properties n Strength higher than grey cast iron n Ductility up to 6% as cast or 20% annealed n Low cost n n Simple manufacturing process makes complex shapes Machinability better than steel
 Applications n Automotive industry 55% of ductile iron in USA n Crankshafts, front wheel spindle supports, steering knuckles, disc brake callipers n Pipe and pipe fittings (joined by welding) see AS 2280
Applications n Automotive industry 55% of ductile iron in USA n Crankshafts, front wheel spindle supports, steering knuckles, disc brake callipers n Pipe and pipe fittings (joined by welding) see AS 2280
 Malleable iron n Graphite in nodular form n Produced by heat treatment of white cast iron n Graphite nodules are irregular clusters n Similar properties to ductile iron. 31
Malleable iron n Graphite in nodular form n Produced by heat treatment of white cast iron n Graphite nodules are irregular clusters n Similar properties to ductile iron. 31
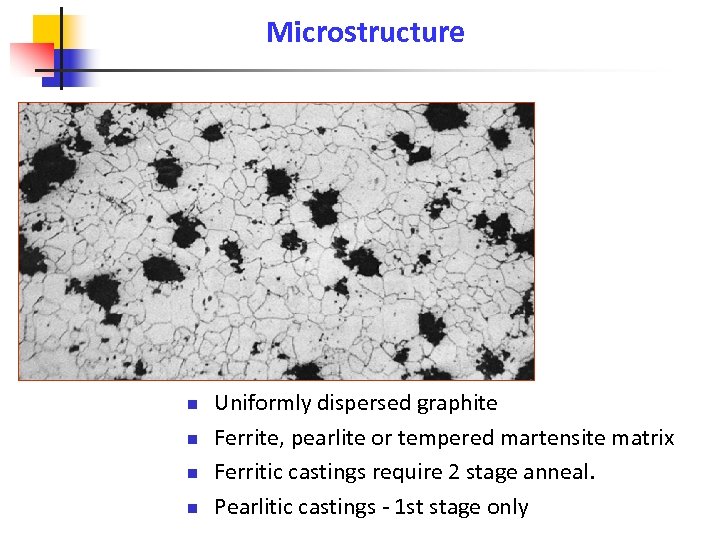 Microstructure n n Uniformly dispersed graphite Ferrite, pearlite or tempered martensite matrix Ferritic castings require 2 stage anneal. Pearlitic castings - 1 st stage only
Microstructure n n Uniformly dispersed graphite Ferrite, pearlite or tempered martensite matrix Ferritic castings require 2 stage anneal. Pearlitic castings - 1 st stage only
 Properties n n Similar to ductile iron Good shock resistance Good ductility Good machinability Applications n n n Similar applications to ductile iron Malleable iron is better for thinner castings Ductile iron better for thicker castings >40 mm Vehicle components n Power trains, frames, suspensions and wheels n Steering components, transmission and differential parts, connecting rods Railway components
Properties n n Similar to ductile iron Good shock resistance Good ductility Good machinability Applications n n n Similar applications to ductile iron Malleable iron is better for thinner castings Ductile iron better for thicker castings >40 mm Vehicle components n Power trains, frames, suspensions and wheels n Steering components, transmission and differential parts, connecting rods Railway components
 Alloy Cast Irons q Cr, Mn, Si, Ni, Al q the range of microstructures q Beneficial effect on many properties high temperature oxidation resistance corrosion resistance in acidic environments wear/abrasion resistance Graphite free Alloy Cast Irons Graphite bearing
Alloy Cast Irons q Cr, Mn, Si, Ni, Al q the range of microstructures q Beneficial effect on many properties high temperature oxidation resistance corrosion resistance in acidic environments wear/abrasion resistance Graphite free Alloy Cast Irons Graphite bearing
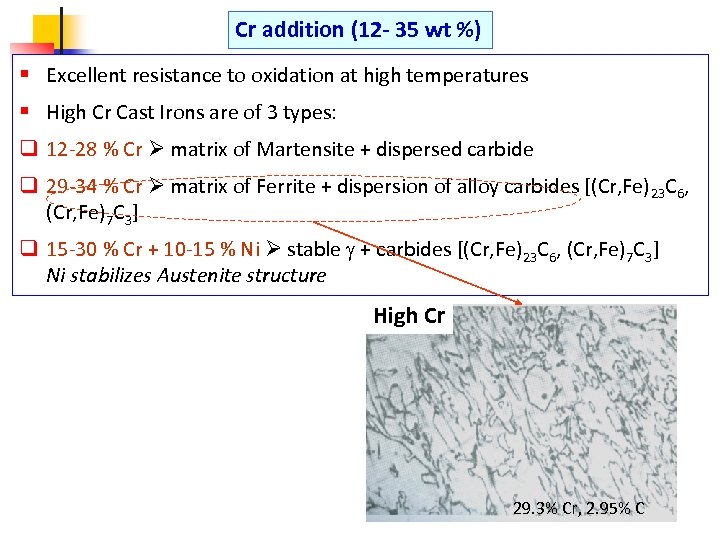 Cr addition (12 - 35 wt %) § Excellent resistance to oxidation at high temperatures § High Cr Cast Irons are of 3 types: q 12 -28 % Cr matrix of Martensite + dispersed carbide q 29 -34 % Cr matrix of Ferrite + dispersion of alloy carbides [(Cr, Fe)23 C 6, (Cr, Fe)7 C 3] q 15 -30 % Cr + 10 -15 % Ni stable + carbides [(Cr, Fe)23 C 6, (Cr, Fe)7 C 3] Ni stabilizes Austenite structure High Cr 29. 3% Cr, 2. 95% C
Cr addition (12 - 35 wt %) § Excellent resistance to oxidation at high temperatures § High Cr Cast Irons are of 3 types: q 12 -28 % Cr matrix of Martensite + dispersed carbide q 29 -34 % Cr matrix of Ferrite + dispersion of alloy carbides [(Cr, Fe)23 C 6, (Cr, Fe)7 C 3] q 15 -30 % Cr + 10 -15 % Ni stable + carbides [(Cr, Fe)23 C 6, (Cr, Fe)7 C 3] Ni stabilizes Austenite structure High Cr 29. 3% Cr, 2. 95% C
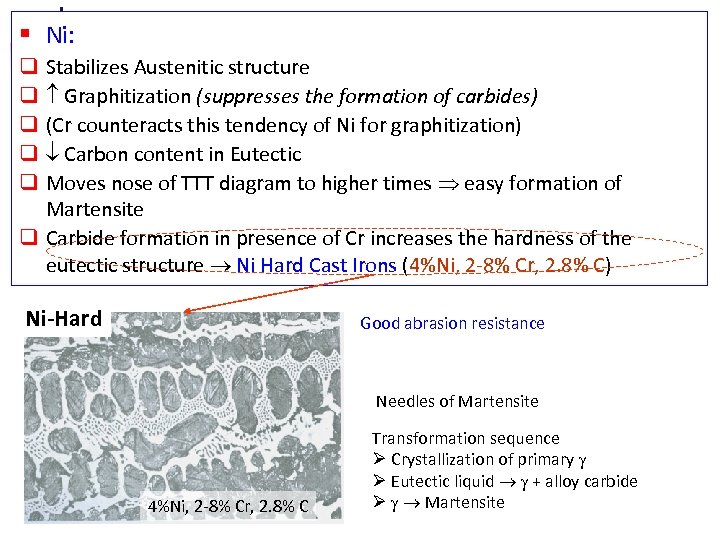 § Ni: Stabilizes Austenitic structure Graphitization (suppresses the formation of carbides) (Cr counteracts this tendency of Ni for graphitization) Carbon content in Eutectic Moves nose of TTT diagram to higher times easy formation of Martensite q Carbide formation in presence of Cr increases the hardness of the eutectic structure Ni Hard Cast Irons (4%Ni, 2 -8% Cr, 2. 8% C) q q q Ni-Hard Good abrasion resistance Needles of Martensite 4%Ni, 2 -8% Cr, 2. 8% C Transformation sequence Crystallization of primary Eutectic liquid + alloy carbide Martensite
§ Ni: Stabilizes Austenitic structure Graphitization (suppresses the formation of carbides) (Cr counteracts this tendency of Ni for graphitization) Carbon content in Eutectic Moves nose of TTT diagram to higher times easy formation of Martensite q Carbide formation in presence of Cr increases the hardness of the eutectic structure Ni Hard Cast Irons (4%Ni, 2 -8% Cr, 2. 8% C) q q q Ni-Hard Good abrasion resistance Needles of Martensite 4%Ni, 2 -8% Cr, 2. 8% C Transformation sequence Crystallization of primary Eutectic liquid + alloy carbide Martensite
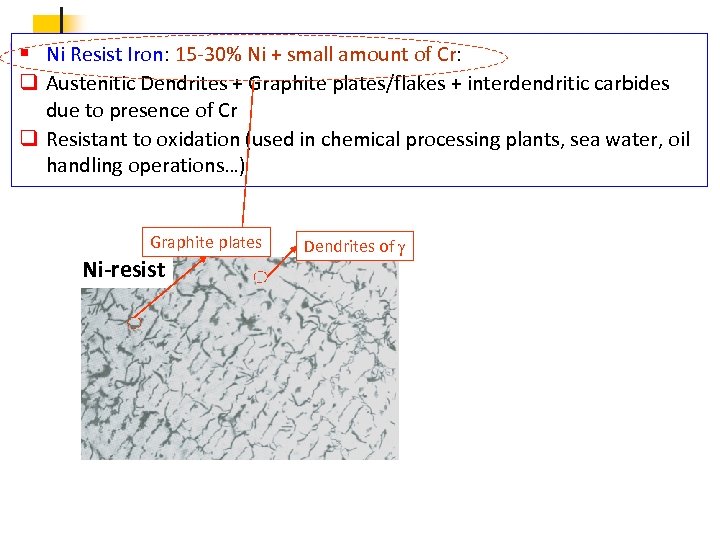 § Ni Resist Iron: 15 -30% Ni + small amount of Cr: q Austenitic Dendrites + Graphite plates/flakes + interdendritic carbides due to presence of Cr q Resistant to oxidation (used in chemical processing plants, sea water, oil handling operations…) Graphite plates Ni-resist Dendrites of
§ Ni Resist Iron: 15 -30% Ni + small amount of Cr: q Austenitic Dendrites + Graphite plates/flakes + interdendritic carbides due to presence of Cr q Resistant to oxidation (used in chemical processing plants, sea water, oil handling operations…) Graphite plates Ni-resist Dendrites of
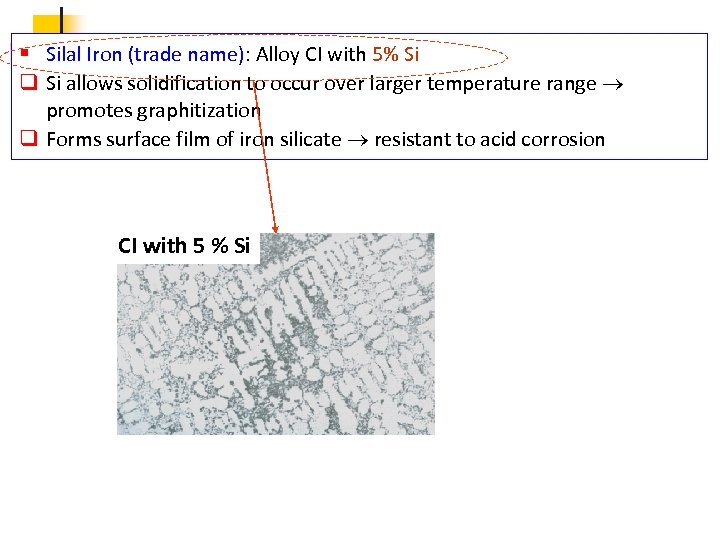 § Silal Iron (trade name): Alloy CI with 5% Si q Si allows solidification to occur over larger temperature range promotes graphitization q Forms surface film of iron silicate resistant to acid corrosion CI with 5 % Si
§ Silal Iron (trade name): Alloy CI with 5% Si q Si allows solidification to occur over larger temperature range promotes graphitization q Forms surface film of iron silicate resistant to acid corrosion CI with 5 % Si
 General Properties and Applications of Ferrous Alloys Ferrous alloys are useful metals in terms of mechanical, physical and chemical properties. Alloys contain iron as their base metal. Carbon steels are least expensive of all metals while stainless steels is costly.
General Properties and Applications of Ferrous Alloys Ferrous alloys are useful metals in terms of mechanical, physical and chemical properties. Alloys contain iron as their base metal. Carbon steels are least expensive of all metals while stainless steels is costly.
