1743d7c01a5e1c3039e08ccec1098a96.ppt
- Количество слайдов: 105

February, the 16 th 2007 A Clinical Trial Disaster in UK Laura BATIQUE, Kelly BROWN, Céline DELPLACE, Bénédicte PALADINI, & Pauline SALADIN

Introduction The « Te. Genero matter » in few words
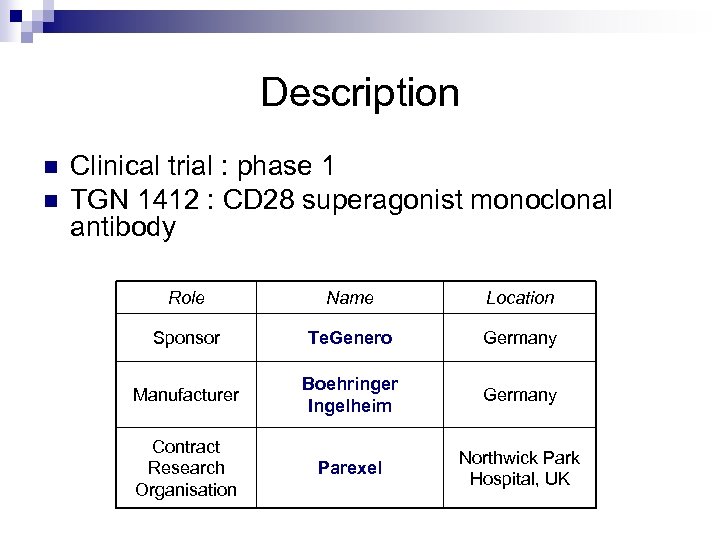
Description n n Clinical trial : phase 1 TGN 1412 : CD 28 superagonist monoclonal antibody Role Name Location Sponsor Te. Genero Germany Manufacturer Boehringer Ingelheim Germany Contract Research Organisation Parexel Northwick Park Hospital, UK

What's happened ? 13 th March 2006 Six healthy volunteers received TGN 1412 at Northwick Park Hospital Phase 1 : Cytokine storm, lymphopenia, monocytopenia Phase 2 : Reactive phase, multiorgan failure Phase 3 : Recovery phase Phase 4 : Steady state

PLAN n n n n Part I : Media’s reaction Part II : Scientific context & mechanism of action Part III : Preclinical studies Part IV : Transition to clinical development Part V : Clinical trial Part VI : Protagonist reaction Part VII : Discussion

Part I : Media’s Reaction
MEDIA Telegraph. co. uk

Media’s reaction Some days after the trial n n Violent Reaction to monoclonal antibody therapy remains a Mystery (Science 15/03/06) Tragic drug trial spotlights potent molecule (Nature 13/03/06 ) « There a number questions that need answering […] what happened […] monkeys were an appropriate animal model to test […] Te. Genero had tested the antibody on human blood […] why Parexel did not leave a longer gap between doses » (Pharmatimes 26/05/06) British trial disaster casts doubts on testing guidelines (Nature 01/04/06 )

What caused this event ? n Errors in manufacture, formulation or administration? n Contamination with endotoxin, pyrogen, microbiologic or other agents? n Mechanism of action?

Part II : Scientific context & mechanism of action

Costimulation of Lymphocyte T
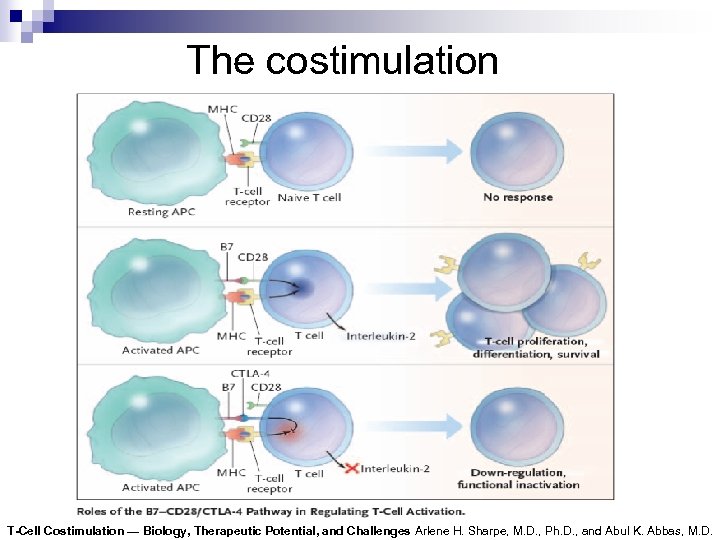
The costimulation T-Cell Costimulation — Biology, Therapeutic Potential, and Challenges Arlene H. Sharpe, M. D. , Ph. D. , and Abul K. Abbas, M. D.
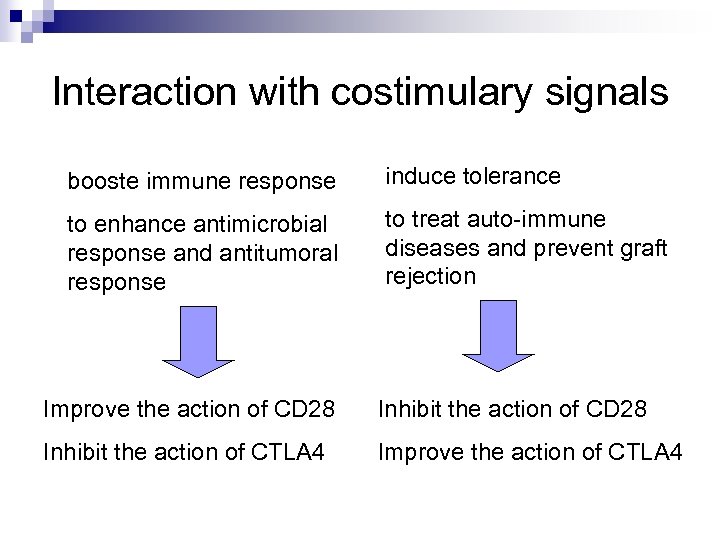
Interaction with costimulary signals booste immune response induce tolerance to enhance antimicrobial response and antitumoral response to treat auto-immune diseases and prevent graft rejection Improve the action of CD 28 Inhibit the action of CTLA 4 Improve the action of CTLA 4

The first target: CTLA 4 Abatacept Orencia® Ipilimumab and ticilimumab
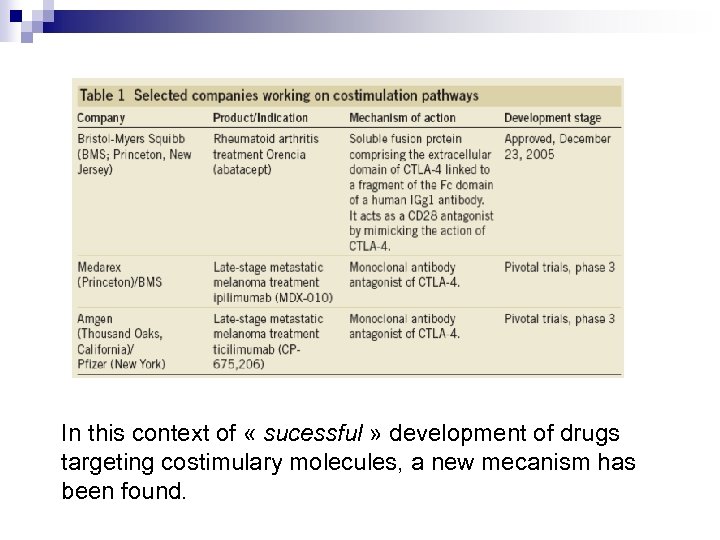
In this context of « sucessful » development of drugs targeting costimulary molecules, a new mecanism has been found.

The second target: CD 28 n What is a superagonist antibody? n The first indication: leukemia n The second indication: autoimmune diseases n Risks of TGN 1412
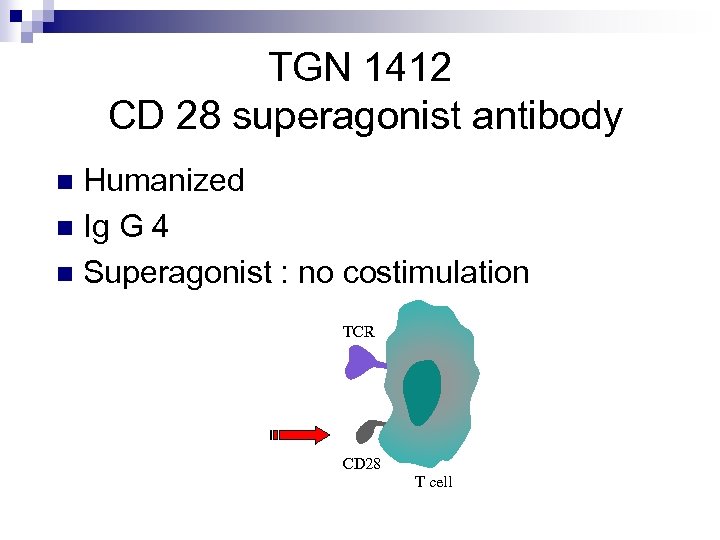
TGN 1412 CD 28 superagonist antibody Humanized n Ig G 4 n Superagonist : no costimulation n TCR CD 28 T cell

