eb356ac07b37e5e716bddf3005678760.ppt
- Количество слайдов: 1
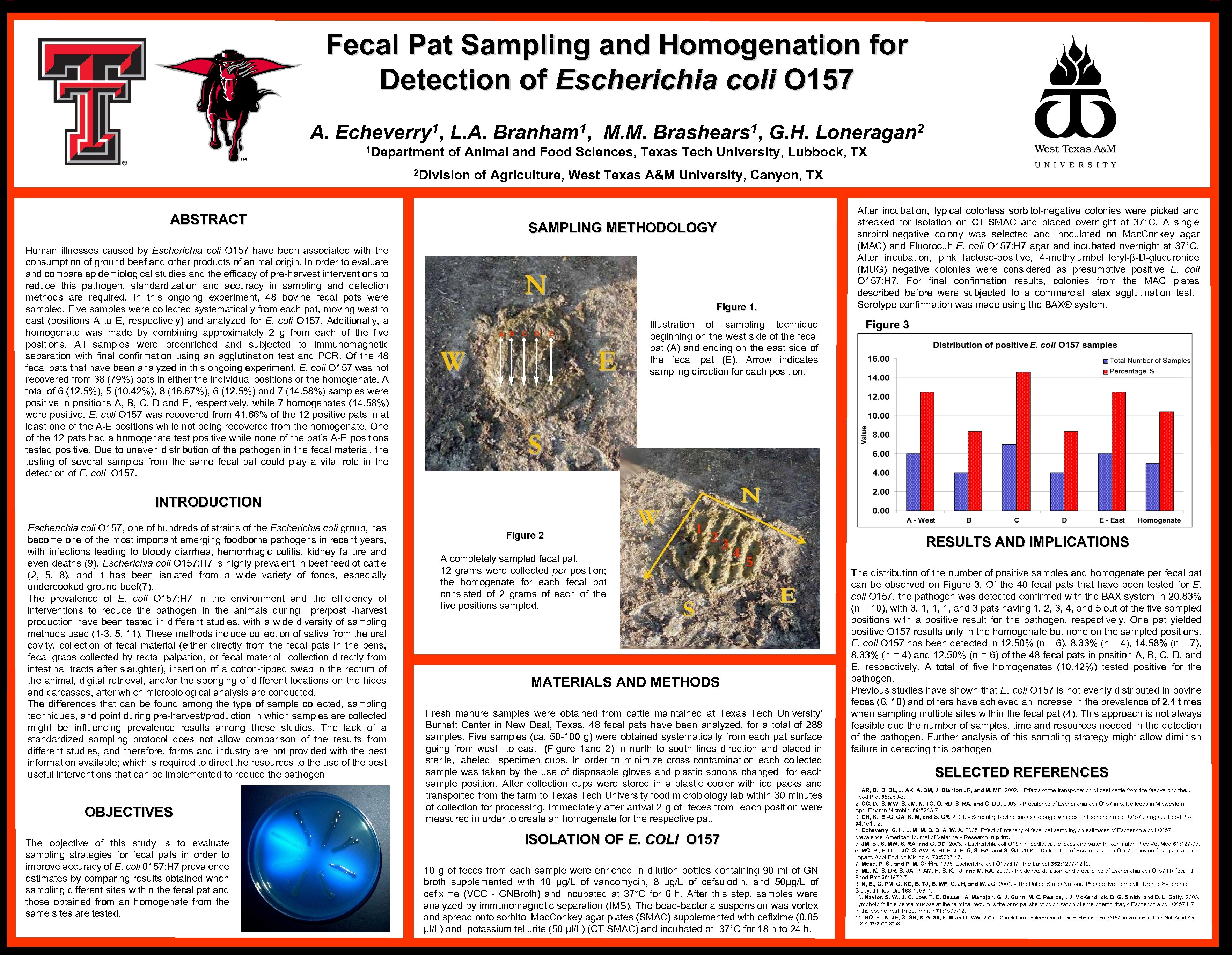 Fecal Pat Sampling and Homogenation for Detection of Escherichia coli O 157 A. 1, Echeverry 1 Department L. A. 1, Branham G. H. 2 Loneragan of Animal and Food Sciences, Texas Tech University, Lubbock, TX 2 Division of Agriculture, West Texas A&M University, Canyon, TX ABSTRACT Human illnesses caused by Escherichia coli O 157 have been associated with the consumption of ground beef and other products of animal origin. In order to evaluate and compare epidemiological studies and the efficacy of pre-harvest interventions to reduce this pathogen, standardization and accuracy in sampling and detection methods are required. In this ongoing experiment, 48 bovine fecal pats were sampled. Five samples were collected systematically from each pat, moving west to east (positions A to E, respectively) and analyzed for E. coli O 157. Additionally, a homogenate was made by combining approximately 2 g from each of the five positions. All samples were preenriched and subjected to immunomagnetic separation with final confirmation using an agglutination test and PCR. Of the 48 fecal pats that have been analyzed in this ongoing experiment, E. coli O 157 was not recovered from 38 (79%) pats in either the individual positions or the homogenate. A total of 6 (12. 5%), 5 (10. 42%), 8 (16. 67%), 6 (12. 5%) and 7 (14. 58%) samples were positive in positions A, B, C, D and E, respectively, while 7 homogenates (14. 58%) were positive. E. coli O 157 was recovered from 41. 66% of the 12 positive pats in at least one of the A-E positions while not being recovered from the homogenate. One of the 12 pats had a homogenate test positive while none of the pat’s A-E positions tested positive. Due to uneven distribution of the pathogen in the fecal material, the testing of several samples from the same fecal pat could play a vital role in the detection of E. coli O 157. M. M. 1, Brashears SAMPLING METHODOLOGY Figure 1. A B C D E Illustration of sampling technique beginning on the west side of the fecal pat (A) and ending on the east side of the fecal pat (E). Arrow indicates sampling direction for each position. After incubation, typical colorless sorbitol-negative colonies were picked and streaked for isolation on CT-SMAC and placed overnight at 37°C. A single sorbitol-negative colony was selected and inoculated on Mac. Conkey agar (MAC) and Fluorocult E. coli O 157: H 7 agar and incubated overnight at 37°C. After incubation, pink lactose-positive, 4 -methylumbelliferyl-β-D-glucuronide (MUG) negative colonies were considered as presumptive positive E. coli O 157: H 7. For final confirmation results, colonies from the MAC plates described before were subjected to a commercial latex agglutination test. Serotype confirmation was made using the BAX® system. Figure 3 INTRODUCTION Escherichia coli O 157, one of hundreds of strains of the Escherichia coli group, has become one of the most important emerging foodborne pathogens in recent years, with infections leading to bloody diarrhea, hemorrhagic colitis, kidney failure and even deaths (9). Escherichia coli O 157: H 7 is