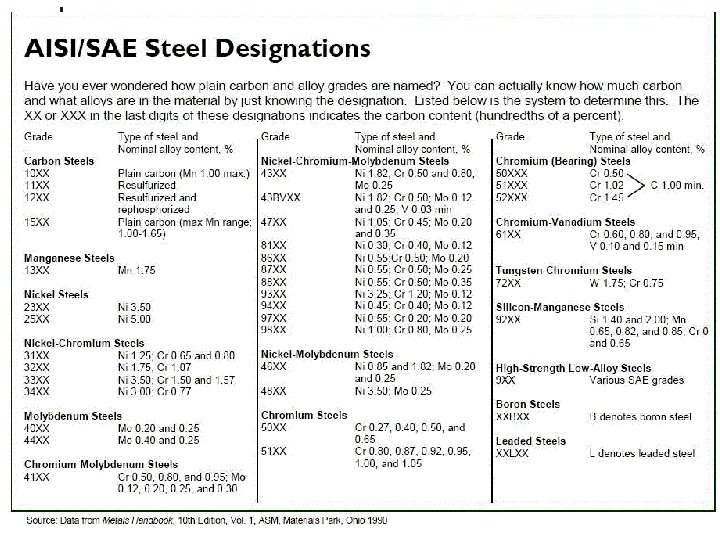
 Grades of Steel Over 3, 500 different grades of steel exist. Commercial steel is generally classified into four groups depending on their metal alloy content and end-use applications: 1. Carbon Steels (including low carbon, medium carbon and high carbon steels). 2. Alloy Steels (common alloy metals; manganese, silicon, nickel and chromium). 3. Stainless Steels (contain about 10% chromium and classified as austenitic, ferritic and martensitic). 4. Tool Steels (alloyed with high temperature and hard metals, such as molybdenum and tungsten). 40
Grades of Steel Over 3, 500 different grades of steel exist. Commercial steel is generally classified into four groups depending on their metal alloy content and end-use applications: 1. Carbon Steels (including low carbon, medium carbon and high carbon steels). 2. Alloy Steels (common alloy metals; manganese, silicon, nickel and chromium). 3. Stainless Steels (contain about 10% chromium and classified as austenitic, ferritic and martensitic). 4. Tool Steels (alloyed with high temperature and hard metals, such as molybdenum and tungsten). 40
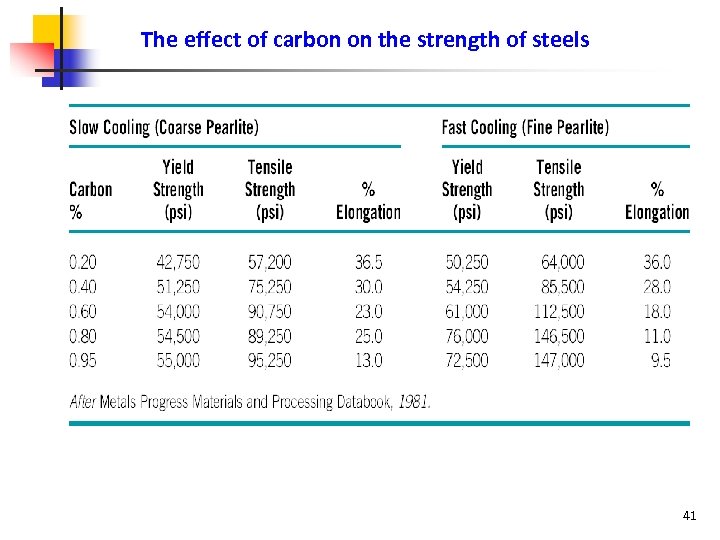 The effect of carbon on the strength of steels 41
The effect of carbon on the strength of steels 41
 Carbon and alloy steels Carbon steels • Classified as low, medium and high: 1. Low-carbon steel or mild steel, < 0. 3%C, bolts, nuts and sheet plates. 2. Medium-carbon steel, 0. 3% ~ 0. 6%C, machinery, automotive and agricultural equipment. 3. High-carbon steel, > 0. 60% C, springs, cutlery, cable.
Carbon and alloy steels Carbon steels • Classified as low, medium and high: 1. Low-carbon steel or mild steel, < 0. 3%C, bolts, nuts and sheet plates. 2. Medium-carbon steel, 0. 3% ~ 0. 6%C, machinery, automotive and agricultural equipment. 3. High-carbon steel, > 0. 60% C, springs, cutlery, cable.
 Carbon and alloy steels Alloy steels • Steels containing significant amounts of alloying elements. • Structural-grade alloy steels used for construction industries due to high strength. • Other alloy steels are used for its strength, hardness, resistance to creep and fatigue, and toughness. • It may heat treated to obtain the desired properties.
Carbon and alloy steels Alloy steels • Steels containing significant amounts of alloying elements. • Structural-grade alloy steels used for construction industries due to high strength. • Other alloy steels are used for its strength, hardness, resistance to creep and fatigue, and toughness. • It may heat treated to obtain the desired properties.
 Carbon and alloy steels High-strength low-alloy steels • Improved strength-to-weight ratio. • Used in automobile bodies to reduce weight and in agricultural equipment. • Some examples are: 1. Dual-phase steels 2. Micro alloyed steels 3. Nano-alloyed steels
Carbon and alloy steels High-strength low-alloy steels • Improved strength-to-weight ratio. • Used in automobile bodies to reduce weight and in agricultural equipment. • Some examples are: 1. Dual-phase steels 2. Micro alloyed steels 3. Nano-alloyed steels
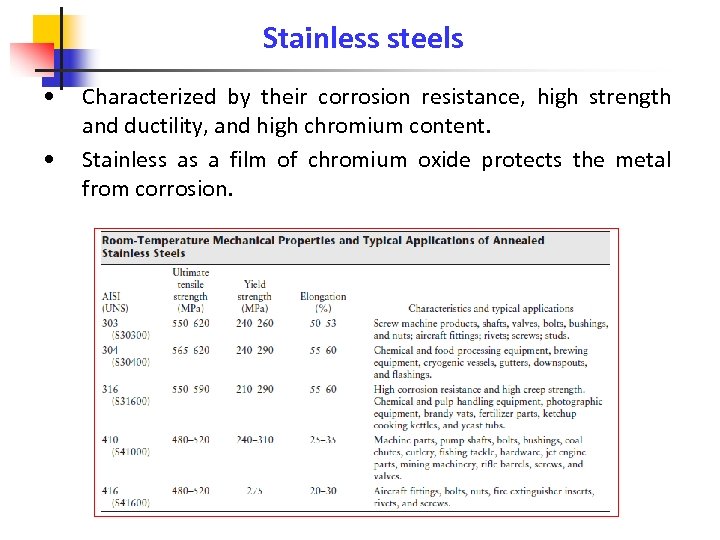 Stainless steels • • Characterized by their corrosion resistance, high strength and ductility, and high chromium content. Stainless as a film of chromium oxide protects the metal from corrosion.
Stainless steels • • Characterized by their corrosion resistance, high strength and ductility, and high chromium content. Stainless as a film of chromium oxide protects the metal from corrosion.
 Stainless steels • Five types of stainless steels: 1. Austenitic steels 2. Ferritic steels 3. Martensitic steels 4. Precipitation-hardening (PH) steels 5. Duplex-structure steels
Stainless steels • Five types of stainless steels: 1. Austenitic steels 2. Ferritic steels 3. Martensitic steels 4. Precipitation-hardening (PH) steels 5. Duplex-structure steels
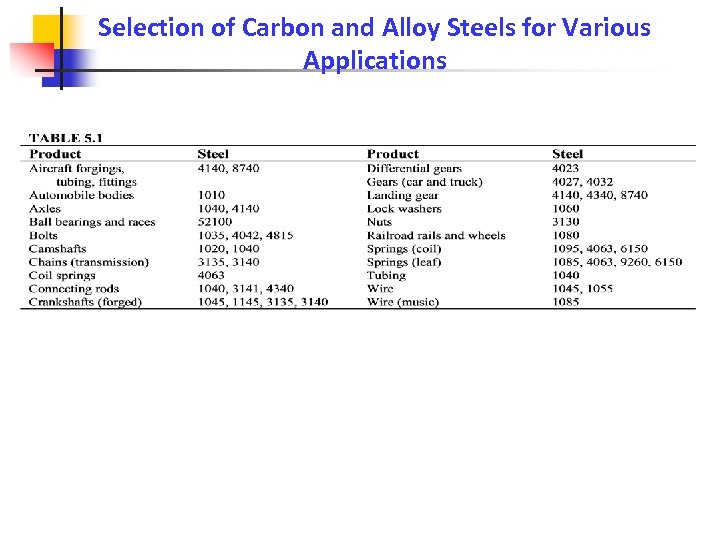 Selection of Carbon and Alloy Steels for Various Applications
Selection of Carbon and Alloy Steels for Various Applications
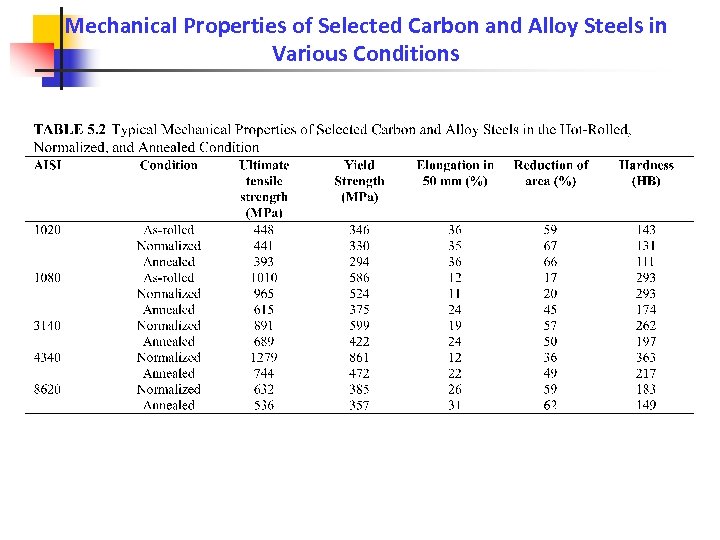 Mechanical Properties of Selected Carbon and Alloy Steels in Various Conditions
Mechanical Properties of Selected Carbon and Alloy Steels in Various Conditions
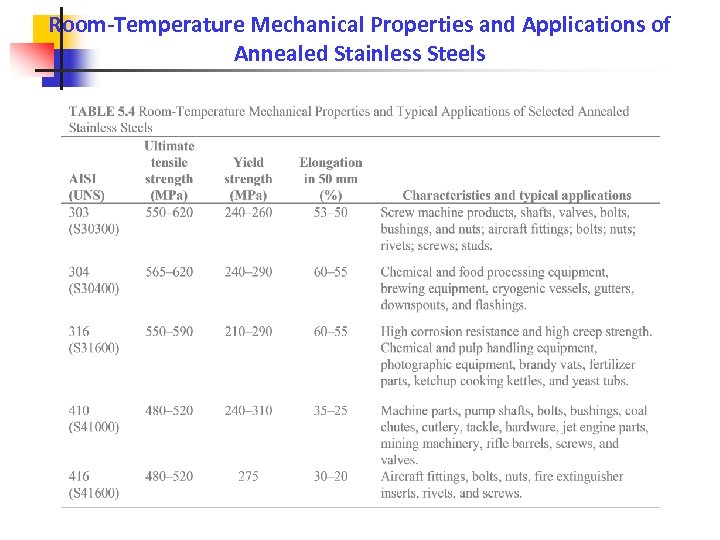 Room-Temperature Mechanical Properties and Applications of Annealed Stainless Steels
Room-Temperature Mechanical Properties and Applications of Annealed Stainless Steels
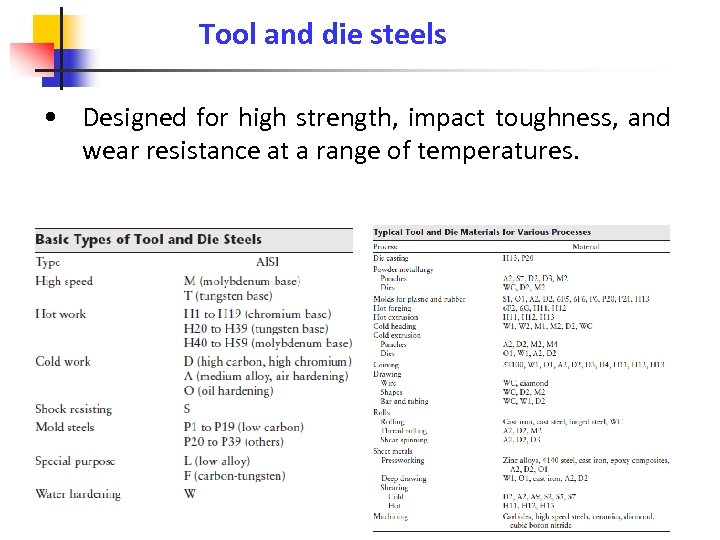 Tool and die steels • Designed for high strength, impact toughness, and wear resistance at a range of temperatures.