The first indication: chronic lymphocytic leukemia
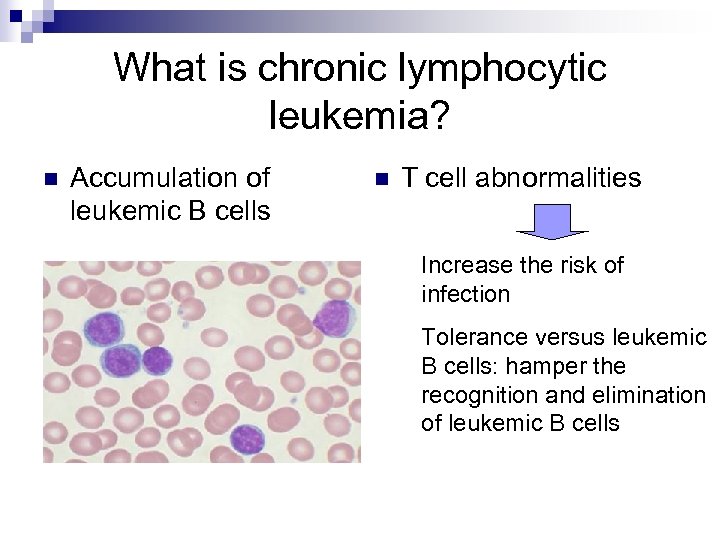
What is chronic lymphocytic leukemia? n Accumulation of leukemic B cells n T cell abnormalities Increase the risk of infection Tolerance versus leukemic B cells: hamper the recognition and elimination of leukemic B cells
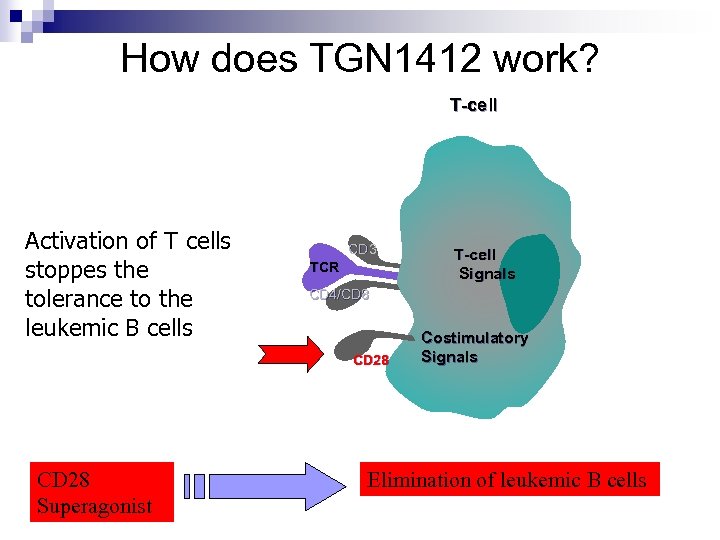
How does TGN 1412 work? T-cell Activation of T cells stoppes the tolerance to the leukemic B cells CD 3 TCR CD 4/CD 8 CD 28 Superagonist T-cell Signals Costimulatory Signals Elimination of leukemic B cells

The second indication: autoimmune diseases
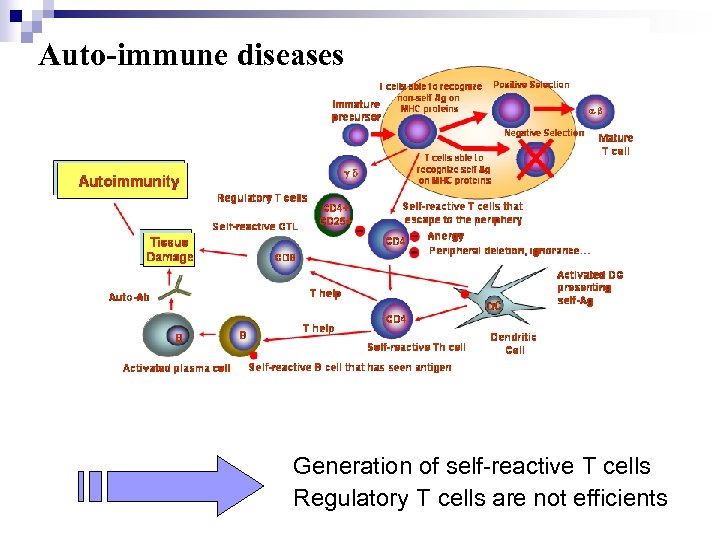
Auto-immune diseases Generation of self-reactive T cells Regulatory T cells are not efficients

How does a CD 28 superagonist work? What are regulatory T cells? Theoric effect of TGN 1412 on the Treg
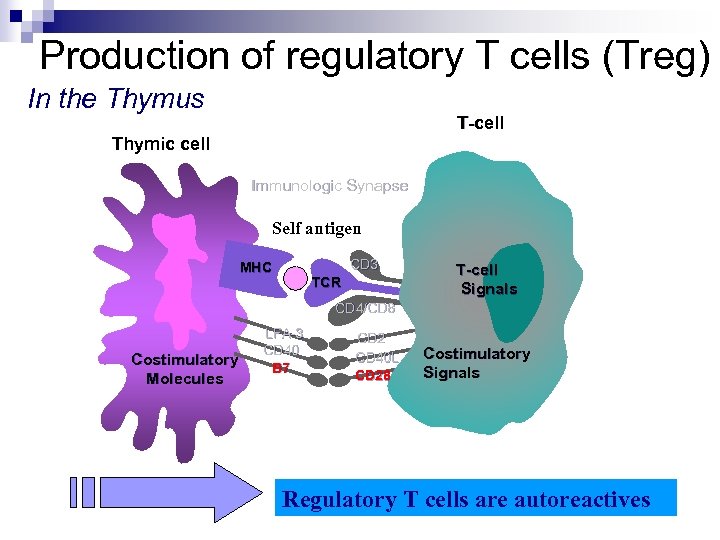
Production of regulatory T cells (Treg) In the Thymus T-cell T- Thymic cell Immunologic Synapse Self antigen CD 3 MHC TCR T-cell Signals CD 4/CD 8 Costimulatory Molecules LFA-3 CD 40 B 7 CD 2 CD 40 L CD 28 Costimulatory Signals Regulatory T cells are autoreactives
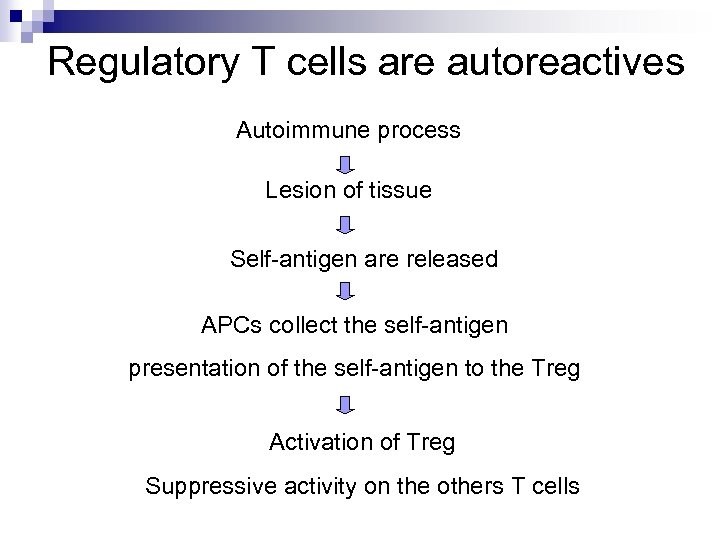
Regulatory T cells are autoreactives Autoimmune process Lesion of tissue Self-antigen are released APCs collect the self-antigen presentation of the self-antigen to the Treg Activation of Treg Suppressive activity on the others T cells
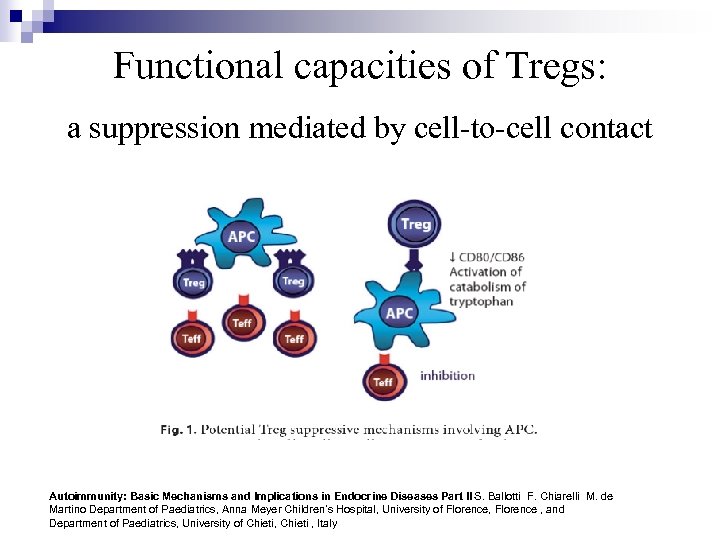
Functional capacities of Tregs: a suppression mediated by cell-to-cell contact Autoimmunity: Basic Mechanisms and Implications in Endocrine Diseases Part II S. Ballotti F. Chiarelli M. de Martino Department of Paediatrics, Anna Meyer Children’s Hospital, University of Florence, Florence , and Department of Paediatrics, University of Chieti, Chieti , Italy
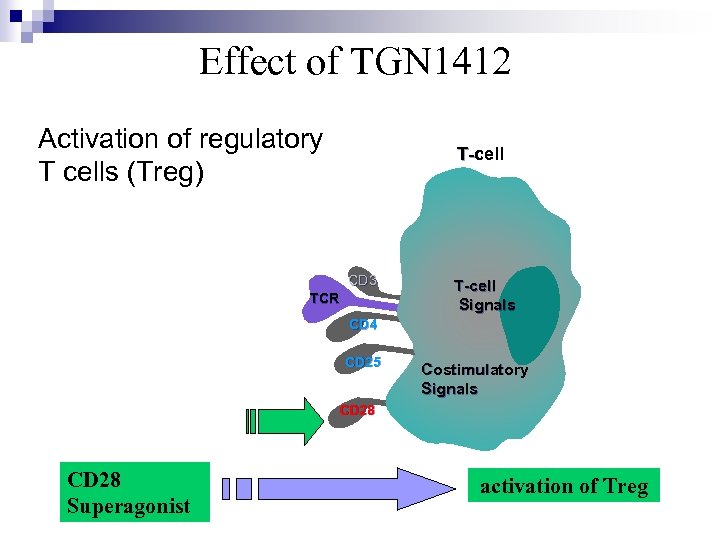
Effect of TGN 1412 Activation of regulatory T cells (Treg) T-cell T- CD 3 TCR T-cell Signals CD 4 CD 25 Costimulatory Signals CD 28 Superagonist activation of Treg

Risks of a CD 28 superagonist
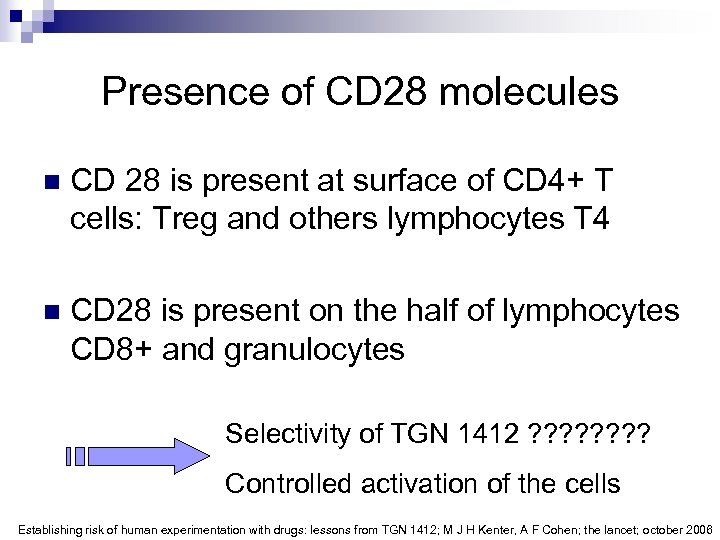
Presence of CD 28 molecules n CD 28 is present at surface of CD 4+ T cells: Treg and others lymphocytes T 4 n CD 28 is present on the half of lymphocytes CD 8+ and granulocytes Selectivity of TGN 1412 ? ? ? ? Controlled activation of the cells Establishing risk of human experimentation with drugs: lessons from TGN 1412; M J H Kenter, A F Cohen; the lancet; october 2006