highly prevalent in beef feedlot cattle (2, 5, 8), and it has been isolated from a wide variety of foods, especially undercooked ground beef(7). The prevalence of E. coli O 157: H 7 in the environment and the efficiency of interventions to reduce the pathogen in the animals during pre/post -harvest production have been tested in different studies, with a wide diversity of sampling methods used (1 -3, 5, 11). These methods include collection of saliva from the oral cavity, collection of fecal material (either directly from the fecal pats in the pens, fecal grabs collected by rectal palpation, or fecal material collection directly from intestinal tracts after slaughter), insertion of a cotton-tipped swab in the rectum of the animal, digital retrieval, and/or the sponging of different locations on the hides and carcasses, after which microbiological analysis are conducted. The differences that can be found among the type of sample collected, sampling techniques, and point during pre-harvest/production in which samples are collected might be influencing prevalence results among these studies. The lack of a standardized sampling protocol does not allow comparison of the results from different studies, and therefore, farms and industry are not provided with the best information available; which is required to direct the resources to the use of the best useful interventions that can be implemented to reduce the pathogen OBJECTIVES The objective of this study is to evaluate sampling strategies for fecal pats in order to improve accuracy of E. coli 0157: H 7 prevalence estimates by comparing results obtained when sampling different sites within the fecal pat and those obtained from an homogenate from the same sites are tested. Figure 2 A completely sampled fecal pat. 12 grams were collected per position; the homogenate for each fecal pat consisted of 2 grams of each of the five positions sampled. MATERIALS AND METHODS Fresh manure samples were obtained from cattle maintained at Texas Tech University’ Burnett Center in New Deal, Texas. 48 fecal pats have been analyzed, for a total of 288 samples. Five samples (ca. 50 -100 g) were obtained systematically from each pat surface going from west to east (Figure 1 and 2) in north to south lines direction and placed in sterile, labeled specimen cups. In order to minimize cross-contamination each collected sample was taken by the use of disposable gloves and plastic spoons changed for each sample position. After collection cups were stored in a plastic cooler with ice packs and transported from the farm to Texas Tech University food microbiology lab within 30 minutes of collection for processing. Immediately after arrival 2 g of feces from each position were measured in order to create an homogenate for the respective pat. ISOLATION OF E. COLI O 157 10 g of feces from each sample were enriched in dilution bottles containing 90 ml of GN broth supplemented with 10 μg/L of vancomycin, 8 μg/L of cefsulodin, and 50μg/L of cefixime (VCC - GNBroth) and incubated at 37°C for 6 h. After this step, samples were analyzed by immunomagnetic separation (IMS). The bead-bacteria suspension was vortex and spread onto sorbitol Mac. Conkey agar plates (SMAC) supplemented with cefixime (0. 