Tool and die steels • Designed for high strength, impact toughness, and wear resistance at a range of temperatures.
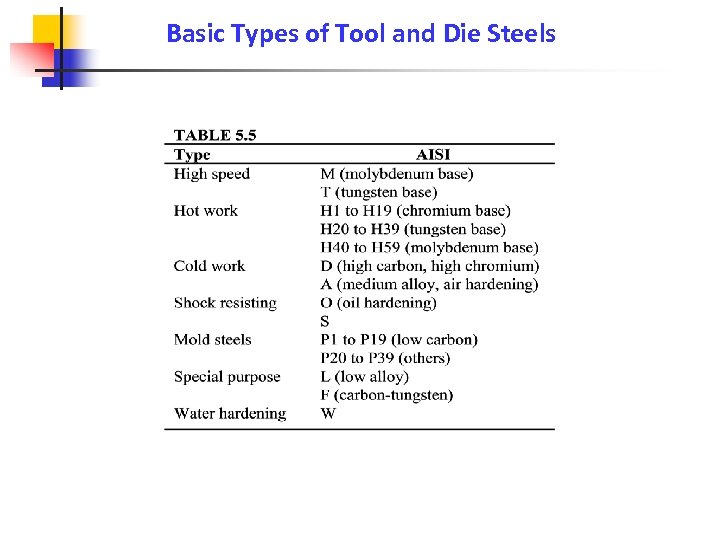 Basic Types of Tool and Die Steels
Basic Types of Tool and Die Steels
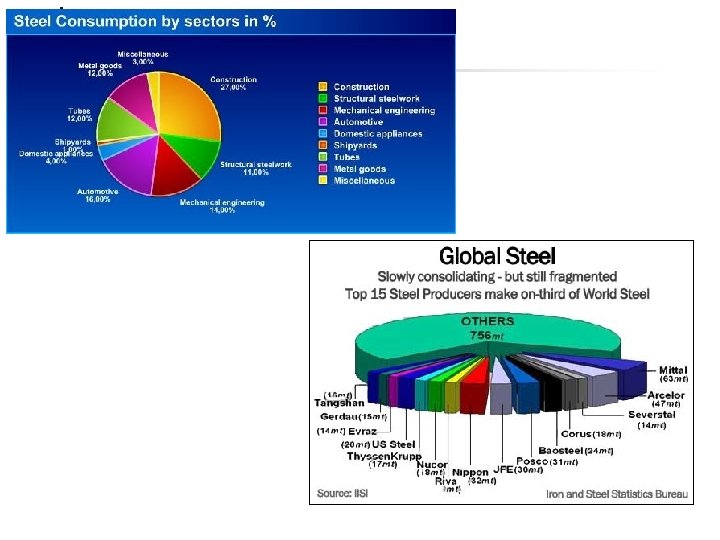
 Nonferrous Metals
Nonferrous Metals
 Nonferrous Metals In metallurgy, a non-ferrous metal is any metal, including alloys, that does not contain iron in appreciable amounts. Generally more expensive than ferrous metals, non-ferrous metals are used because of desirable properties such as low weight (e. g. , aluminium), higher conductivity (e. g. , copper), non-magnetic property or resistance to corrosion (e. g. , zinc). Important non-ferrous metals include aluminium, copper, lead, nickel, tin, titanium and zinc, and alloys such as brass. Precious metals such as gold, silver and platinum are also nonferrous.
Nonferrous Metals In metallurgy, a non-ferrous metal is any metal, including alloys, that does not contain iron in appreciable amounts. Generally more expensive than ferrous metals, non-ferrous metals are used because of desirable properties such as low weight (e. g. , aluminium), higher conductivity (e. g. , copper), non-magnetic property or resistance to corrosion (e. g. , zinc). Important non-ferrous metals include aluminium, copper, lead, nickel, tin, titanium and zinc, and alloys such as brass. Precious metals such as gold, silver and platinum are also nonferrous.
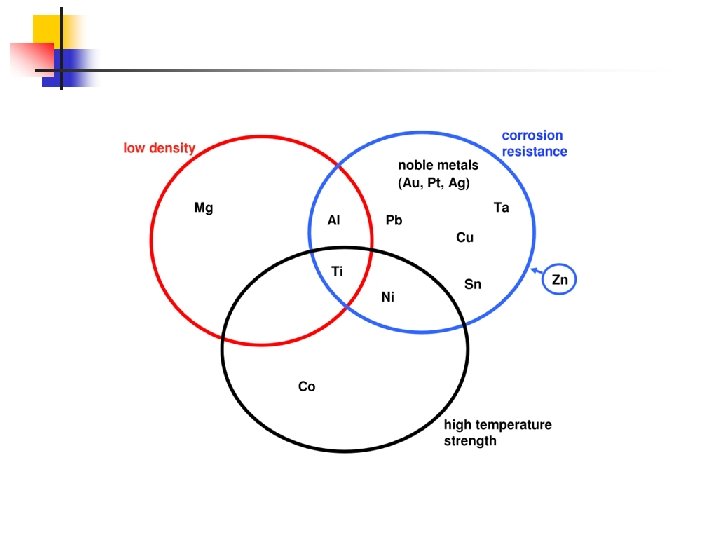
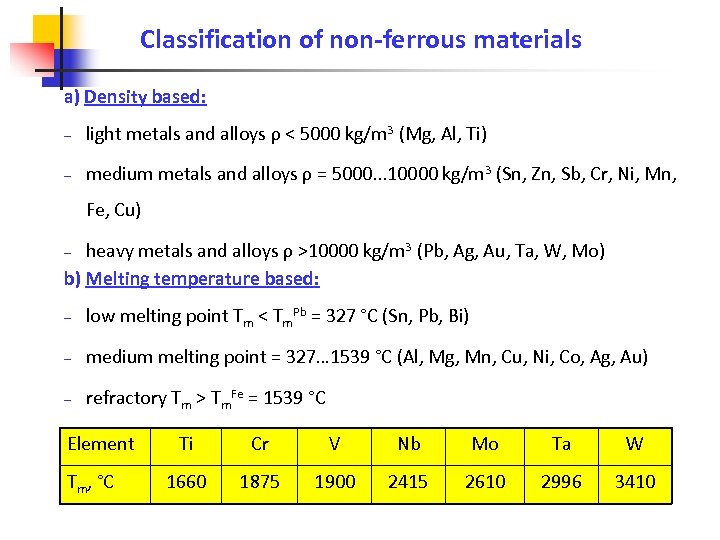 Classification of non-ferrous materials a) Density based: – light metals and alloys ρ < 5000 kg/m 3 (Mg, Al, Ti) – medium metals and alloys ρ = 5000. . . 10000 kg/m 3 (Sn, Zn, Sb, Cr, Ni, Mn, Fe, Cu) heavy metals and alloys ρ >10000 kg/m 3 (Pb, Ag, Au, Ta, W, Mo) b) Melting temperature based: – – low melting point Tm < Tm. Pb = 327 °C (Sn, Pb, Bi) – medium melting point = 327… 1539 °C (Al, Mg, Mn, Cu, Ni, Co, Ag, Au) – refractory Tm > Tm. Fe = 1539 °C Element Tm, °C Ti Cr V Nb Mo Ta W 1660 1875 1900 2415 2610 2996 3410
Classification of non-ferrous materials a) Density based: – light metals and alloys ρ < 5000 kg/m 3 (Mg, Al, Ti) – medium metals and alloys ρ = 5000. . . 10000 kg/m 3 (Sn, Zn, Sb, Cr, Ni, Mn, Fe, Cu) heavy metals and alloys ρ >10000 kg/m 3 (Pb, Ag, Au, Ta, W, Mo) b) Melting temperature based: – – low melting point Tm < Tm. Pb = 327 °C (Sn, Pb, Bi) – medium melting point = 327… 1539 °C (Al, Mg, Mn, Cu, Ni, Co, Ag, Au) – refractory Tm > Tm. Fe = 1539 °C Element Tm, °C Ti Cr V Nb Mo Ta W 1660 1875 1900 2415 2610 2996 3410
 Aluminium and aluminium alloys
Aluminium and aluminium alloys
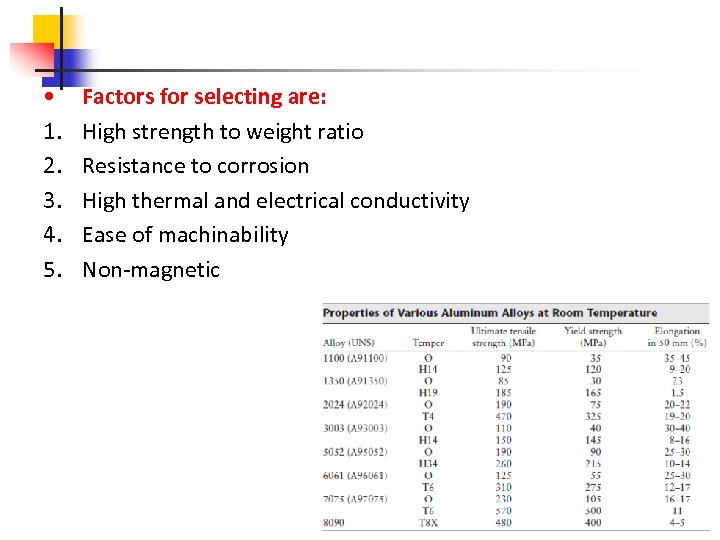 • 1. 2. 3. 4. 5. Factors for selecting are: High strength to weight ratio Resistance to corrosion High thermal and electrical conductivity Ease of machinability Non-magnetic
• 1. 2. 3. 4. 5. Factors for selecting are: High strength to weight ratio Resistance to corrosion High thermal and electrical conductivity Ease of machinability Non-magnetic
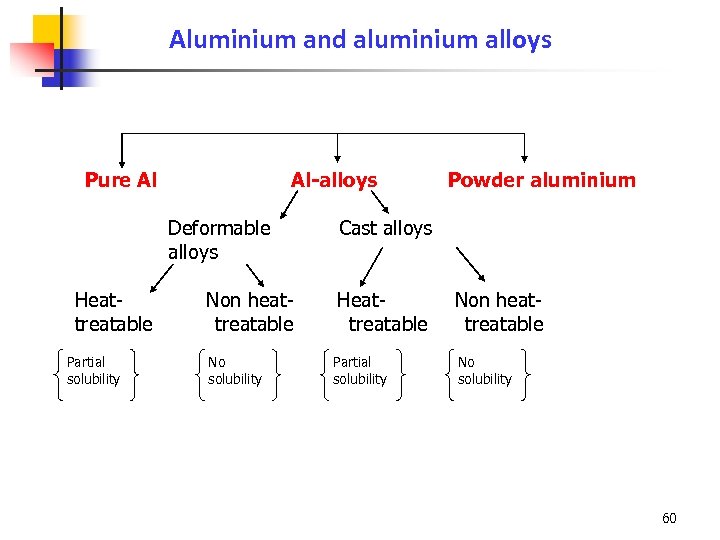 Aluminium and aluminium alloys Pure Al Al-alloys Deformable alloys Heattreatable Partial solubility Non heattreatable No solubility Powder aluminium Cast alloys Heattreatable Partial solubility Non heattreatable No solubility 60
Aluminium and aluminium alloys Pure Al Al-alloys Deformable alloys Heattreatable Partial solubility Non heattreatable No solubility Powder aluminium Cast alloys Heattreatable Partial solubility Non heattreatable No solubility 60
 Aluminium is the third most abundant element in the earth's crust, behind silicon and oxygen. It is the most abundant metal. It is strong, lightweight, electrically- and thermally-conductive, and corrosion resistant. It is often anodized to help prevent corrosion also its electrical conductivity make it an excellent choice for electrical applications such as wiring and conductors. Its strength-to-weight ratio makes it attractive in structural applications as well as cast aluminum engine components, e. g. blocks, heads, and manifolds. Its high reflectivity of infrared and visible radiation makes it desirable in headlights, light fixtures, and many insulations. It is also used as a paint pigment.