Superagonist Antibodies ?
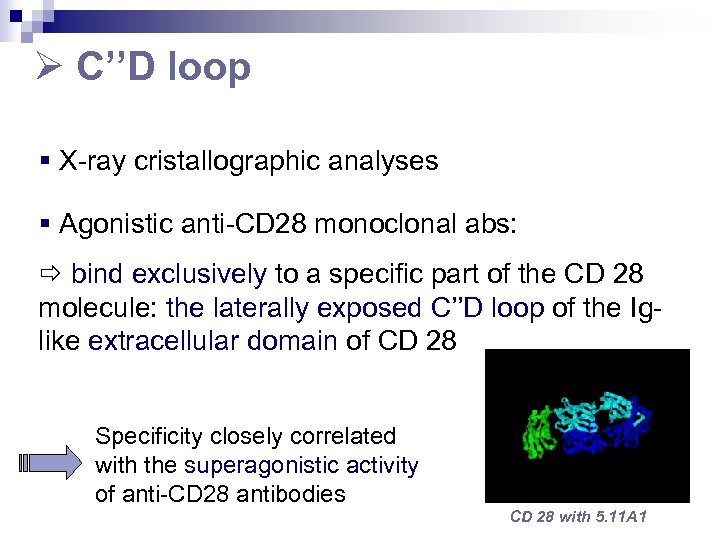
C’’D loop § X-ray cristallographic analyses § Agonistic anti-CD 28 monoclonal abs: bind exclusively to a specific part of the CD 28 molecule: the laterally exposed C’’D loop of the Iglike extracellular domain of CD 28 Specificity closely correlated with the superagonistic activity of anti-CD 28 antibodies CD 28 with 5. 11 A 1
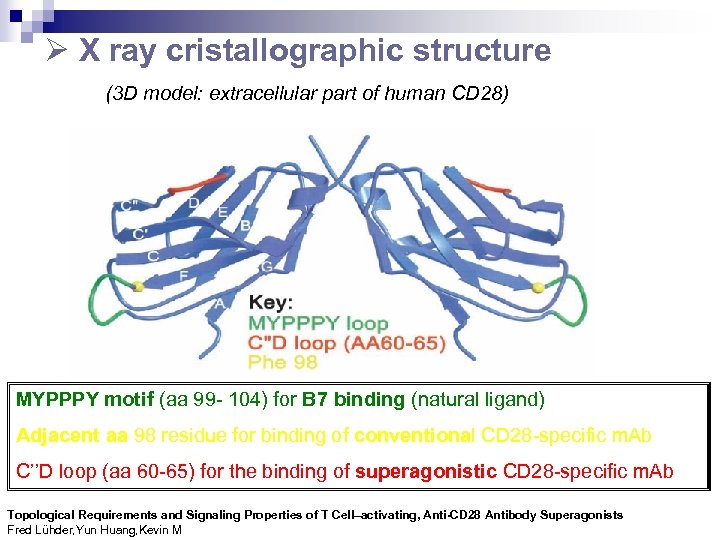
X ray cristallographic structure (3 D model: extracellular part of human CD 28) MYPPPY motif (aa 99 - 104) for B 7 binding (natural ligand) Adjacent aa 98 residue for binding of conventional CD 28 -specific m. Ab C’’D loop (aa 60 -65) for the binding of superagonistic CD 28 -specific m. Ab Topological Requirements and Signaling Properties of T Cell–activating, Anti-CD 28 Antibody Superagonists Fred Lühder, Yun Huang, Kevin M

Part III : Preclinical Studies (Toxicology) § 1. Rodent: rat § 2. Non human primate: monkey

1. Toxicology On Rats
Preclinical On Rats § Presentation of molecules § Trials on healthy rats in vivo § Trials on unwell rats in vivo
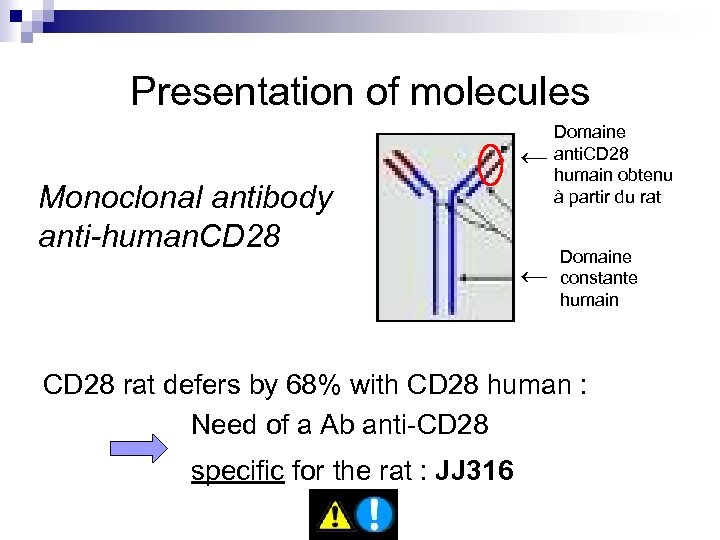
Presentation of molecules ← Monoclonal antibody anti-human. CD 28 Domaine anti. CD 28 humain obtenu à partir du rat ← Domaine constante humain CD 28 rat defers by 68% with CD 28 human : Need of a Ab anti-CD 28 specific for the rat : JJ 316
Trials on healthy rats § The 2 phenotypes of CD 4+Tcells § Identification of regulatory Tcells (Treg) § JJ 316’s action on CD 4+Tcells
The phenotypes 2 phenotypes are possible for CD 4+Tcells : CD 25+ ou CD 25 - Experiment : - isolation of Tcells lymph nodes from Lewis rats - marking : CD 4+ with CFSE CD 25+ with anti-CD 25 m. Ab
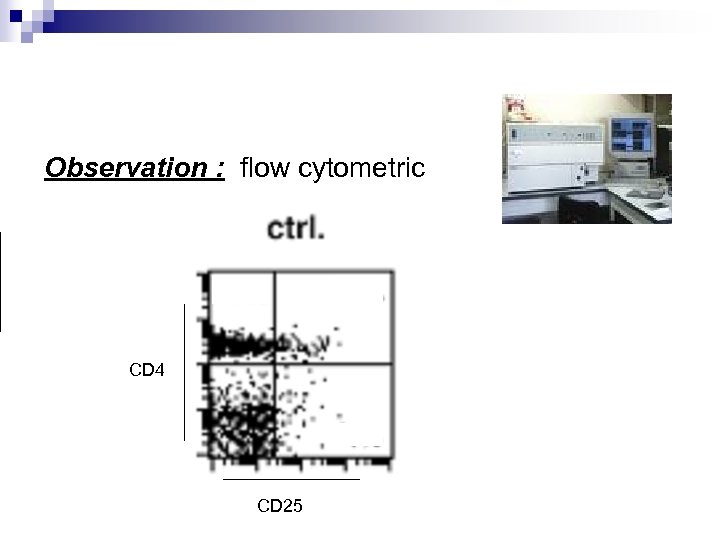
Observation : flow cytometric CD 4 CD 25
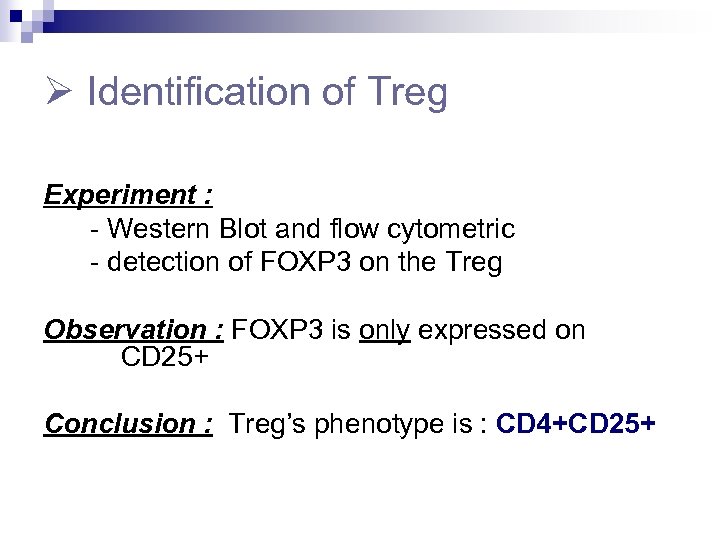
Identification of Treg Experiment : - Western Blot and flow cytometric - detection of FOXP 3 on the Treg Observation : FOXP 3 is only expressed on CD 25+ Conclusion : Treg’s phenotype is : CD 4+CD 25+
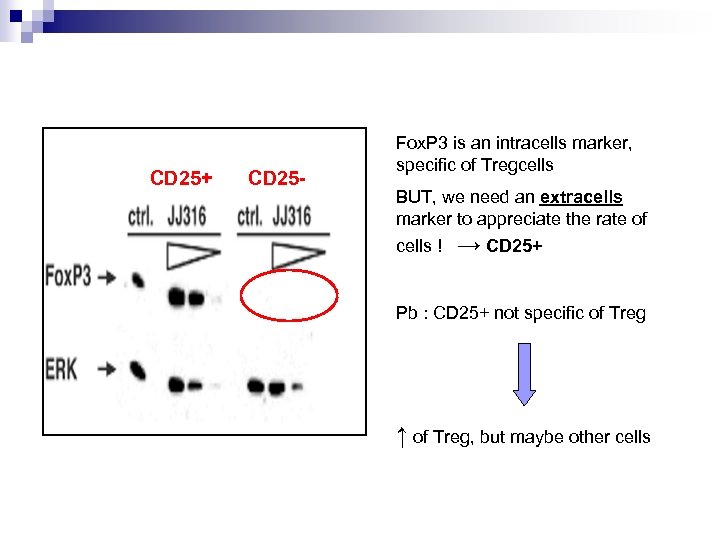
CD 25+ CD 25 - Fox. P 3 is an intracells marker, specific of Tregcells BUT, we need an extracells marker to appreciate the rate of cells ! → CD 25+ Pb : CD 25+ not specific of Treg ↑ of Treg, but maybe other cells
Ab’s action Aim : to quantify the JJ 316’s action Experiment : - injections of several JJ 316 or MOPC-31 C dosages to Lewis rats (0. 1 à 1 mg/kg) - isolation of lymph node and spleen - flow cytometric
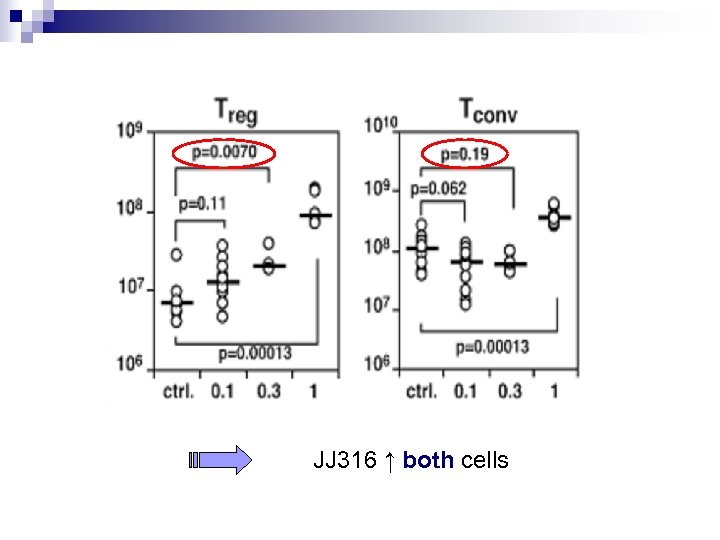
JJ 316 ↑ both cells
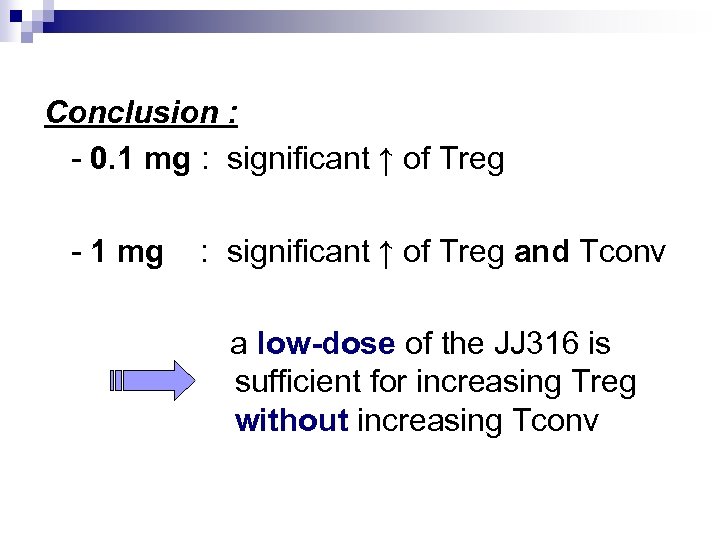
Conclusion : - 0. 1 mg : significant ↑ of Treg - 1 mg : significant ↑ of Treg and Tconv a low-dose of the JJ 316 is sufficient for increasing Treg without increasing Tconv
Abstract § Identification of different CD 4+ Tcells (due to their phenotype) : Treg and Tconv § To show clearly the selective ↑ of Treg with dosage ≤ 0. 3 mg/kg of JJ 316 So don’t make mistakes in doses !