05 μl/L) and potassium tellurite (50 μl/L) (CT-SMAC) and incubated at 37°C for 18 h to 24 h. RESULTS AND IMPLICATIONS The distribution of the number of positive samples and homogenate per fecal pat can be observed on Figure 3. Of the 48 fecal pats that have been tested for E. coli O 157, the pathogen was detected confirmed with the BAX system in 20. 83% (n = 10), with 3, 1, 1, 1, and 3 pats having 1, 2, 3, 4, and 5 out of the five sampled positions with a positive result for the pathogen, respectively. One pat yielded positive O 157 results only in the homogenate but none on the sampled positions. E. coli O 157 has been detected in 12. 50% (n = 6), 8. 33% (n = 4), 14. 58% (n = 7), 8. 33% (n = 4) and 12. 50% (n = 6) of the 48 fecal pats in position A, B, C, D, and E, respectively. A total of five homogenates (10. 42%) tested positive for the pathogen. Previous studies have shown that E. coli O 157 is not evenly distributed in bovine feces (6, 10) and others have achieved an increase in the prevalence of 2. 4 times when sampling multiple sites within the fecal pat (4). This approach is not always feasible due the number of samples, time and resources needed in the detection of the pathogen. Further analysis of this sampling strategy might allow diminish failure in detecting this pathogen. SELECTED REFERENCES 1. AR, B. BL, J. AK, A. DM, J. Blanton JR, and M. MF. 2002. - Effects of the transportation of beef cattle from the feedyard to the. J Food Prot 65: 280 -3. 2. CC, D. , S. MW, S. JM, N. TG, O. RD, S. RA, and G. DD. 2003. - Prevalence of Escherichia coli O 157 in cattle feeds in Midwestern. Appl Environ Microbiol 69: 5243 -7. 3. DH, K. , B. -G. GA, K. M, and S. GR. 2001. - Screening bovine carcass sponge samples for Escherichia coli O 157 using a. J Food Prot 64: 1610 -2. 4. Echeverry, G. H. L. M. M. B. B. A. W. A. 2005. Effect of intensity of fecal-pat sampling on estimates of Escherichia coli O 157 prevalence. American Journal of Veterinary Research In print. 5. JM, S. MW, S. RA, and G. DD. 2003. - Escherichia coli O 157 in feedlot cattle feces and water in four major. Prev Vet Med 61: 127 -35. 6. MC, P. , F. D, L. JC, S. AW, K. HI, E. J, F. G, S. BA, and G. GJ. 2004. - Distribution of Escherichia coli O 157 in bovine fecal pats and its impact. Appl Environ Microbiol 70: 5737 -43. 7. Mead, P. S. , and P. M. Griffin. 1998. Escherichia coli O 157: H 7. The Lancet 352: 1207 -1212. 8. ML, K. , S. DR, S. JA, P. AM, H. S, K. TJ, and M. RA. 2003. - Incidence, duration, and prevalence of Escherichia coli O 157: H 7 fecal. J Food Prot 66: 1972 -7. 9. N, B. , G. PM, G. KD, B. TJ, B. WF, G. JH, and W. JG. 2001. - The United States National Prospective Hemolytic Uremic Syndrome Study. J Infect Dis 183: 1063 -70. 10. Naylor, S. W. , J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. Mc. Kendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O 157: H 7 in the bovine host. Infect Immun 71: 1505 -12. 11. RO, E. , K. JE, S. GR, B. -G. GA, K. M, and L. WW. 2000. - Correlation of enterohemorrhagic Escherichia coli O 157 prevalence in. Proc Natl Acad Sci U S A 97: 2999 -3003.