Aluminium is the third most abundant element in the earth's crust, behind silicon and oxygen. It is the most abundant metal. It is strong, lightweight, electrically- and thermally-conductive, and corrosion resistant. It is often anodized to help prevent corrosion also its electrical conductivity make it an excellent choice for electrical applications such as wiring and conductors. Its strength-to-weight ratio makes it attractive in structural applications as well as cast aluminum engine components, e. g. blocks, heads, and manifolds. Its high reflectivity of infrared and visible radiation makes it desirable in headlights, light fixtures, and many insulations. It is also used as a paint pigment.
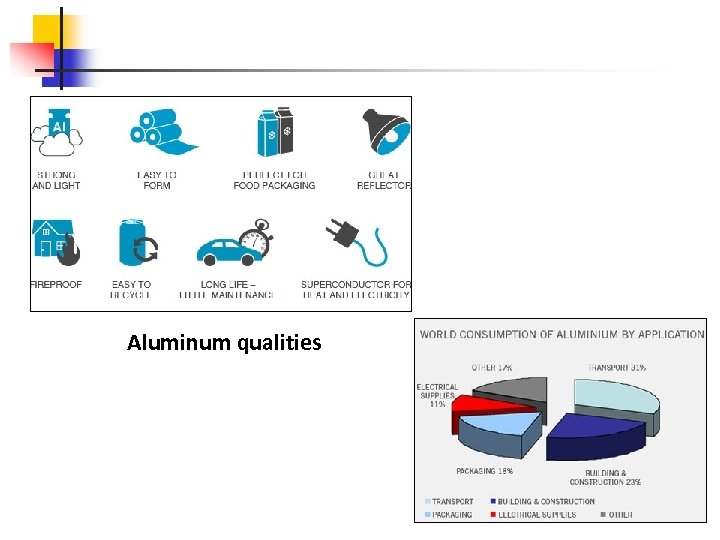 Aluminum qualities
Aluminum qualities
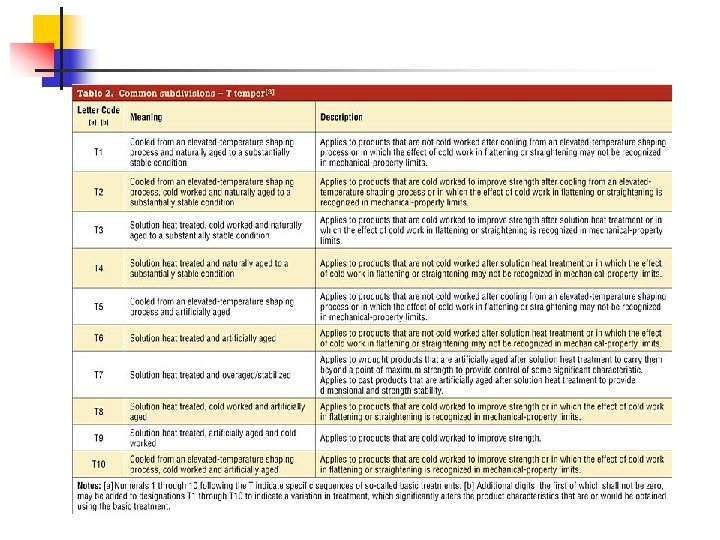
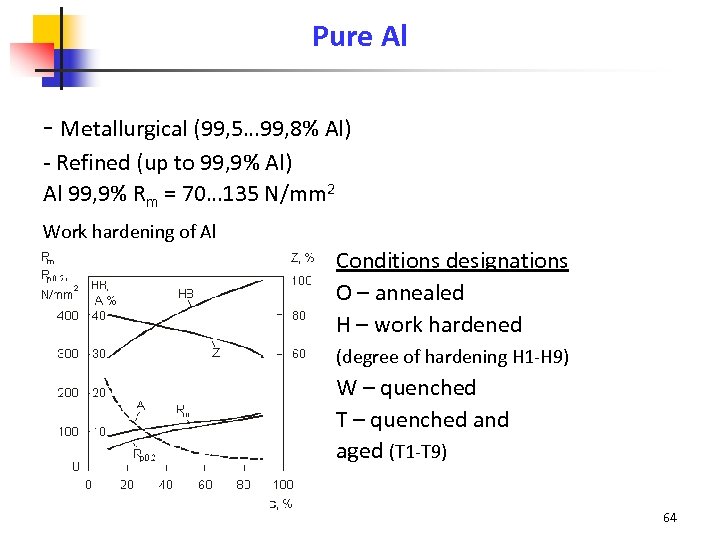 Pure Al - Metallurgical (99, 5… 99, 8% Al) - Refined (up to 99, 9% Al) Al 99, 9% Rm = 70… 135 N/mm 2 Work hardening of Al Conditions designations O – annealed H – work hardened (degree of hardening H 1 -H 9) W – quenched T – quenched and aged (T 1 -T 9) 64
Pure Al - Metallurgical (99, 5… 99, 8% Al) - Refined (up to 99, 9% Al) Al 99, 9% Rm = 70… 135 N/mm 2 Work hardening of Al Conditions designations O – annealed H – work hardened (degree of hardening H 1 -H 9) W – quenched T – quenched and aged (T 1 -T 9) 64
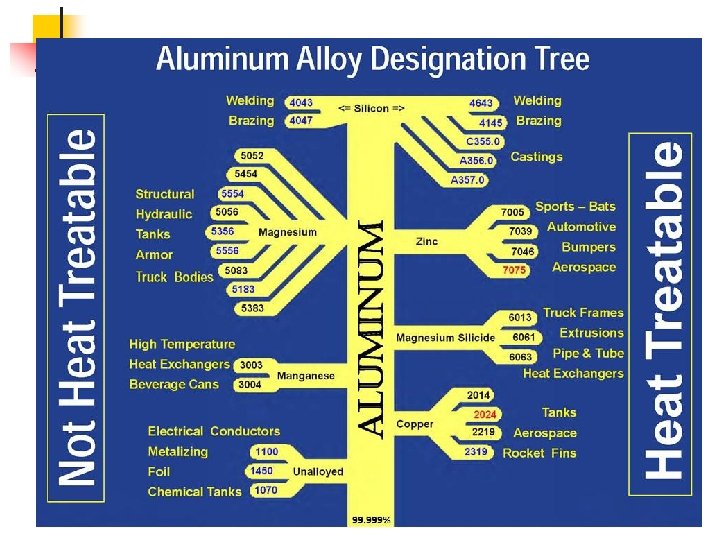
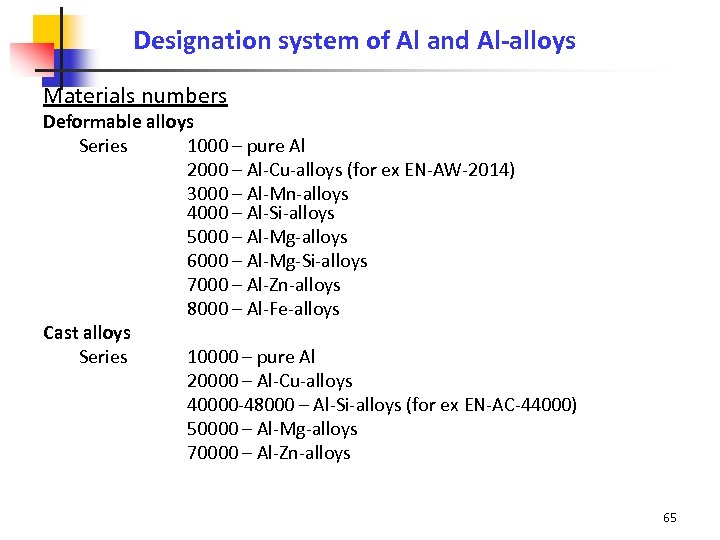 Designation system of Al and Al-alloys Materials numbers Deformable alloys Series 1000 – pure Al 2000 – Al-Cu-alloys (for ex EN-AW-2014) 3000 – Al-Mn-alloys 4000 – Al-Si-alloys 5000 – Al-Mg-alloys 6000 – Al-Mg-Si-alloys 7000 – Al-Zn-alloys 8000 – Al-Fe-alloys Cast alloys Series 10000 – pure Al 20000 – Al-Cu-alloys 40000 -48000 – Al-Si-alloys (for ex EN-AC-44000) 50000 – Al-Mg-alloys 70000 – Al-Zn-alloys 65
Designation system of Al and Al-alloys Materials numbers Deformable alloys Series 1000 – pure Al 2000 – Al-Cu-alloys (for ex EN-AW-2014) 3000 – Al-Mn-alloys 4000 – Al-Si-alloys 5000 – Al-Mg-alloys 6000 – Al-Mg-Si-alloys 7000 – Al-Zn-alloys 8000 – Al-Fe-alloys Cast alloys Series 10000 – pure Al 20000 – Al-Cu-alloys 40000 -48000 – Al-Si-alloys (for ex EN-AC-44000) 50000 – Al-Mg-alloys 70000 – Al-Zn-alloys 65
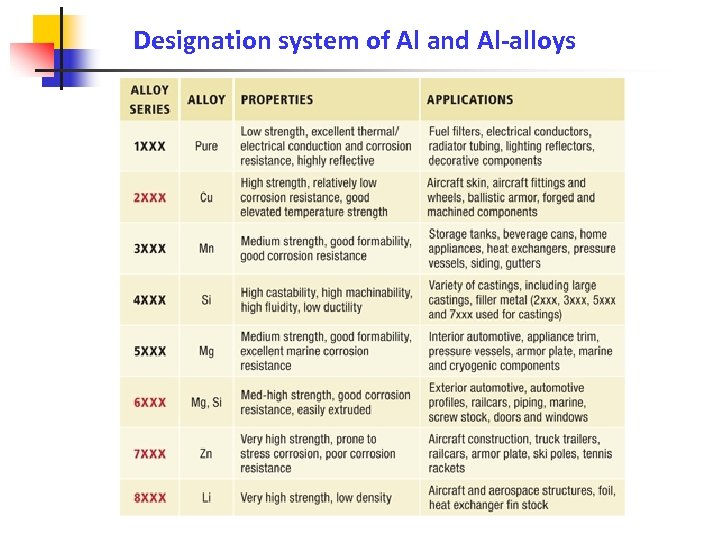 Designation system of Al and Al-alloys
Designation system of Al and Al-alloys
 Application of Al alloys n n n n Aluminum alloys are widely used for aeronautical applications because of high strength weight ratio. For automobiles for reducing weight of the vehicle thus reducing fuel consumption. For applications as electrical conductors including overhead transmission lines. House hold and consumer items such as utensils. Used as sacrificial anode. Marine applications. For surface transport such as fittings in railway coaches and buses. Aluminum is also used in making windows, doors and roofs of factories. Also in Sporting Equipments.
Application of Al alloys n n n n Aluminum alloys are widely used for aeronautical applications because of high strength weight ratio. For automobiles for reducing weight of the vehicle thus reducing fuel consumption. For applications as electrical conductors including overhead transmission lines. House hold and consumer items such as utensils. Used as sacrificial anode. Marine applications. For surface transport such as fittings in railway coaches and buses. Aluminum is also used in making windows, doors and roofs of factories. Also in Sporting Equipments.
 Common Applications of Aluminium • aluminium foils • beverage cans * Both are alloys of 92% to 99% aluminium
Common Applications of Aluminium • aluminium foils • beverage cans * Both are alloys of 92% to 99% aluminium
 Newer Applications of Aluminium • • drive shafts Radiators cylinder heads suspension systems *proven to be most advantageous when dealing with weight versus strength versus cost considerations.
Newer Applications of Aluminium • • drive shafts Radiators cylinder heads suspension systems *proven to be most advantageous when dealing with weight versus strength versus cost considerations.