Trials on unwell rats Experiment : determination of the DME - treatment of rats with different doses : 0. 03 / 0. 1 / 0. 3 mg/kg of JJ 316 - evaluation of clinical symptoms
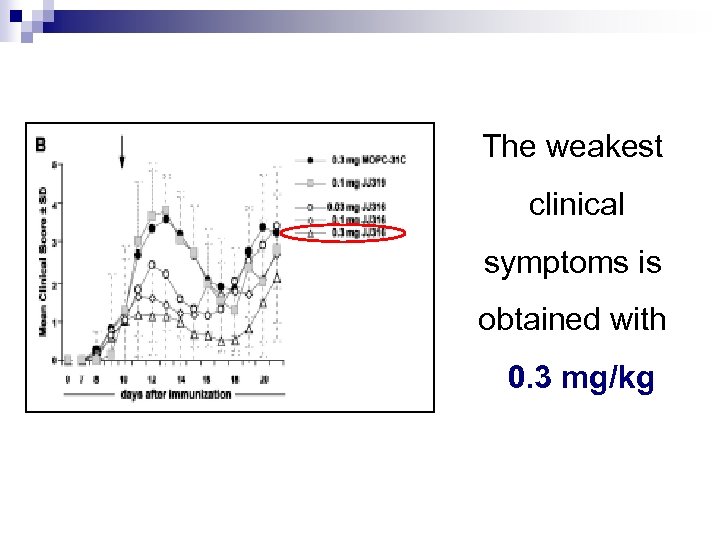
The weakest clinical symptoms is obtained with 0. 3 mg/kg
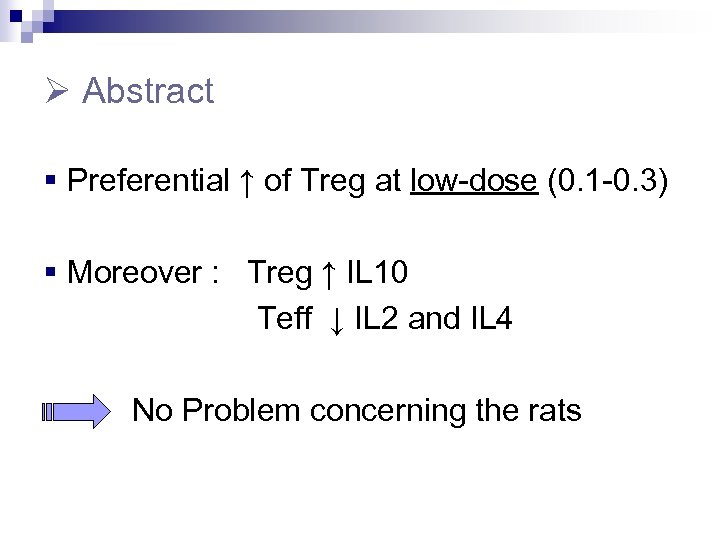
Abstract § Preferential ↑ of Treg at low-dose (0. 1 -0. 3) § Moreover : Treg ↑ IL 10 Teff ↓ IL 2 and IL 4 No Problem concerning the rats