Fecal Pat Sampling and Homogenation for Detection of Escherichia coli O 157 A. 1, Echeverry 1 Department L. A. 1, Branham G. H. 2 Loneragan of Animal and Food Sciences, Texas Tech University, Lubbock, TX 2 Division of Agriculture, West Texas A&M University, Canyon, TX ABSTRACT Human illnesses caused by Escherichia coli O 157 have been associated with the consumption of ground beef and other products of animal origin. In order to evaluate and compare epidemiological studies and the efficacy of pre-harvest interventions to reduce this pathogen, standardization and accuracy in sampling and detection methods are required. In this ongoing experiment, 48 bovine fecal pats were sampled. Five samples were collected systematically from each pat, moving west to east (positions A to E, respectively) and analyzed for E. coli O 157. Additionally, a homogenate was made by combining approximately 2 g from each of the five positions. All samples were preenriched and subjected to immunomagnetic separation with final confirmation using an agglutination test and PCR. Of the 48 fecal pats that have been analyzed in this ongoing experiment, E. coli O 157 was not recovered from 38 (79%) pats in either the individual positions or the homogenate. A total of 6 (12. 5%), 5 (10. 42%), 8 (16. 67%), 6 (12. 5%) and 7 (14. 58%) samples were positive in positions A, B, C, D and E, respectively, while 7 homogenates (14. 58%) were positive. E. coli O 157 was recovered from 41. 66% of the 12 positive pats in at least one of the A-E positions while not being recovered from the homogenate. One of the 12 pats had a homogenate test positive while none of the pat’s A-E positions tested positive. Due to uneven distribution of the pathogen in the fecal material, the testing of several samples from the same fecal pat could play a vital role in the detection of E. coli O 157. M. M. 1, Brashears SAMPLING METHODOLOGY Figure 1. A B C D E Illustration of sampling technique beginning on the west side of the fecal pat (A) and ending on the east side of the fecal pat (E). Arrow indicates sampling direction for each position. After incubation, typical colorless sorbitol-negative colonies were picked and streaked for isolation on CT-SMAC and placed overnight at 37°C. A single sorbitol-negative colony was selected and inoculated on Mac. Conkey agar (MAC) and Fluorocult E. coli O 157: H 7 agar and incubated overnight at 37°C. After incubation, pink lactose-positive, 4 -methylumbelliferyl-β-D-glucuronide (MUG) negative colonies were considered as presumptive positive E. coli O 157: H 7. For final confirmation results, colonies from the MAC plates described before were subjected to a commercial latex agglutination test. Serotype confirmation was made using the BAX® system. Figure 3 INTRODUCTION Escherichia coli O 157, one of hundreds of strains of the Escherichia coli group, has become one of the most important emerging foodborne pathogens in recent years, with infections leading to bloody diarrhea, hemorrhagic colitis, kidney failure and even deaths (9). Escherichia coli O 157: H 7 is highly prevalent in beef feedlot cattle (2, 5, 8), and it has been isolated from a wide variety of foods, especially undercooked ground beef(7). The prevalence of E. coli O 157: H 7 in the environment and the efficiency of interventions to reduce the pathogen in the animals during pre/post -harvest production have been tested in different studies, with a wide diversity of sampling methods used (1 -3, 5, 11). These methods include collection of saliva from the oral cavity, collection of fecal material (either directly from the fecal pats in the pens, fecal grabs collected by rectal palpation, or fecal material collection directly from intestinal tracts after slaughter), insertion of a cotton-tipped swab in the rectum of the animal, digital retrieval, and/or the sponging of different locations on the hides and carcasses, after which microbiological analysis are conducted. The differences that can be found among the type of sample collected, sampling techniques, and point during pre-harvest/production in which samples are collected might be influencing prevalence results among these studies. The lack of a standardized sampling protocol does not allow comparison of the results from different studies, and therefore, farms and industry are not provided with the best information available; which is required to direct the resources to the use of the best useful interventions that can be implemented to reduce the pathogen OBJECTIVES The objective of this study is to evaluate sampling strategies for fecal pats in order to improve accuracy of E. coli 0157: H 7 prevalence estimates by comparing results obtained when sampling different sites within the fecal pat and those obtained from an homogenate from the same sites are tested. Figure 2 A completely sampled fecal pat. 12 grams were collected per position; the homogenate for each fecal pat consisted of 2 grams of each of the five positions sampled. MATERIALS AND METHODS Fresh manure samples were obtained from cattle maintained at Texas Tech University’ Burnett Center in New Deal, Texas. 