 Some of the major uses of aluminium and its alloys Packaging (drinking cans and foil) Building and construction (siding and roofing, doors and windows, building wiring) Transportation (sheet, tube, castings, bodies, firm, and mechanical parts of cars, boats and planes) Electrical applications (overhead transmission lines, cable sheathing and wiring)
Some of the major uses of aluminium and its alloys Packaging (drinking cans and foil) Building and construction (siding and roofing, doors and windows, building wiring) Transportation (sheet, tube, castings, bodies, firm, and mechanical parts of cars, boats and planes) Electrical applications (overhead transmission lines, cable sheathing and wiring)
 Some of the major uses of aluminium and its alloys Household items (cooking utensils) musical instruments (guitars) sports equipment (baseball bats) Paint and pyrotechnics Currency (coins)
Some of the major uses of aluminium and its alloys Household items (cooking utensils) musical instruments (guitars) sports equipment (baseball bats) Paint and pyrotechnics Currency (coins)
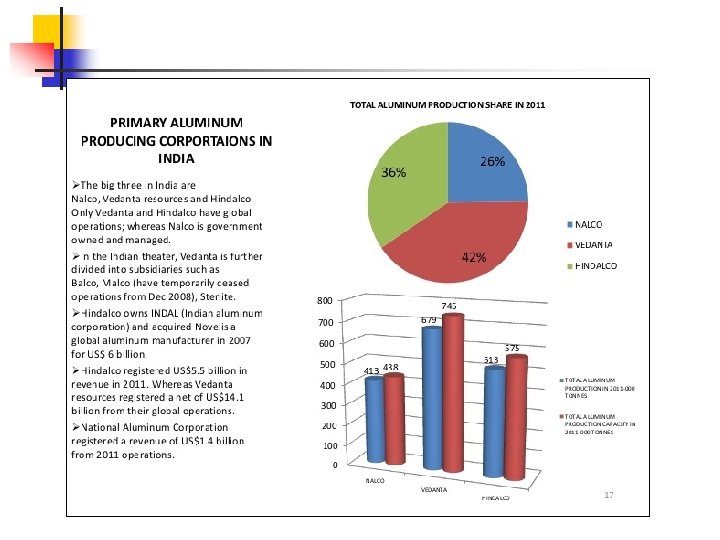
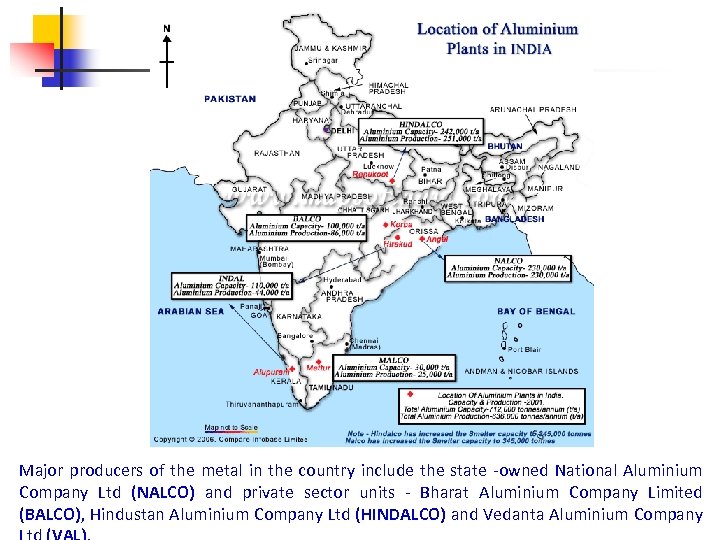 Major producers of the metal in the country include the state -owned National Aluminium Company Ltd (NALCO) and private sector units - Bharat Aluminium Company Limited (BALCO), Hindustan Aluminium Company Ltd (HINDALCO) and Vedanta Aluminium Company
Major producers of the metal in the country include the state -owned National Aluminium Company Ltd (NALCO) and private sector units - Bharat Aluminium Company Limited (BALCO), Hindustan Aluminium Company Ltd (HINDALCO) and Vedanta Aluminium Company
 Copper and copper alloys
Copper and copper alloys
 • Copper alloys have electrical and mechanical properties, corrosion resistance, thermal conductivity and wear resistance. • Applications are electronic components, springs and heat exchangers. • Brass is an alloy of copper and zinc. • Bronze is an alloy of copper and tin.
• Copper alloys have electrical and mechanical properties, corrosion resistance, thermal conductivity and wear resistance. • Applications are electronic components, springs and heat exchangers. • Brass is an alloy of copper and zinc. • Bronze is an alloy of copper and tin.
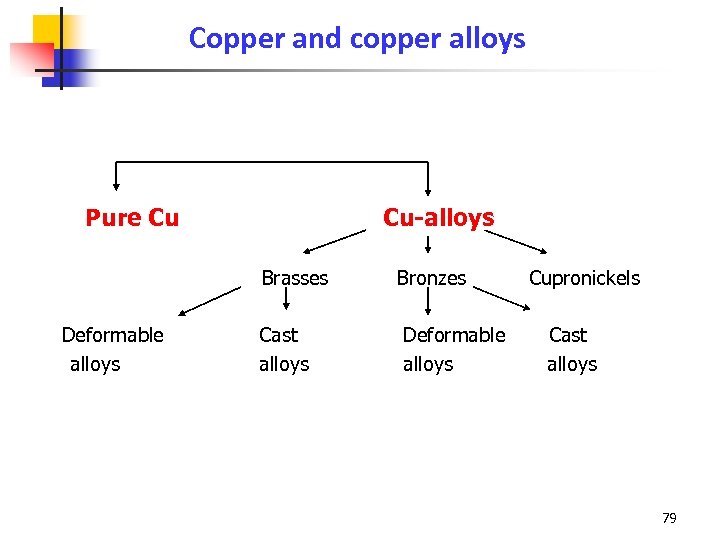 Copper and copper alloys Pure Cu Cu-alloys Brasses Deformable alloys Cast alloys Bronzes Deformable alloys Cupronickels Cast alloys 79
Copper and copper alloys Pure Cu Cu-alloys Brasses Deformable alloys Cast alloys Bronzes Deformable alloys Cupronickels Cast alloys 79
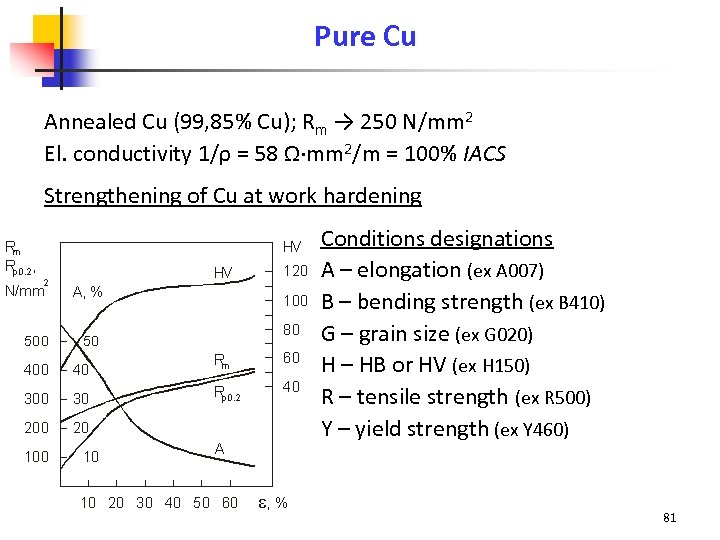 Pure Cu Annealed Cu (99, 85% Cu); Rm → 250 N/mm 2 El. conductivity 1/ρ = 58 Ω·mm 2/m = 100% IACS Strengthening of Cu at work hardening Conditions designations A – elongation (ex A 007) B – bending strength (ex B 410) G – grain size (ex G 020) H – HB or HV (ex H 150) R – tensile strength (ex R 500) Y – yield strength (ex Y 460) 81
Pure Cu Annealed Cu (99, 85% Cu); Rm → 250 N/mm 2 El. conductivity 1/ρ = 58 Ω·mm 2/m = 100% IACS Strengthening of Cu at work hardening Conditions designations A – elongation (ex A 007) B – bending strength (ex B 410) G – grain size (ex G 020) H – HB or HV (ex H 150) R – tensile strength (ex R 500) Y – yield strength (ex Y 460) 81
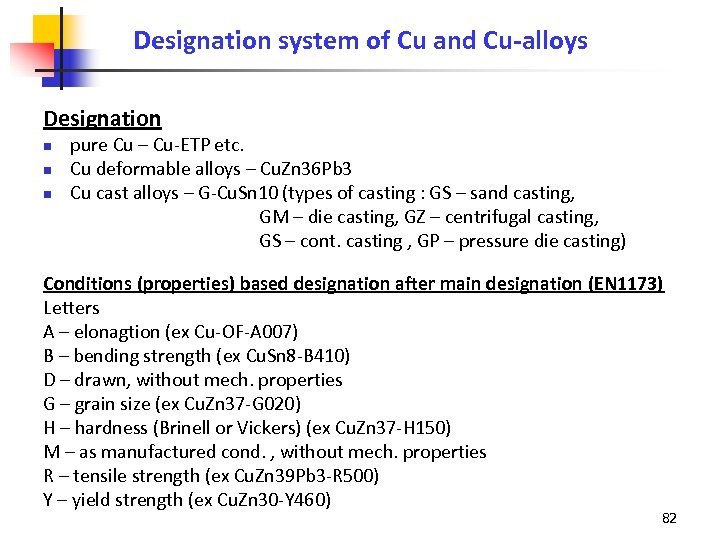 Designation system of Cu and Cu-alloys Designation n pure Cu – Cu-ETP etc. Cu deformable alloys – Cu. Zn 36 Pb 3 Cu cast alloys – G-Cu. Sn 10 (types of casting : GS – sand casting, GM – die casting, GZ – centrifugal casting, GS – cont. casting , GP – pressure die casting) Conditions (properties) based designation after main designation (EN 1173) Letters A – elonagtion (ex Cu-OF-A 007) B – bending strength (ex Cu. Sn 8 -B 410) D – drawn, without mech. properties G – grain size (ex Cu. Zn 37 -G 020) H – hardness (Brinell or Vickers) (ex Cu. Zn 37 -H 150) M – as manufactured cond. , without mech. properties R – tensile strength (ex Cu. Zn 39 Pb 3 -R 500) Y – yield strength (ex Cu. Zn 30 -Y 460) 82
Designation system of Cu and Cu-alloys Designation n pure Cu – Cu-ETP etc. Cu deformable alloys – Cu. Zn 36 Pb 3 Cu cast alloys – G-Cu. Sn 10 (types of casting : GS – sand casting, GM – die casting, GZ – centrifugal casting, GS – cont. casting , GP – pressure die casting) Conditions (properties) based designation after main designation (EN 1173) Letters A – elonagtion (ex Cu-OF-A 007) B – bending strength (ex Cu. Sn 8 -B 410) D – drawn, without mech. properties G – grain size (ex Cu. Zn 37 -G 020) H – hardness (Brinell or Vickers) (ex Cu. Zn 37 -H 150) M – as manufactured cond. , without mech. properties R – tensile strength (ex Cu. Zn 39 Pb 3 -R 500) Y – yield strength (ex Cu. Zn 30 -Y 460) 82