2. Toxicology On Monkeys and Limits § In vitro & Failure in Investigator’s brochure § In vivo § Pharmacokinetics & Toxicokinetics
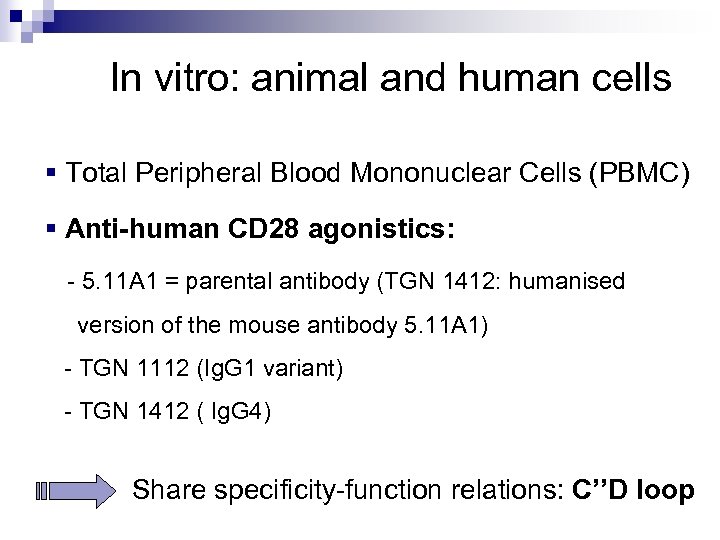
In vitro: animal and human cells § Total Peripheral Blood Mononuclear Cells (PBMC) § Anti-human CD 28 agonistics: - 5. 11 A 1 = parental antibody (TGN 1412: humanised version of the mouse antibody 5. 11 A 1) - TGN 1112 (Ig. G 1 variant) - TGN 1412 ( Ig. G 4) Share specificity-function relations: C’’D loop
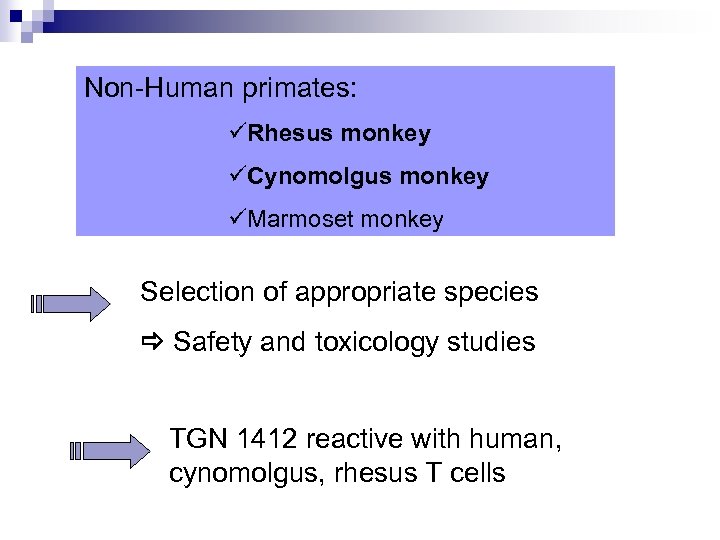
Non-Human primates: üRhesus monkey üCynomolgus monkey üMarmoset monkey Selection of appropriate species Safety and toxicology studies TGN 1412 reactive with human, cynomolgus, rhesus T cells
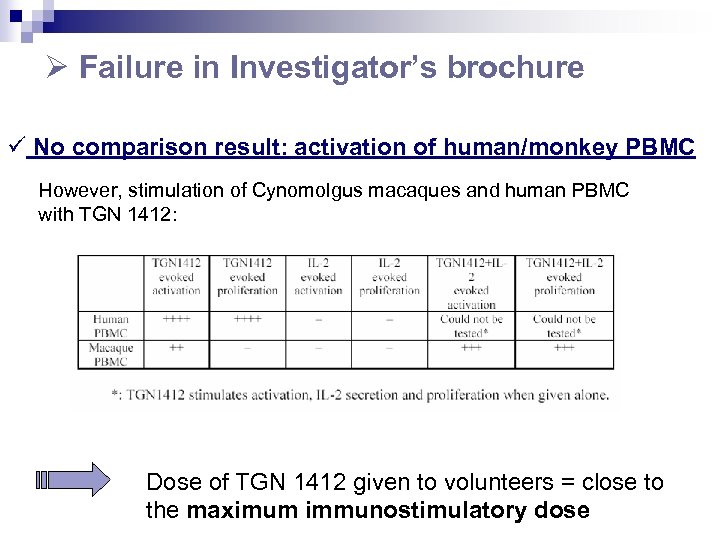
Failure in Investigator’s brochure ü No comparison result: activation of human/monkey PBMC However, stimulation of Cynomolgus macaques and human PBMC with TGN 1412: Dose of TGN 1412 given to volunteers = close to the maximum immunostimulatory dose
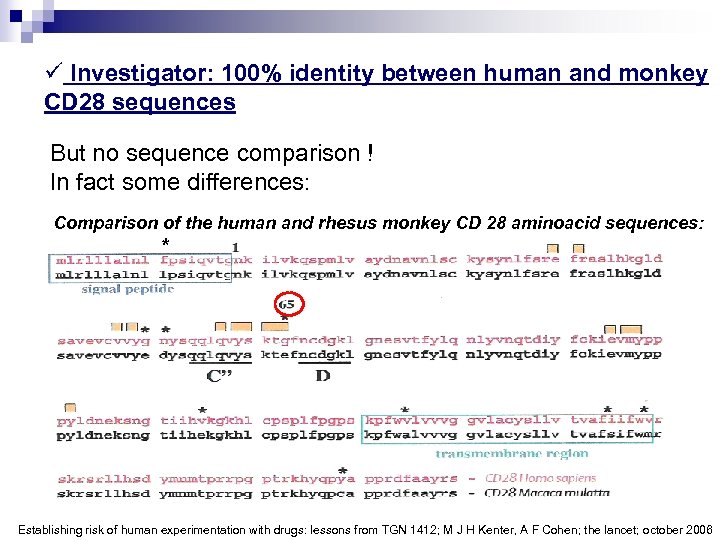
ü Investigator: 100% identity between human and monkey CD 28 sequences But no sequence comparison ! In fact some differences: Comparison of the human and rhesus monkey CD 28 aminoacid sequences: * Establishing risk of human experimentation with drugs: lessons from TGN 1412; M J H Kenter, A F Cohen; the lancet; october 2006
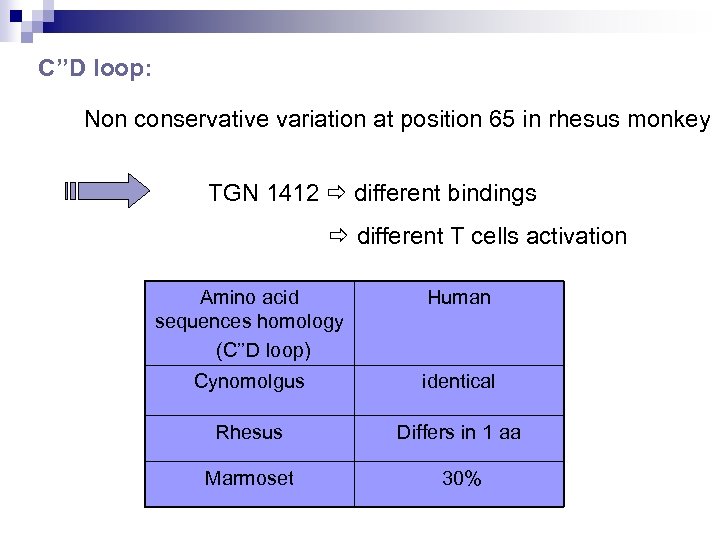
C’’D loop: Non conservative variation at position 65 in rhesus monkey TGN 1412 different bindings different T cells activation Amino acid sequences homology (C’’D loop) Human Cynomolgus identical Rhesus Differs in 1 aa Marmoset 30%

Failure in Investigator’s brochure ü No test done to demonstrate identity CD 28 sequences between cynomolgus monkeys and humans ü No comparison between rhesus, cynomolgus monkeys and human CD 28 sequences ü No comparison: affinity of TGN 1412 on human and monkey CD 28
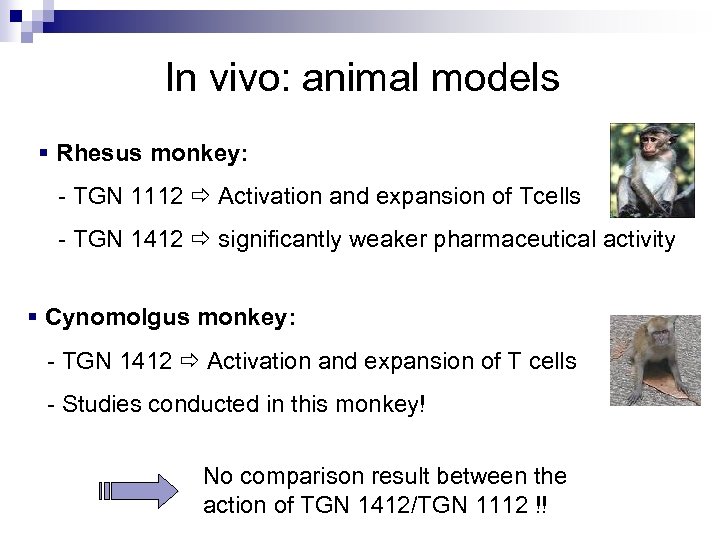
In vivo: animal models § Rhesus monkey: - TGN 1112 Activation and expansion of Tcells - TGN 1412 significantly weaker pharmaceutical activity § Cynomolgus monkey: - TGN 1412 Activation and expansion of T cells - Studies conducted in this monkey! No comparison result between the action of TGN 1412/TGN 1112 !!

Pharmacokinetics and Toxicokinetics § Cynomolgus monkey: the most predictive § Repeat-dose toxicity study: - IV - 5 up to 50 mg/kg TGN 1412 : well tolerated up to 50 mg/kg/week 50 mg/kg = NOAEL (No-Observed-Adverse-Effect Level) Half life = 8 days Full removal = 1 month
Part IV : Transition to Clinical Development of TGN 1412 § Differences between mouse/rat models & humans § Affinity differences between antibodies § Use of healthy volunteers § Dose calculation
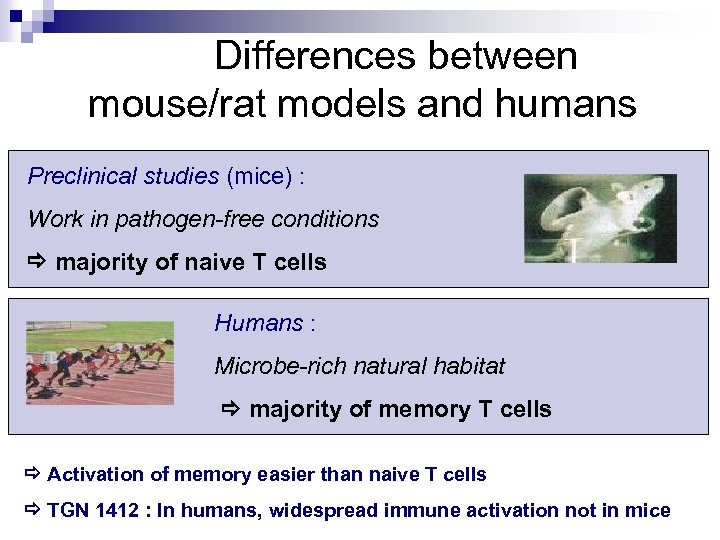
Differences between mouse/rat models and humans Preclinical studies (mice) : Work in pathogen-free conditions majority of naive T cells Humans : Microbe-rich natural habitat majority of memory T cells Activation of memory easier than naive T cells TGN 1412 : In humans, widespread immune activation not in mice
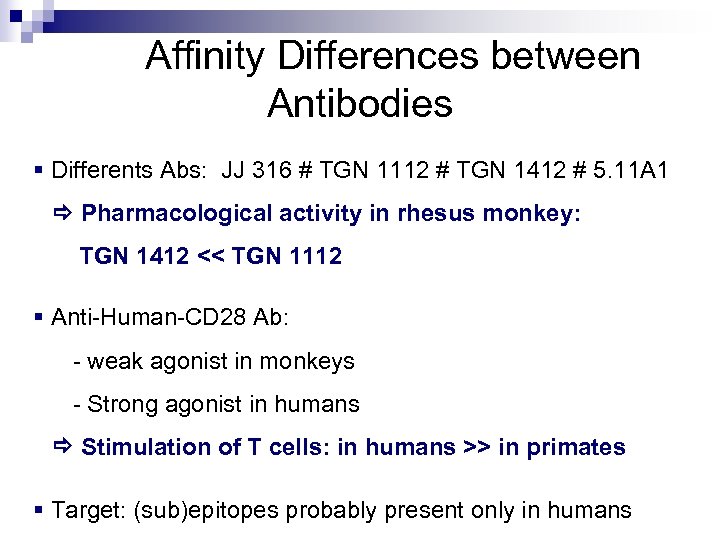
Affinity Differences between Antibodies § Differents Abs: JJ 316 # TGN 1112 # TGN 1412 # 5. 11 A 1 Pharmacological activity in rhesus monkey: TGN 1412 << TGN 1112 § Anti-Human-CD 28 Ab: - weak agonist in monkeys - Strong agonist in humans Stimulation of T cells: in humans >> in primates § Target: (sub)epitopes probably present only in humans