48 fecal pats have been analyzed, for a total of 288 samples. Five samples (ca. 50 -100 g) were obtained systematically from each pat surface going from west to east (Figure 1 and 2) in north to south lines direction and placed in sterile, labeled specimen cups. In order to minimize cross-contamination each collected sample was taken by the use of disposable gloves and plastic spoons changed for each sample position. After collection cups were stored in a plastic cooler with ice packs and transported from the farm to Texas Tech University food microbiology lab within 30 minutes of collection for processing. Immediately after arrival 2 g of feces from each position were measured in order to create an homogenate for the respective pat. ISOLATION OF E. COLI O 157 10 g of feces from each sample were enriched in dilution bottles containing 90 ml of GN broth supplemented with 10 μg/L of vancomycin, 8 μg/L of cefsulodin, and 50μg/L of cefixime (VCC - GNBroth) and incubated at 37°C for 6 h. After this step, samples were analyzed by immunomagnetic separation (IMS). The bead-bacteria suspension was vortex and spread onto sorbitol Mac. Conkey agar plates (SMAC) supplemented with cefixime (0. 05 μl/L) and potassium tellurite (50 μl/L) (CT-SMAC) and incubated at 37°C for 18 h to 24 h. RESULTS AND IMPLICATIONS The distribution of the number of positive samples and homogenate per fecal pat can be observed on Figure 3. Of the 48 fecal pats that have been tested for E. coli O 157, the pathogen was detected confirmed with the BAX system in 20. 83% (n = 10), with 3, 1, 1, 1, and 3 pats having 1, 2, 3, 4, and 5 out of the five sampled positions with a positive result for the pathogen, respectively. One pat yielded positive O 157 results only in the homogenate but none on the sampled positions. E. coli O 157 has been detected in 12. 50% (n = 6), 8. 33% (n = 4), 14. 58% (n = 7), 8. 33% (n = 4) and 12. 50% (n = 6) of the 48 fecal pats in position A, B, C, D, and E, respectively. A total of five homogenates (10. 42%) tested positive for the pathogen. Previous studies have shown that E. coli O 157 is not evenly distributed in bovine feces (6, 10) and others have achieved an increase in the prevalence of 2. 4 times when sampling multiple sites within the fecal pat (4). This approach is not always feasible due the number of samples, time and resources needed in the detection of the pathogen. Further analysis of this sampling strategy might allow diminish failure in detecting this pathogen. SELECTED REFERENCES 1. AR, B. BL, J. AK, A. DM, J. Blanton JR, and M. MF. 2002. - Effects of the transportation of beef cattle from the feedyard to the. J Food Prot 65: 280 -3. 2. CC, D. , S. MW, S. JM, N. TG, O. RD, S. RA, and G. DD. 2003. - Prevalence of Escherichia coli O 157 in cattle feeds in Midwestern. Appl Environ Microbiol 69: 5243 -7. 3. DH, K. , B. -G. GA, K. M, and S. GR. 2001. - Screening bovine carcass sponge samples for Escherichia coli O 157 using a. J Food Prot 64: 1610 -2. 4. Echeverry, G. H. L. M. M. B. B. A. W. A. 2005. Effect of intensity of fecal-pat sampling on estimates of Escherichia coli O 157 prevalence. American Journal of Veterinary Research In print. 5. JM, S. MW, S. RA, and G. DD. 2003. - Escherichia coli O 157 in feedlot cattle feces and water in four major. Prev Vet Med 61: 127 -35. 6. MC, P. , F. D, L. JC, S. AW, K. HI, E. J, F. G, S. BA, and G. GJ. 2004. - Distribution of Escherichia coli O 157 in bovine fecal pats and its impact. Appl Environ Microbiol 70: 5737 -43. 7. Mead, P. S. , and P. M. Griffin. 1998. Escherichia coli O 157: H 7. The Lancet 352: 1207 -1212. 8. ML, K. , S. DR, S. JA, P. AM, H. S, K. TJ, and M. RA. 2003. - Incidence, duration, and prevalence of Escherichia coli O 157: H 7 fecal. J Food Prot 66: 1972 -7. 9. N, B. , G. PM, G. KD, B. TJ, B. WF, G. JH, and W. JG. 2001. - The United States National Prospective Hemolytic Uremic Syndrome Study. J Infect Dis 183: 1063 -70. 10. Naylor, S. W. , J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. Mc. Kendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O 157: H 7 in the bovine host. Infect Immun 71: 1505 -12. 11. RO, E. , K. JE, S. GR, B. -G. GA, K. M, and L. WW. 2000. - Correlation of enterohemorrhagic Escherichia coli O 157 prevalence in. Proc Natl Acad Sci U S A 97: 2999 -3003.