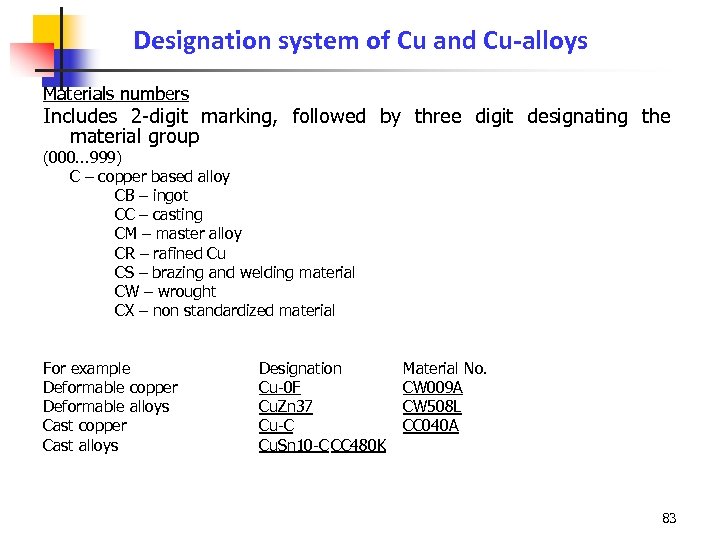 Designation system of Cu and Cu-alloys Materials numbers Includes 2 -digit marking, followed by three digit designating the material group (000. . . 999) C – copper based alloy CB – ingot CC – casting CM – master alloy CR – rafined Cu CS – brazing and welding material CW – wrought CX – non standardized material For example Deformable copper Deformable alloys Cast copper Cast alloys Designation Cu-0 F Cu. Zn 37 Cu-C Cu. Sn 10 -C CC 480 K Material No. CW 009 A CW 508 L CC 040 A 83
Designation system of Cu and Cu-alloys Materials numbers Includes 2 -digit marking, followed by three digit designating the material group (000. . . 999) C – copper based alloy CB – ingot CC – casting CM – master alloy CR – rafined Cu CS – brazing and welding material CW – wrought CX – non standardized material For example Deformable copper Deformable alloys Cast copper Cast alloys Designation Cu-0 F Cu. Zn 37 Cu-C Cu. Sn 10 -C CC 480 K Material No. CW 009 A CW 508 L CC 040 A 83
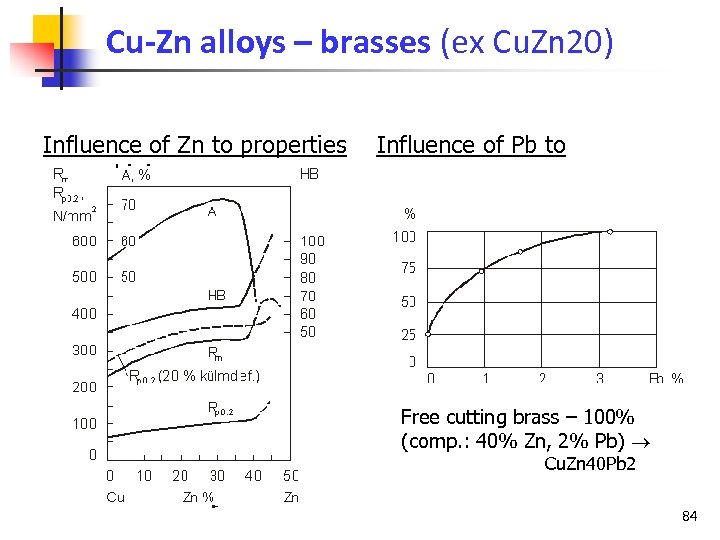 Cu-Zn alloys – brasses (ex Cu. Zn 20) Influence of Zn to properties machining Influence of Pb to Free cutting brass – 100% (comp. : 40% Zn, 2% Pb) Cu. Zn 40 Pb 2 84
Cu-Zn alloys – brasses (ex Cu. Zn 20) Influence of Zn to properties machining Influence of Pb to Free cutting brass – 100% (comp. : 40% Zn, 2% Pb) Cu. Zn 40 Pb 2 84
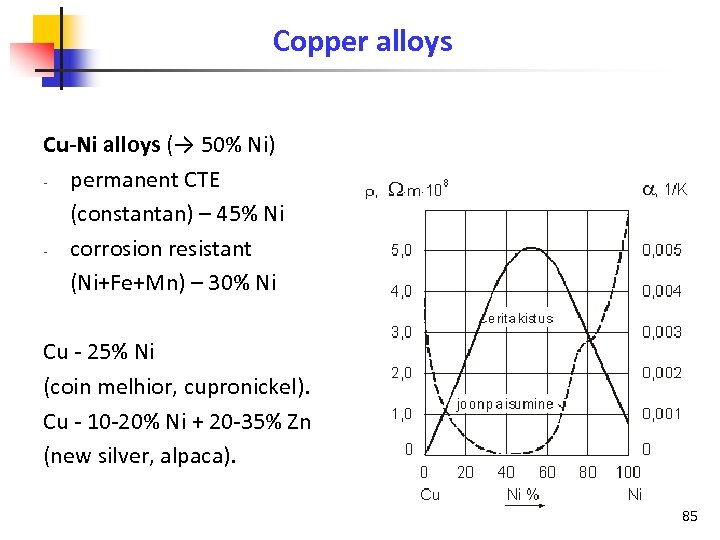 Copper alloys Cu-Ni alloys (→ 50% Ni) permanent CTE (constantan) – 45% Ni corrosion resistant (Ni+Fe+Mn) – 30% Ni Cu - 25% Ni (coin melhior, cupronickel). Cu - 10 -20% Ni + 20 -35% Zn (new silver, alpaca). 85
Copper alloys Cu-Ni alloys (→ 50% Ni) permanent CTE (constantan) – 45% Ni corrosion resistant (Ni+Fe+Mn) – 30% Ni Cu - 25% Ni (coin melhior, cupronickel). Cu - 10 -20% Ni + 20 -35% Zn (new silver, alpaca). 85
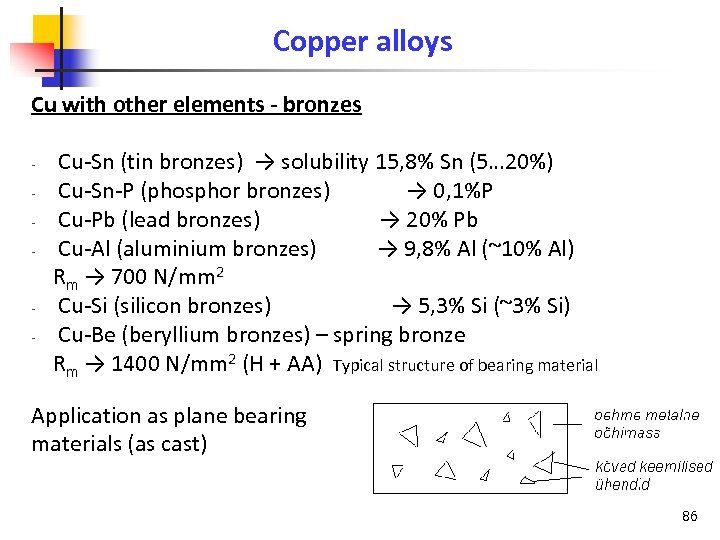 Copper alloys Cu with other elements - bronzes Cu-Sn (tin bronzes) → solubility 15, 8% Sn (5… 20%) Cu-Sn-P (phosphor bronzes) → 0, 1%P Cu-Pb (lead bronzes) → 20% Pb Cu-Al (aluminium bronzes) → 9, 8% Al (~10% Al) Rm → 700 N/mm 2 Cu-Si (silicon bronzes) → 5, 3% Si (~3% Si) Cu-Be (beryllium bronzes) – spring bronze Rm → 1400 N/mm 2 (H + AA) Typical structure of bearing material Application as plane bearing materials (as cast) 86
Copper alloys Cu with other elements - bronzes Cu-Sn (tin bronzes) → solubility 15, 8% Sn (5… 20%) Cu-Sn-P (phosphor bronzes) → 0, 1%P Cu-Pb (lead bronzes) → 20% Pb Cu-Al (aluminium bronzes) → 9, 8% Al (~10% Al) Rm → 700 N/mm 2 Cu-Si (silicon bronzes) → 5, 3% Si (~3% Si) Cu-Be (beryllium bronzes) – spring bronze Rm → 1400 N/mm 2 (H + AA) Typical structure of bearing material Application as plane bearing materials (as cast) 86
 Magnesium and magnesium alloys
Magnesium and magnesium alloys
 • • Magnesium (Mg) is the lightest metal. Alloys are used in structural and non-structural applications. Typical uses of magnesium alloys are aircraft and missile components. Also has good vibration-damping characteristics.
• • Magnesium (Mg) is the lightest metal. Alloys are used in structural and non-structural applications. Typical uses of magnesium alloys are aircraft and missile components. Also has good vibration-damping characteristics.
 Why ? Lowest density structural alloys 1. 738 g per cm 3 High specific strength Superior damping properties Excellent Castability Highly recyclable Good Machinability Good Weldability under inert atmosphere
Why ? Lowest density structural alloys 1. 738 g per cm 3 High specific strength Superior damping properties Excellent Castability Highly recyclable Good Machinability Good Weldability under inert atmosphere
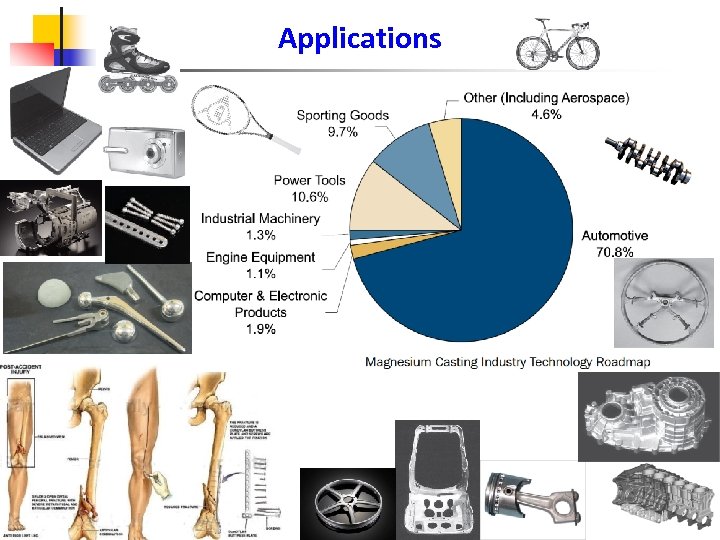 Applications
Applications
 Chevrolet Corvette C 5 Z 06 single-piece die-cast magnesium engine cradle by high temperature Mg alloy AE 44 : 33% (10. 85 Kg) weight savings The Mg gearbox and clutch housing unit is used with VW engines Volkswagen Golf Mg shifter support bracket beneath the shifter that has twice the stiffness of its welded steel counterpart. Acura ZDX Mg third-row seat framing optimizes strength and functionality without adding weight. Ford explorer Mg instrument panel has improved durability and lighter weight, connecting to navigation, safety and audio systems. Ford Explorer Jeep Grand Cherokee Improvements in high-pressure die casting, Mg extrusion and twin roll cast Mg sheet technology are underway to offer for structural automotive components The global voice of the magnesium industry © 2011, International Magnesium Association