Why did the compagny choose healthy volunteers? § No adverse effects in healthy animals § No pre-activation or dysfunction of T cells § No pre-existing imbalance: Tconv/Treg cells § Homogenous population: No impact of pre/co-medication and/or disease activity No interference with interpretation of TGN 1412 safety and pharmacology
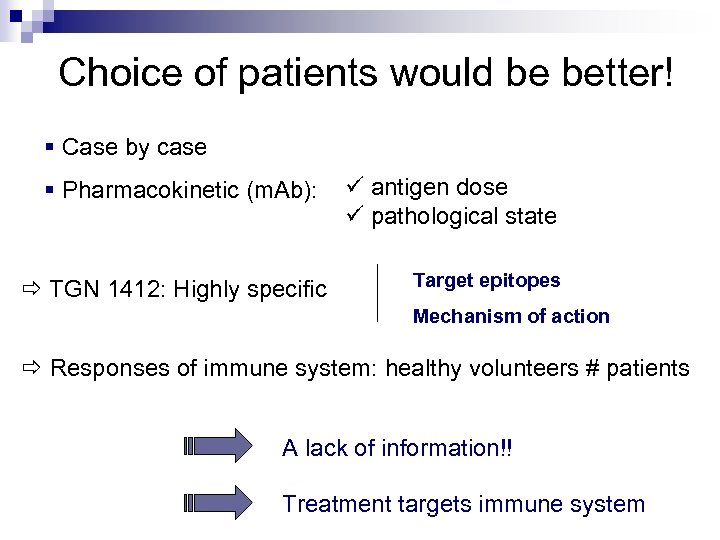
Choice of patients would be better! § Case by case § Pharmacokinetic (m. Ab): TGN 1412: Highly specific ü antigen dose ü pathological state Target epitopes Mechanism of action Responses of immune system: healthy volunteers # patients A lack of information!! Treatment targets immune system
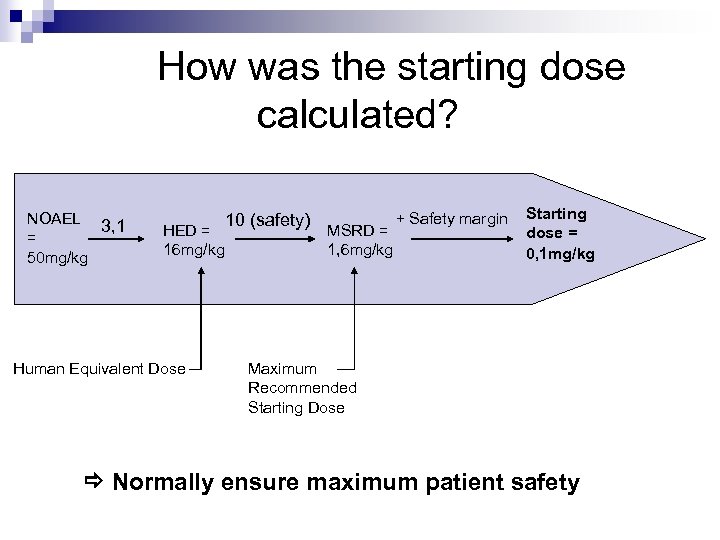
How was the starting dose calculated? NOAEL 3, 1 = 50 mg/kg HED = 16 mg/kg Human Equivalent Dose 10 (safety) MSRD = 1, 6 mg/kg + Safety margin Starting dose = 0, 1 mg/kg Maximum Recommended Starting Dose Normally ensure maximum patient safety
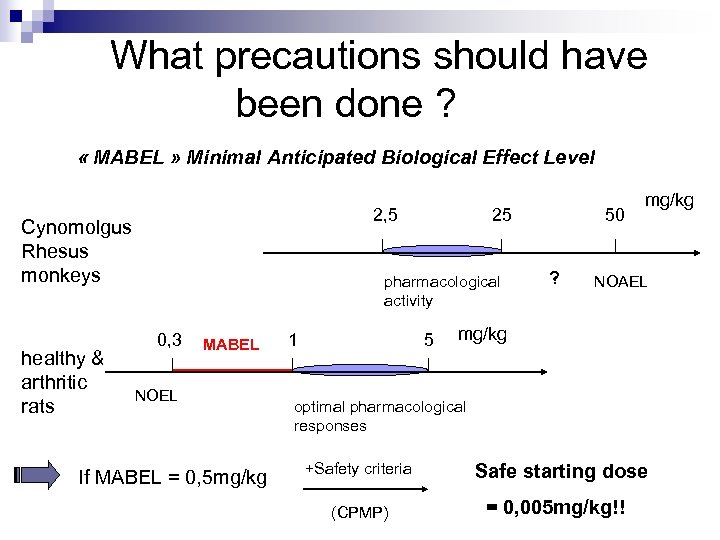
What precautions should have been done ? « MABEL » Minimal Anticipated Biological Effect Level 2, 5 Cynomolgus Rhesus monkeys healthy & arthritic rats 25 pharmacological activity 0, 3 MABEL NOEL If MABEL = 0, 5 mg/kg 1 5 50 ? mg/kg NOAEL mg/kg optimal pharmacological responses +Safety criteria Safe starting dose (CPMP) = 0, 005 mg/kg!!
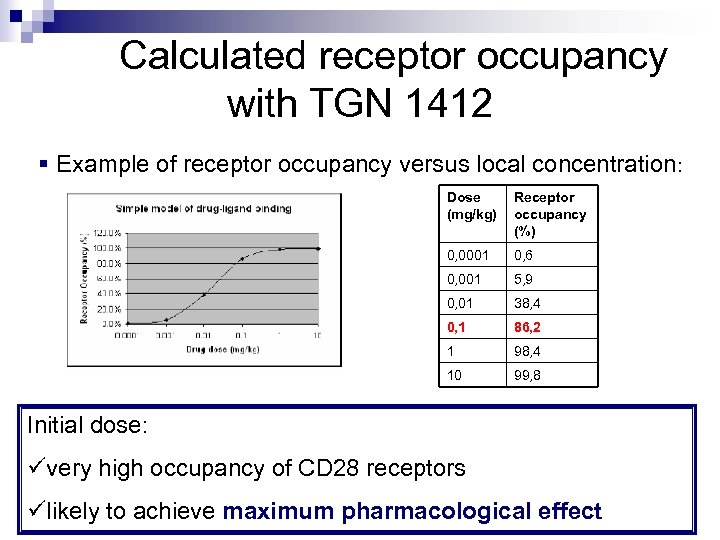
Calculated receptor occupancy with TGN 1412 § Example of receptor occupancy versus local concentration: Dose (mg/kg) Receptor occupancy (%) 0, 0001 0, 6 0, 001 5, 9 0, 01 38, 4 0, 1 86, 2 1 98, 4 10 99, 8 Initial dose: üvery high occupancy of CD 28 receptors ülikely to achieve maximum pharmacological effect

Part V : Clinical Development n Trial protocol n Adverse effects observed n Consequences n Failure in the protocol

Trial Protocol

Design of trial n n n n n Single-centre Double-blind Randomized Placebo-controlled 4 groups of 8 healthy young male volunteers planned Administration : single doses intravenously Dosing range : 0. 1, 0. 5, 2. 0 and 5. 0 mg/kg BW Dosing time : 8 to 10 a. m First day : 13 th March 2006

Volunteers n n n No notable medical history Informed consent £ 2000 fees + £ 30 per visit First group of eight volunteers : n 19 – 34 years old n Six received active agent n Two received placebo
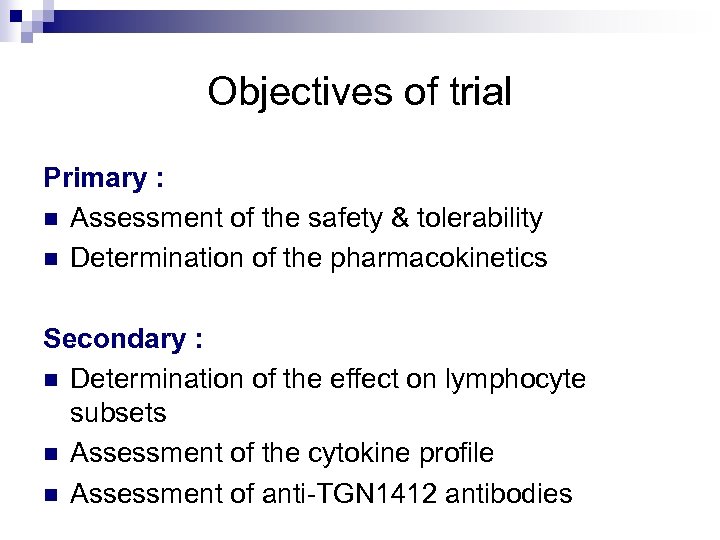
Objectives of trial Primary : n Assessment of the safety & tolerability n Determination of the pharmacokinetics Secondary : n Determination of the effect on lymphocyte subsets n Assessment of the cytokine profile n Assessment of anti-TGN 1412 antibodies

Approval § 27 th January 2006 : Authorization by the Medicines and Healthcare products Regulatory Agency (MHRA) § 14 th February 2006 : Favourable opinion by the Brent Medical Ethics Committee