Chevrolet Corvette C 5 Z 06 single-piece die-cast magnesium engine cradle by high temperature Mg alloy AE 44 : 33% (10. 85 Kg) weight savings The Mg gearbox and clutch housing unit is used with VW engines Volkswagen Golf Mg shifter support bracket beneath the shifter that has twice the stiffness of its welded steel counterpart. Acura ZDX Mg third-row seat framing optimizes strength and functionality without adding weight. Ford explorer Mg instrument panel has improved durability and lighter weight, connecting to navigation, safety and audio systems. Ford Explorer Jeep Grand Cherokee Improvements in high-pressure die casting, Mg extrusion and twin roll cast Mg sheet technology are underway to offer for structural automotive components The global voice of the magnesium industry © 2011, International Magnesium Association
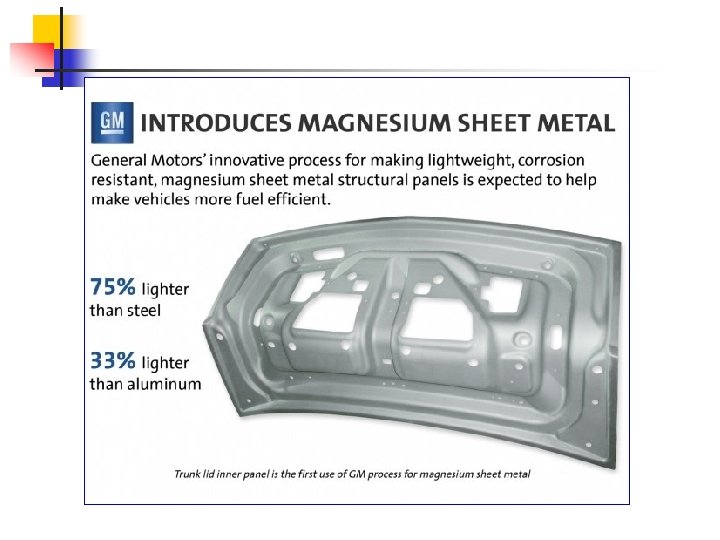
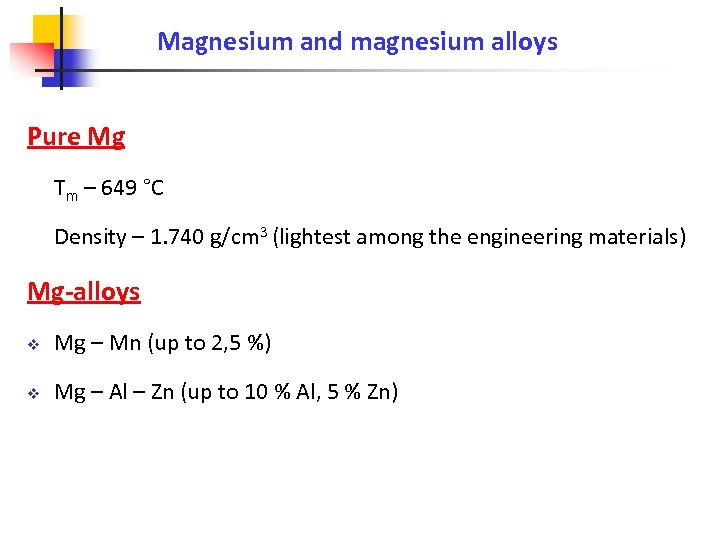 Magnesium and magnesium alloys Pure Mg Tm – 649 °C Density – 1. 740 g/cm 3 (lightest among the engineering materials) Mg-alloys v Mg – Mn (up to 2, 5 %) v Mg – Al – Zn (up to 10 % Al, 5 % Zn)
Magnesium and magnesium alloys Pure Mg Tm – 649 °C Density – 1. 740 g/cm 3 (lightest among the engineering materials) Mg-alloys v Mg – Mn (up to 2, 5 %) v Mg – Al – Zn (up to 10 % Al, 5 % Zn)
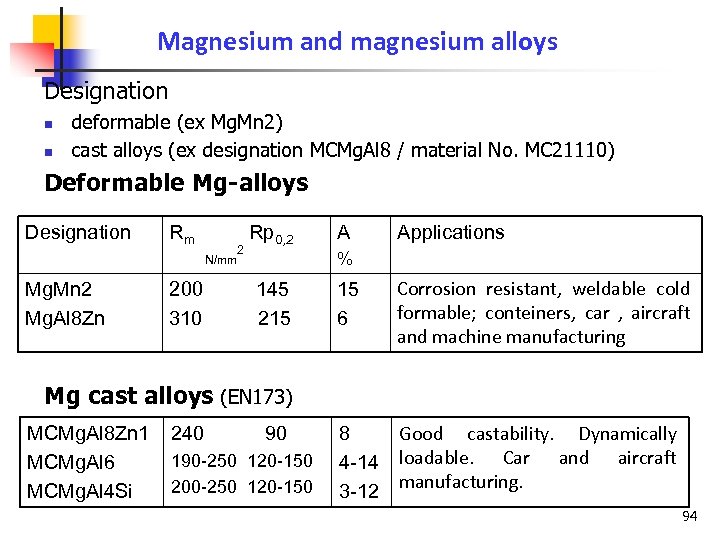 Magnesium and magnesium alloys Designation n n deformable (ex Mg. Mn 2) cast alloys (ex designation MCMg. Al 8 / material No. MC 21110) Deformable Mg-alloys Designation Mg. Mn 2 Mg. Al 8 Zn Rm 200 310 2 N/mm Rp 0, 2 A Applications % 145 215 15 6 Corrosion resistant, weldable cold formable; conteiners, car , aircraft and machine manufacturing Mg cast alloys (EN 173) MCMg. Al 8 Zn 1 MCMg. Al 6 MCMg. Al 4 Si 240 90 190 -250 120 -150 200 -250 120 -150 Good castability. Dynamically 8 4 -14 loadable. Car and aircraft manufacturing. 3 -12 94
Magnesium and magnesium alloys Designation n n deformable (ex Mg. Mn 2) cast alloys (ex designation MCMg. Al 8 / material No. MC 21110) Deformable Mg-alloys Designation Mg. Mn 2 Mg. Al 8 Zn Rm 200 310 2 N/mm Rp 0, 2 A Applications % 145 215 15 6 Corrosion resistant, weldable cold formable; conteiners, car , aircraft and machine manufacturing Mg cast alloys (EN 173) MCMg. Al 8 Zn 1 MCMg. Al 6 MCMg. Al 4 Si 240 90 190 -250 120 -150 200 -250 120 -150 Good castability. Dynamically 8 4 -14 loadable. Car and aircraft manufacturing. 3 -12 94
 Titanium and titanium alloys
Titanium and titanium alloys
 Pure Ti Tm – 1660 °C Density – 4. 540 g/cm 3 Very active to O, C, N → 2 x hardnes increase Ti-alloys, classification Ti – Al – alloys (4… 6 % Al) – -alloys Ti – Al – Cr, V, Cu, Mo - alloys – + -alloys Ti – Al – Mo, Cr, Zr - alloys – -alloys 96
Pure Ti Tm – 1660 °C Density – 4. 540 g/cm 3 Very active to O, C, N → 2 x hardnes increase Ti-alloys, classification Ti – Al – alloys (4… 6 % Al) – -alloys Ti – Al – Cr, V, Cu, Mo - alloys – + -alloys Ti – Al – Mo, Cr, Zr - alloys – -alloys 96
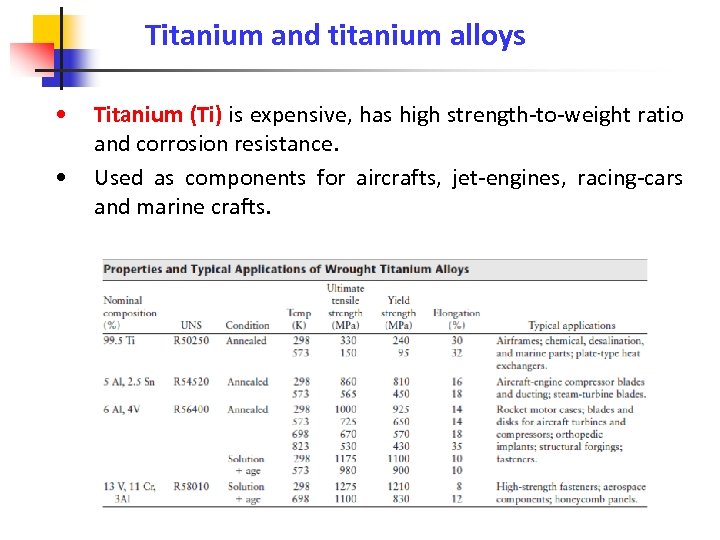 Titanium and titanium alloys • • Titanium (Ti) is expensive, has high strength-to-weight ratio and corrosion resistance. Used as components for aircrafts, jet-engines, racing-cars and marine crafts.
Titanium and titanium alloys • • Titanium (Ti) is expensive, has high strength-to-weight ratio and corrosion resistance. Used as components for aircrafts, jet-engines, racing-cars and marine crafts.
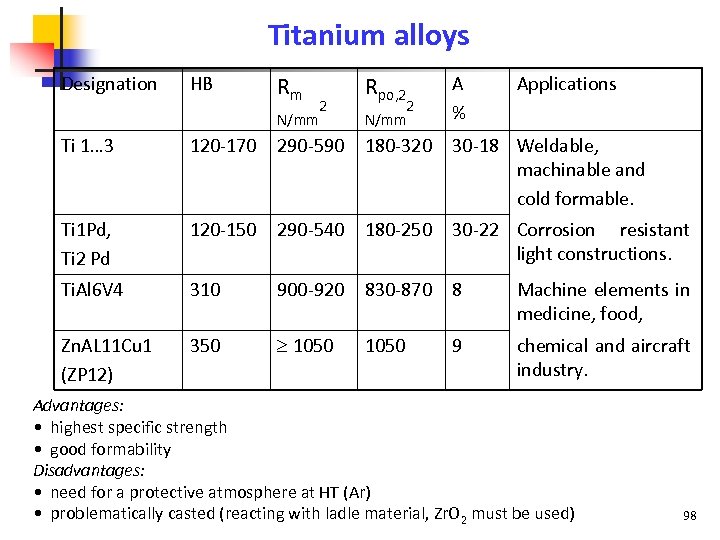 Titanium alloys Designation HB Rm N/mm 2 Rpo, 2 A Applications 2 % N/mm Ti 1… 3 120 -170 290 -590 180 -320 30 -18 Weldable, machinable and cold formable. Ti 1 Pd, Ti 2 Pd 120 -150 290 -540 180 -250 30 -22 Corrosion resistant light constructions. Ti. Al 6 V 4 310 900 -920 830 -870 8 Machine elements in medicine, food, Zn. AL 11 Cu 1 (ZP 12) 350 1050 9 chemical and aircraft industry. Advantages: • highest specific strength • good formability Disadvantages: • need for a protective atmosphere at HT (Ar) • problematically casted (reacting with ladle material, Zr. O 2 must be used) 98
Titanium alloys Designation HB Rm N/mm 2 Rpo, 2 A Applications 2 % N/mm Ti 1… 3 120 -170 290 -590 180 -320 30 -18 Weldable, machinable and cold formable. Ti 1 Pd, Ti 2 Pd 120 -150 290 -540 180 -250 30 -22 Corrosion resistant light constructions. Ti. Al 6 V 4 310 900 -920 830 -870 8 Machine elements in medicine, food, Zn. AL 11 Cu 1 (ZP 12) 350 1050 9 chemical and aircraft industry. Advantages: • highest specific strength • good formability Disadvantages: • need for a protective atmosphere at HT (Ar) • problematically casted (reacting with ladle material, Zr. O 2 must be used) 98
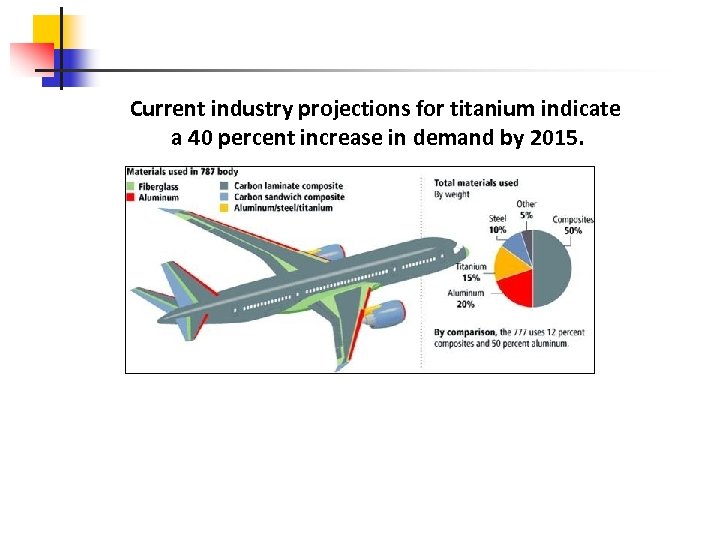 Current industry projections for titanium indicate a 40 percent increase in demand by 2015.
Current industry projections for titanium indicate a 40 percent increase in demand by 2015.
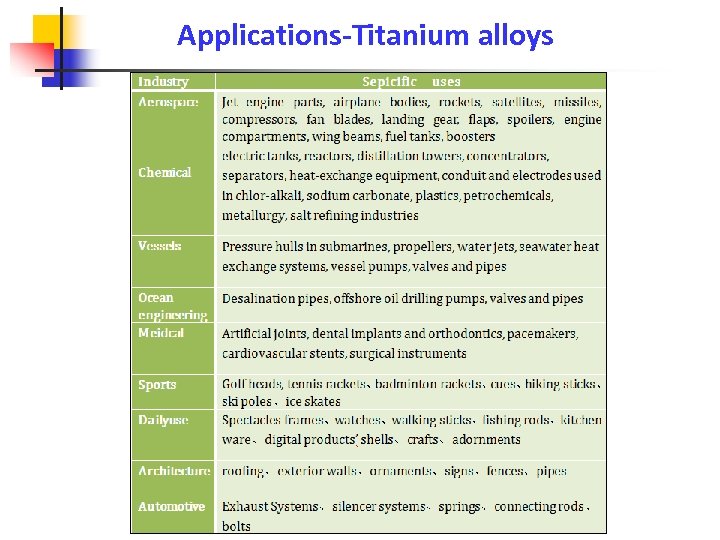 Applications-Titanium alloys
Applications-Titanium alloys
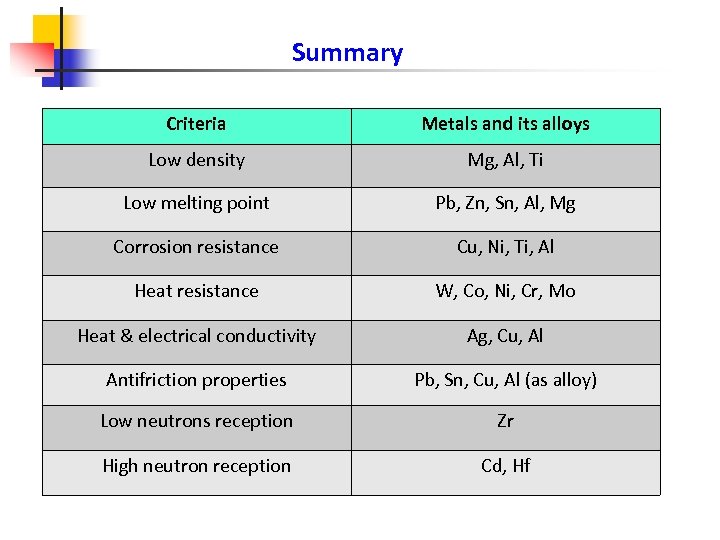 Summary Criteria Metals and its alloys Low density Mg, Al, Ti Low melting point Pb, Zn, Sn, Al, Mg Corrosion resistance Cu, Ni, Ti, Al Heat resistance W, Co, Ni, Cr, Mo Heat & electrical conductivity Ag, Cu, Al Antifriction properties Pb, Sn, Cu, Al (as alloy) Low neutrons reception Zr High neutron reception Cd, Hf
Summary Criteria Metals and its alloys Low density Mg, Al, Ti Low melting point Pb, Zn, Sn, Al, Mg Corrosion resistance Cu, Ni, Ti, Al Heat resistance W, Co, Ni, Cr, Mo Heat & electrical conductivity Ag, Cu, Al Antifriction properties Pb, Sn, Cu, Al (as alloy) Low neutrons reception Zr High neutron reception Cd, Hf
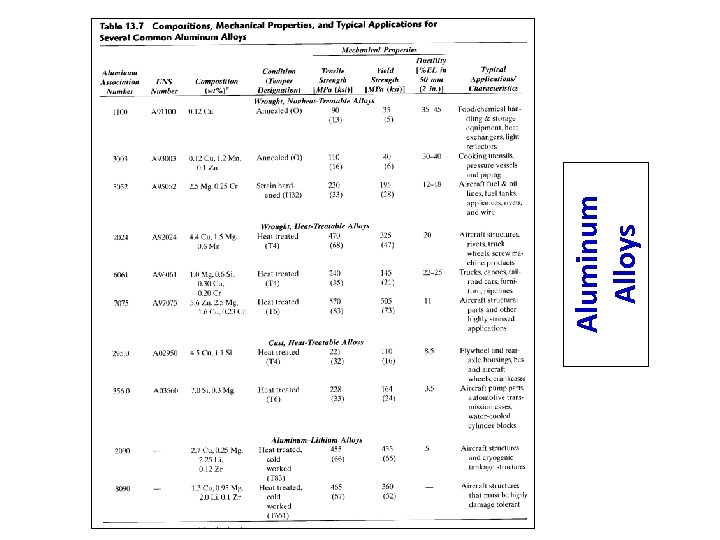 Aluminum Alloys
Aluminum Alloys
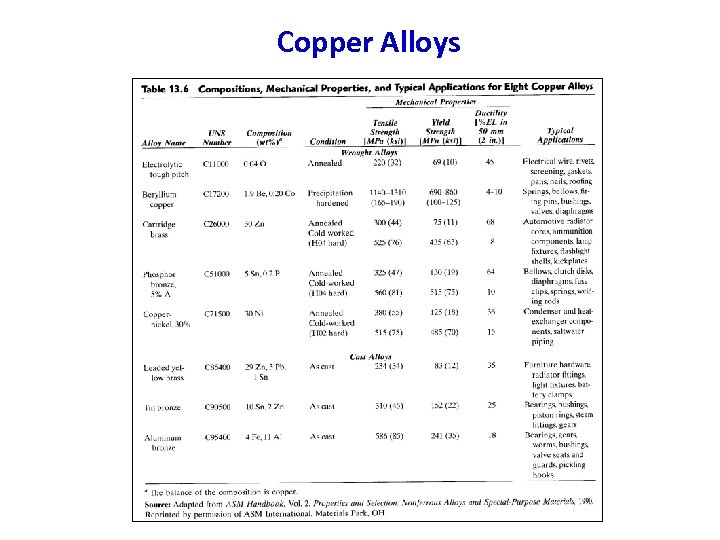 Copper Alloys
Copper Alloys
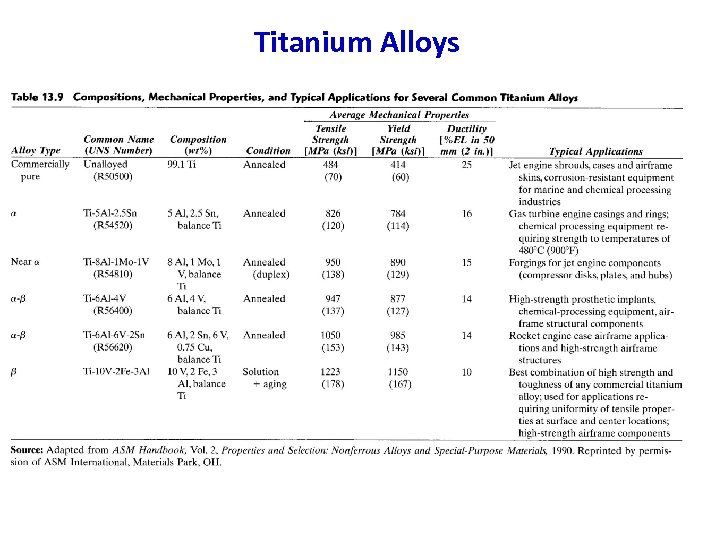 Titanium Alloys
Titanium Alloys
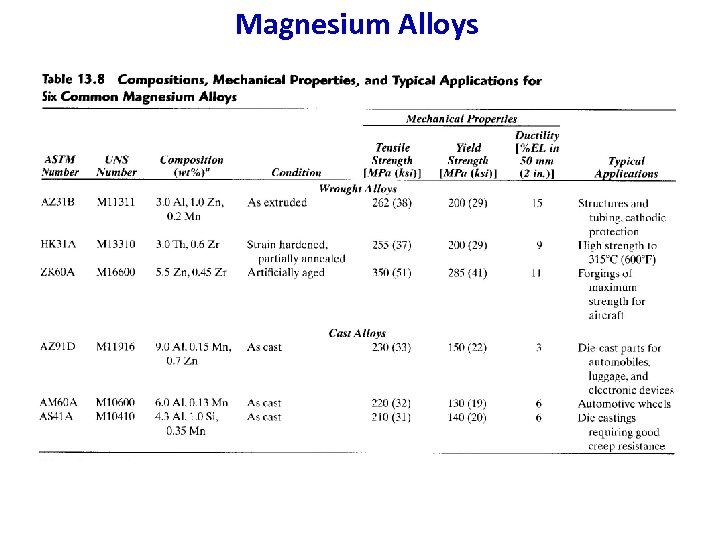 Magnesium Alloys
Magnesium Alloys
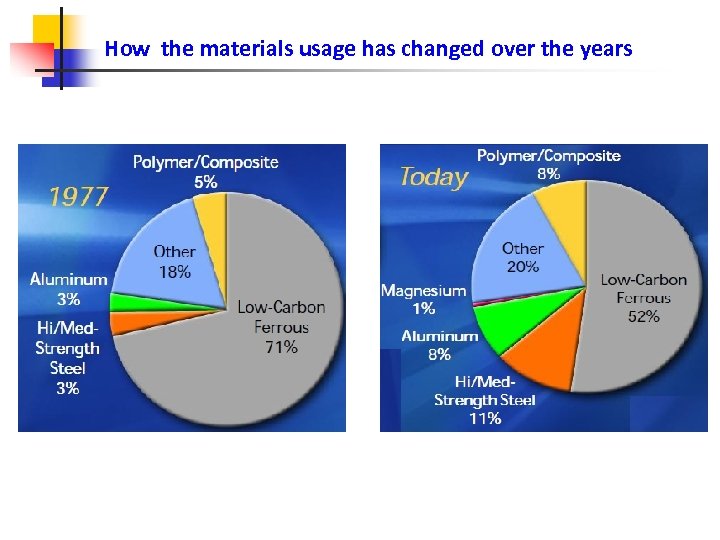 How the materials usage has changed over the years
How the materials usage has changed over the years
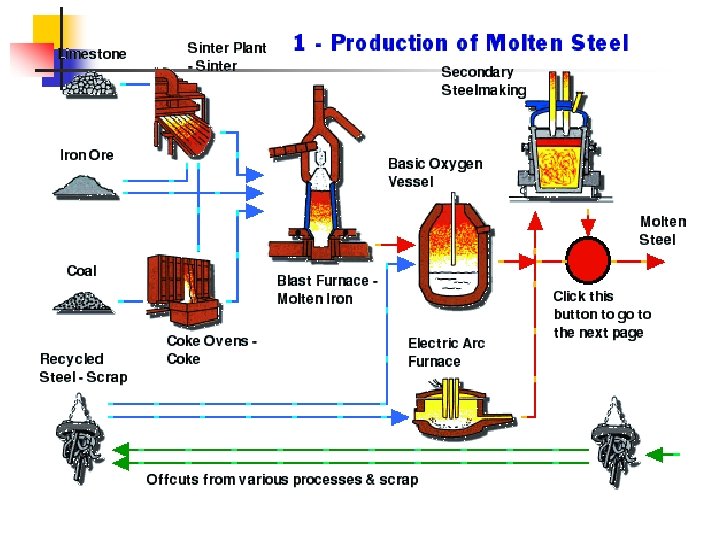
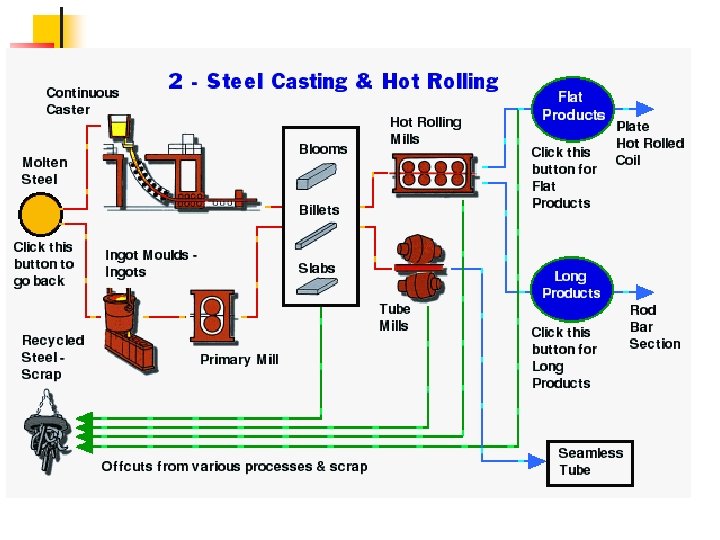
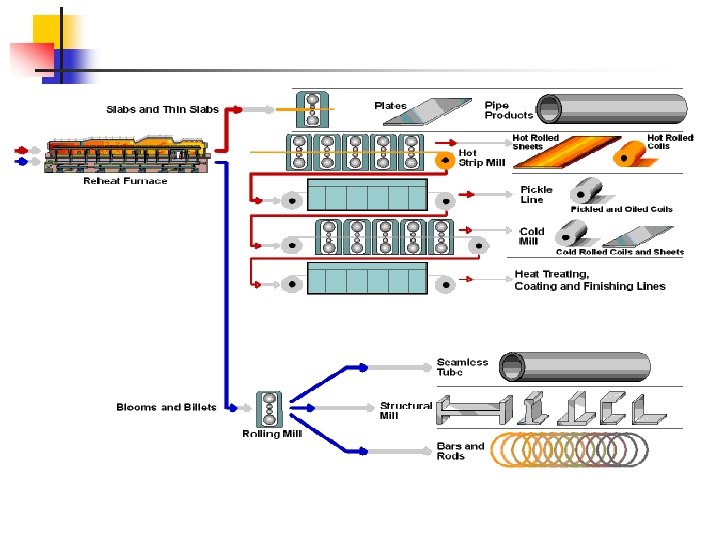
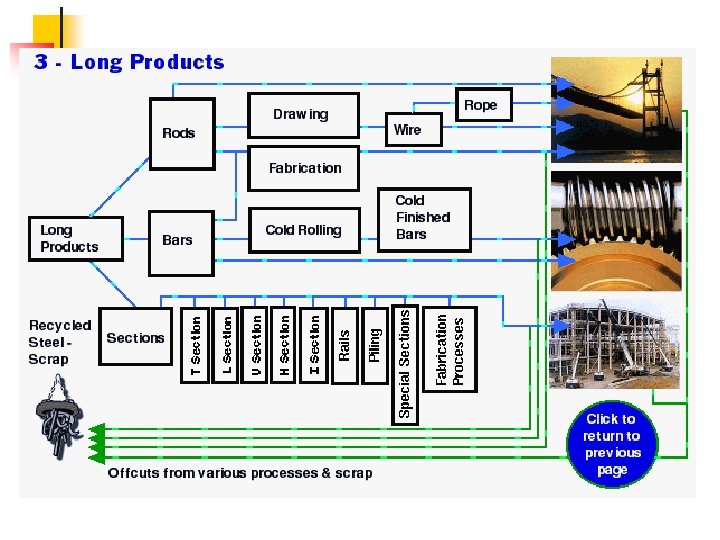
 Supplementary slides Steel Processing and applications
Supplementary slides Steel Processing and applications