Adverse Events observed
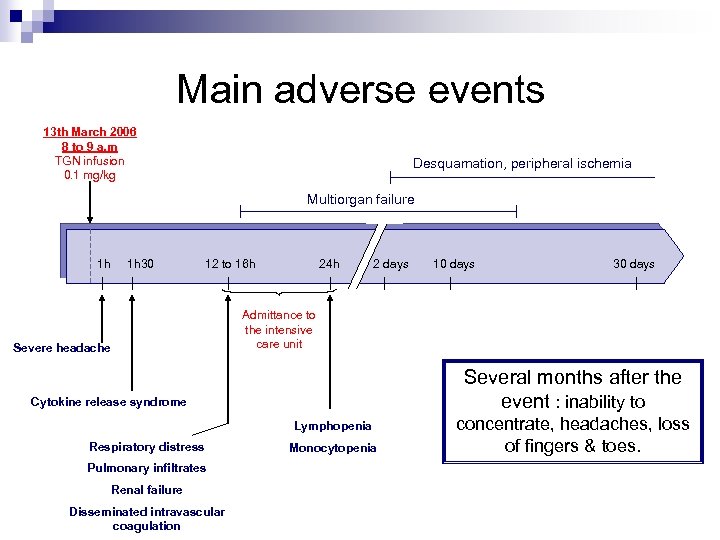
Main adverse events 13 th March 2006 8 to 9 a. m TGN infusion 0. 1 mg/kg Desquamation, peripheral ischemia Multiorgan failure 1 h 1 h 30 12 to 16 h Severe headache 24 h 2 days Several months after the event : inability to Lymphopenia Pulmonary infiltrates Renal failure Disseminated intravascular coagulation 30 days Admittance to the intensive care unit Cytokine release syndrome Respiratory distress 10 days Monocytopenia concentrate, headaches, loss of fingers & toes.
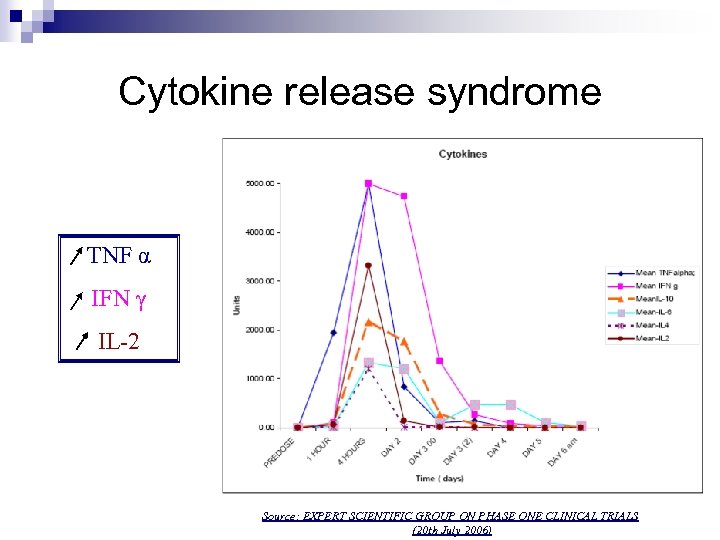
Cytokine release syndrome TNF α IFN γ IL-2 Source: EXPERT SCIENTIFIC GROUP ON PHASE ONE CLINICAL TRIALS (20 th July 2006)
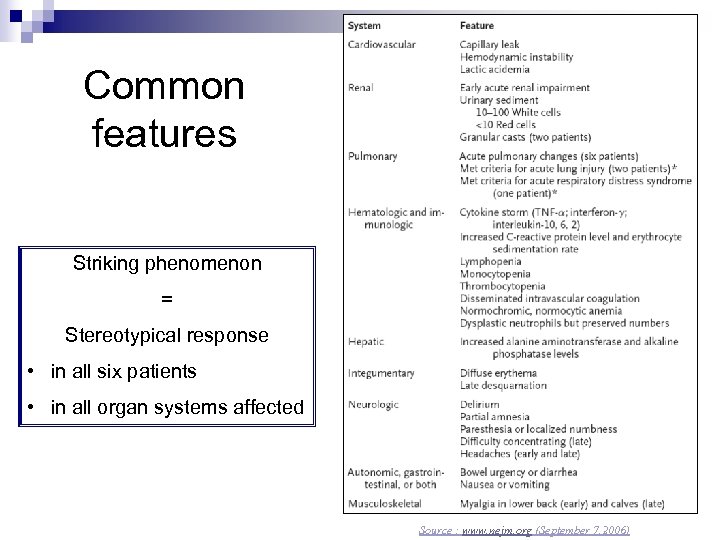
Common features Striking phenomenon = Stereotypical response • in all six patients • in all organ systems affected Source : www. nejm. org (September 7, 2006)
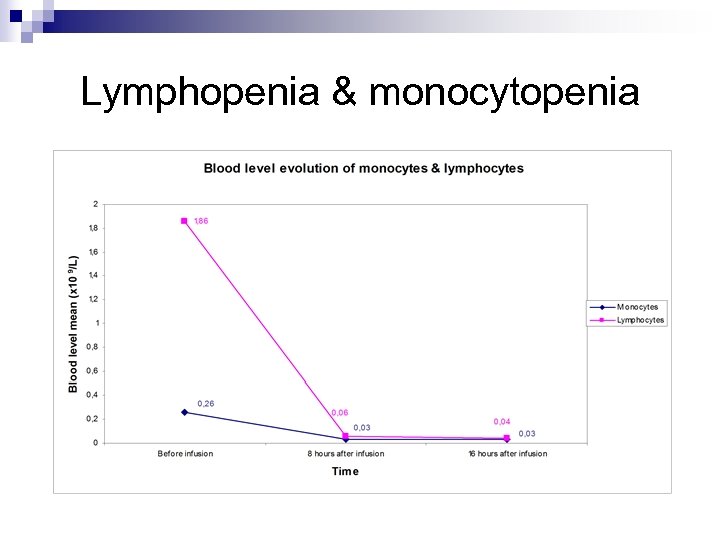
Lymphopenia & monocytopenia

Consequences
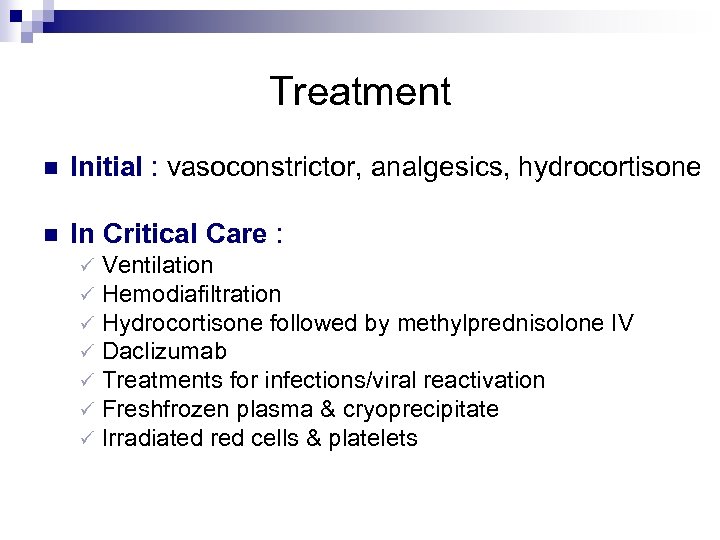
Treatment n Initial : vasoconstrictor, analgesics, hydrocortisone n In Critical Care : ü ü ü ü Ventilation Hemodiafiltration Hydrocortisone followed by methylprednisolone IV Daclizumab Treatments for infections/viral reactivation Freshfrozen plasma & cryoprecipitate Irradiated red cells & platelets

Measures taken by MHRA Incidents reported to the MHRA on the afternoon of 14 th March The MHRA : n n Immediately suspended the authorization Confirmed that the drug was not in use in any other trial Alerted international drug regulatory authorities Sent a team of inspectors to the unit in Northwick Park MHRA = The Medicines and Healthcare products Regulatory Agency

Failure in the Protocol
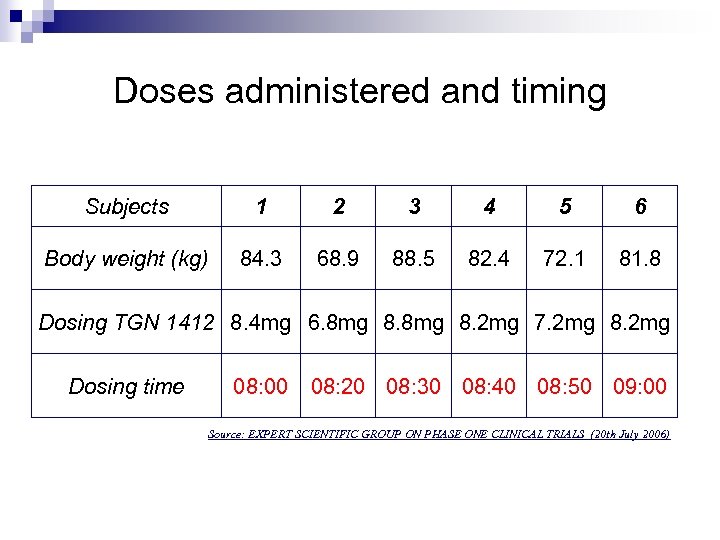
Doses administered and timing Subjects 1 2 3 4 5 6 Body weight (kg) 84. 3 68. 9 88. 5 82. 4 72. 1 81. 8 Dosing TGN 1412 8. 4 mg 6. 8 mg 8. 2 mg 7. 2 mg 8. 2 mg Dosing time 08: 00 08: 20 08: 30 08: 40 08: 50 09: 00 Source: EXPERT SCIENTIFIC GROUP ON PHASE ONE CLINICAL TRIALS (20 th July 2006)
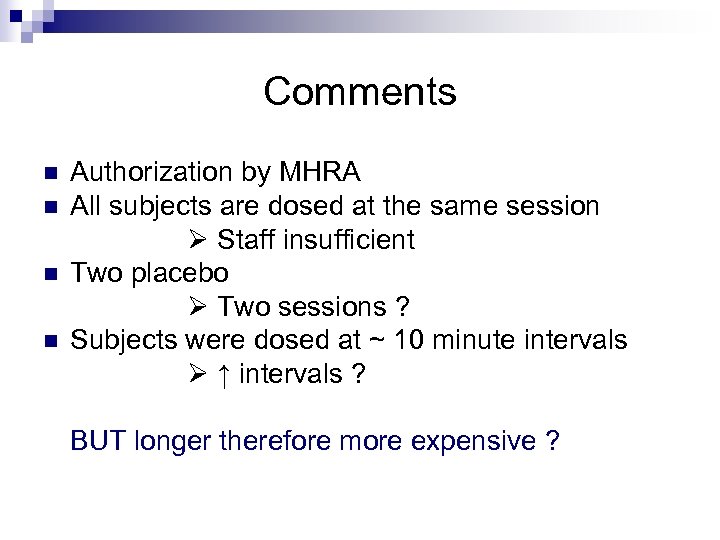
Comments n n Authorization by MHRA All subjects are dosed at the same session Staff insufficient Two placebo Two sessions ? Subjects were dosed at ~ 10 minute intervals ↑ intervals ? BUT longer therefore more expensive ?
Part VI : Protagonist Reaction n Boehringer Ingelheim n Parexel n Te. Genero

Boehringer Ingelheim n Sorry for the patients of trial n Contract manufacturer, the material for investigational use only Conformed to GMP No contamination n n

Parexel n Sorry for the patients of clinical trial n n To assist the pharmaceutical, biotech, and medical device industries Investigator No deficiency during inspection by MHRA Good practices and policies and procedures n MHRA confirmed the approved protocole n

Te Genero’s Team n n n Sorry for trial patients, their family « Develop innovative treatment to help millions of people » « Side effects could have been caused by one of our products » MHRA agreement for protocole used « No sign of risk from preclinical test »

The victims n Tom Edward: lucky escape from trial n. Myfanwy Marshall’s boyfriend §Ryan Flanagan: 21, a student from Highbury §Ryan Wilson: 20, the most seriously ill

• Heads and bodies swelled up • He was suffering from heart, liver and kidney The consequences failure, pneumonia and septicaemia. • Development gangrene Amputation parts on his fingers • Risk infections and cancer
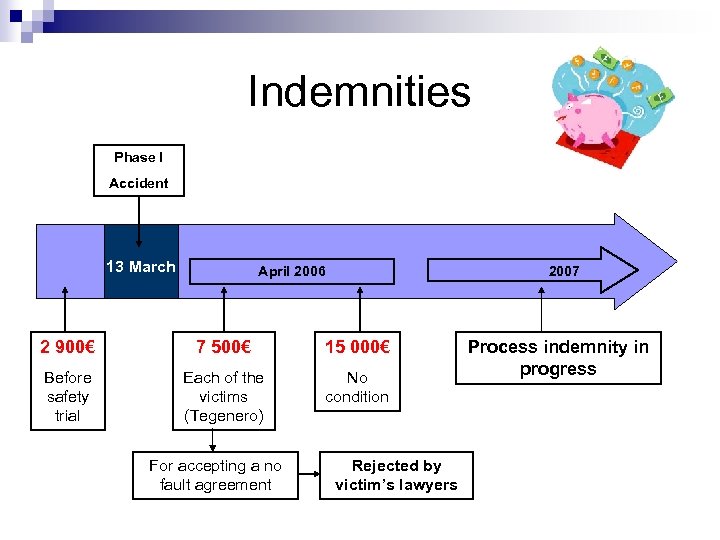
Indemnities Phase I Accident 13 March April 2006 2007 2 900€ 7 500€ 15 000€ Before safety trial Each of the victims (Tegenero) No condition For accepting a no fault agreement Rejected by victim’s lawyers Process indemnity in progress

Part VII : Discussion Solutions to find

ESG’S Objectives (20/12/06) To put in position new recommendations to OPTIMISE the SECURITY of Clinical Trials’Phase I

Recommendations § Characterisation of new agents’risks and Harmonisation between all authorities § Choice of preclinical and clinical models § Doses’schema
§ Wider approach for doses : the MABEL § Staff & Environment § Better communication for intra and inter- tests

§ Creation of the EAG § Time-Scale § Creation of Specialised Centres § Creation of Specialist Centres
Conclusion
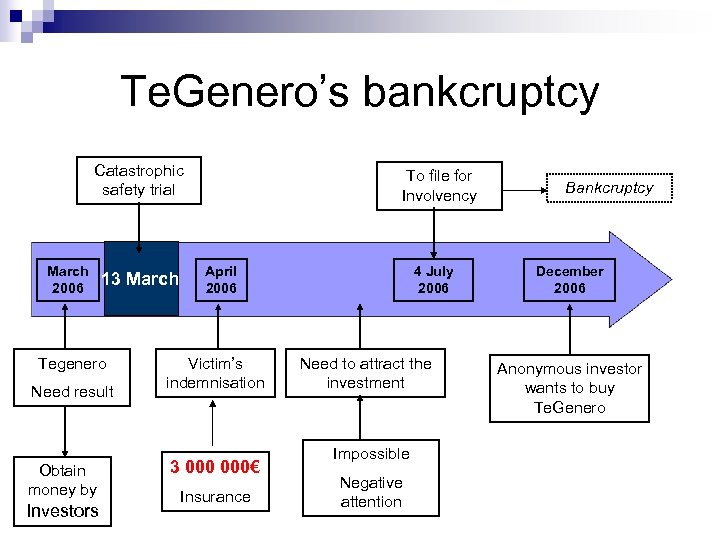
Te. Genero’s bankcruptcy Catastrophic safety trial March 2006 13 March Tegenero Need result Obtain money by Investors To file for Involvency April 2006 Victim’s indemnisation 3 000€ Insurance 4 July 2006 Need to attract the investment Impossible Negative attention Bankcruptcy December 2006 Anonymous investor wants to buy Te. Genero
CONCLUSION n Could this disaster have been avoided? Yes The culpables & sanctions In process n The lessons to remember: n Need changing and Hardening of the system More controls

Merci de votre attention

Powerpoint bonus
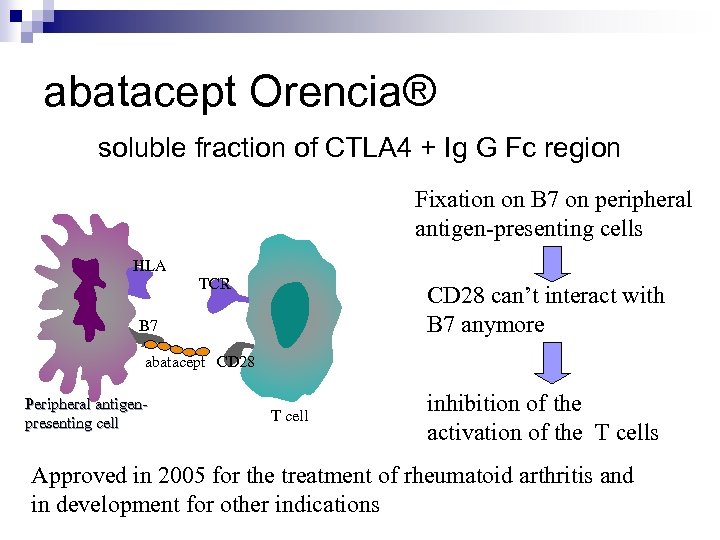
abatacept Orencia® soluble fraction of CTLA 4 + Ig G Fc region Fixation on B 7 on peripheral antigen-presenting cells HLA TCR CD 28 can’t interact with B 7 anymore B 7 abatacept CD 28 Peripheral antigenpresenting cell T cell inhibition of the activation of the T cells Approved in 2005 for the treatment of rheumatoid arthritis and in development for other indications

Adverse events: n n n Phase 4 trial: One year, randomized, multicenter, double-blind, placebo-controlled trial Serious infections were more frequent in the abatacept group than in the placebo group (2. 9% versus 1. 9%). Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo -controlled study. M. Weinblatt 1 *, B. Combe 2, A. Covucci 3, R. Aranda 3, J. C. Becker 3, E. Keystone 4
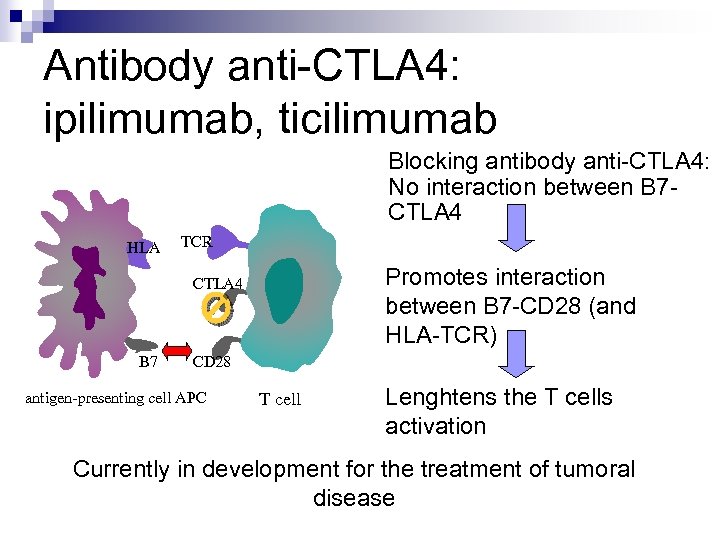
Antibody anti-CTLA 4: ipilimumab, ticilimumab Blocking antibody anti-CTLA 4: No interaction between B 7 CTLA 4 HLA TCR Promotes interaction between B 7 -CD 28 (and HLA-TCR) CTLA 4 B 7 CD 28 antigen-presenting cell APC T cell Lenghtens the T cells activation Currently in development for the treatment of tumoral disease
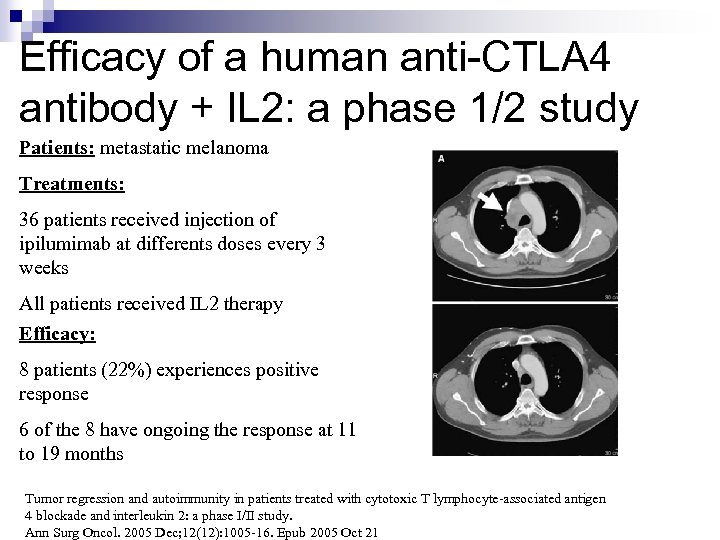
Efficacy of a human anti-CTLA 4 antibody + IL 2: a phase 1/2 study Patients: metastatic melanoma Treatments: 36 patients received injection of ipilumimab at differents doses every 3 weeks All patients received IL 2 therapy Efficacy: 8 patients (22%) experiences positive response 6 of the 8 have ongoing the response at 11 to 19 months Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005 Dec; 12(12): 1005 -16. Epub 2005 Oct 21
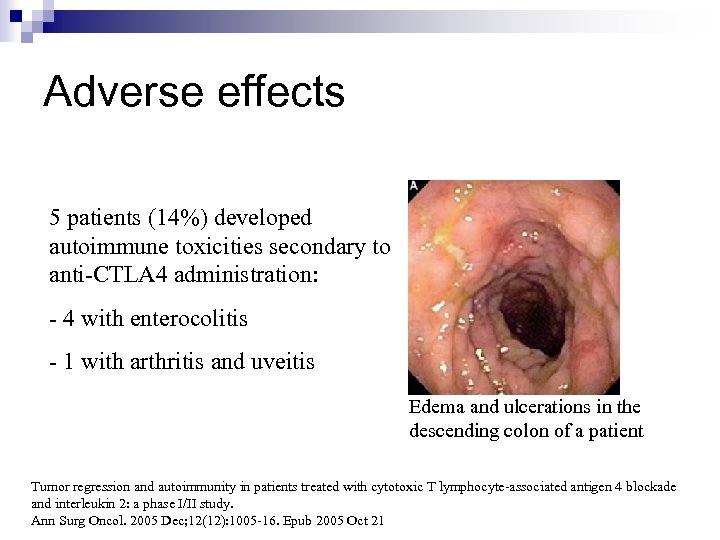
Adverse effects 5 patients (14%) developed autoimmune toxicities secondary to anti-CTLA 4 administration: - 4 with enterocolitis - 1 with arthritis and uveitis Edema and ulcerations in the descending colon of a patient Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005 Dec; 12(12): 1005 -16. Epub 2005 Oct 21
1743d7c01a5e1c3039e08ccec1098a96.ppt