8565627c45cc1c059a09e3b501ff0e9c.ppt
- Количество слайдов: 17
 FDA H 5 N 1 Update: Classification of H 5 N 1 Viruses and Development of Vaccine Reference Strains Nancy J. Cox, Ph. D. Director, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza Centers for Disease Control and Prevention
FDA H 5 N 1 Update: Classification of H 5 N 1 Viruses and Development of Vaccine Reference Strains Nancy J. Cox, Ph. D. Director, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza Centers for Disease Control and Prevention
 History of H 5 N 1 Currently 2 discrete lineages of H 5 HAs descended from the A/Gs/Guangdong/96 have infected humans Ø 1997 poultry outbreak in Hong Kong • 18 human cases, 6 deaths • Direct avian to human transmission: limited H-2 -H transmission Ø 2003 ongoing poultry outbreaks and spread • 2 human cases, 1 death in Hong Kong • 1 death in Beijing (from time of SARS) Ø End of 2003 - Today • 258 human cases, 154 deaths • Azerbaijan, Cambodia, China, Djbouti, Egypt, Indonesia, Iraq, Laos, Nigeria, Thailand, Turkey, Vietnam Ø
History of H 5 N 1 Currently 2 discrete lineages of H 5 HAs descended from the A/Gs/Guangdong/96 have infected humans Ø 1997 poultry outbreak in Hong Kong • 18 human cases, 6 deaths • Direct avian to human transmission: limited H-2 -H transmission Ø 2003 ongoing poultry outbreaks and spread • 2 human cases, 1 death in Hong Kong • 1 death in Beijing (from time of SARS) Ø End of 2003 - Today • 258 human cases, 154 deaths • Azerbaijan, Cambodia, China, Djbouti, Egypt, Indonesia, Iraq, Laos, Nigeria, Thailand, Turkey, Vietnam Ø
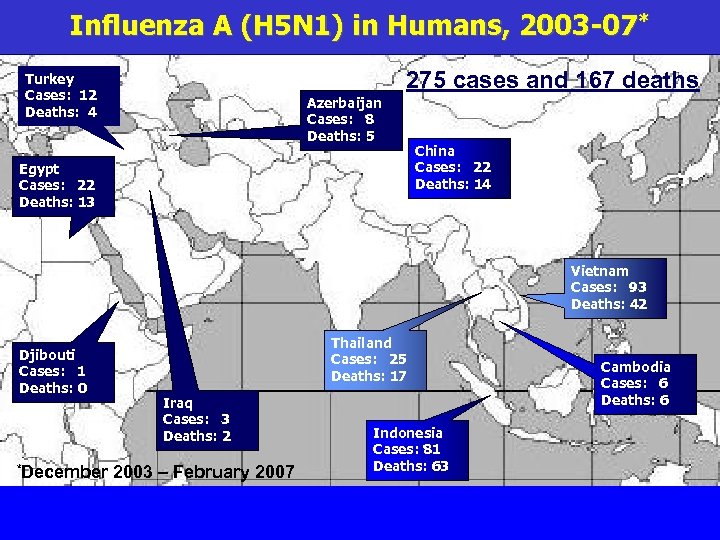 Influenza A (H 5 N 1) in Humans, 2003 -07* 275 cases and 167 deaths Turkey Cases: 12 Deaths: 4 Azerbaijan Cases: 8 Deaths: 5 Egypt Cases: 22 Deaths: 13 China Cases: 22 Deaths: 14 Vietnam Cases: 93 Deaths: 42 Djibouti Cases: 1 Deaths: 0 *December Thailand Cases: 25 Deaths: 17 Iraq Cases: 3 Deaths: 2 2003 – February 2007 Indonesia Cases: 81 Deaths: 63 Cambodia Cases: 6 Deaths: 6
Influenza A (H 5 N 1) in Humans, 2003 -07* 275 cases and 167 deaths Turkey Cases: 12 Deaths: 4 Azerbaijan Cases: 8 Deaths: 5 Egypt Cases: 22 Deaths: 13 China Cases: 22 Deaths: 14 Vietnam Cases: 93 Deaths: 42 Djibouti Cases: 1 Deaths: 0 *December Thailand Cases: 25 Deaths: 17 Iraq Cases: 3 Deaths: 2 2003 – February 2007 Indonesia Cases: 81 Deaths: 63 Cambodia Cases: 6 Deaths: 6
 WHO H 5 N 1 Vaccine Development: Principles and Practices Ø Development of H 5 N 1 vaccines is one component of WHO’s overall strategy for pandemic preparedness Ø WHO’s 4 Collaborating Centers for Influenza Reference and Research along with 4 additional H 5 Reference Laboratories share H 5 N 1 antigenic and genetic data frequently Ø WHO convenes periodic teleconferences of H 5 Reference Laboratory representatives to discuss data and apportion tasks required for vaccine candidate reference virus production Ø Development of appropriate H 5 N 1 vaccines requires integration of antigenic, genetic and epidemiologic data from human and veterinary health sectors Ø H 5 N 1 vaccine candidate reference viruses are chosen on the basis of antigenic and genetic properties and epidemiologic information
WHO H 5 N 1 Vaccine Development: Principles and Practices Ø Development of H 5 N 1 vaccines is one component of WHO’s overall strategy for pandemic preparedness Ø WHO’s 4 Collaborating Centers for Influenza Reference and Research along with 4 additional H 5 Reference Laboratories share H 5 N 1 antigenic and genetic data frequently Ø WHO convenes periodic teleconferences of H 5 Reference Laboratory representatives to discuss data and apportion tasks required for vaccine candidate reference virus production Ø Development of appropriate H 5 N 1 vaccines requires integration of antigenic, genetic and epidemiologic data from human and veterinary health sectors Ø H 5 N 1 vaccine candidate reference viruses are chosen on the basis of antigenic and genetic properties and epidemiologic information
 Circulation of H 5 N 1 Viruses Ø HA sequences of the majority of H 5 N 1 viruses in avian species segregate into 2 distinct phylogenetic clades Ø Clade 1 viruses circulated in Cambodia, Thailand Viet Nam and caused human infections during 2004 and 2005 -and in Thailand in 2006 Ø Clade 2 viruses circulated in birds in China and Indonesia during 2003– 2004 and spread westward during 2005 and 2006 to the Middle East, Europe and Africa Ø Clade 2 viruses have caused the majority of human Infections since late 2005 Ø Multiple sub-clades of clade 2 have been distinguished, three of which (subclades 2. 1, 2. 2 and 2. 3) have been responsible for human cases and differ in geographical distribution
Circulation of H 5 N 1 Viruses Ø HA sequences of the majority of H 5 N 1 viruses in avian species segregate into 2 distinct phylogenetic clades Ø Clade 1 viruses circulated in Cambodia, Thailand Viet Nam and caused human infections during 2004 and 2005 -and in Thailand in 2006 Ø Clade 2 viruses circulated in birds in China and Indonesia during 2003– 2004 and spread westward during 2005 and 2006 to the Middle East, Europe and Africa Ø Clade 2 viruses have caused the majority of human Infections since late 2005 Ø Multiple sub-clades of clade 2 have been distinguished, three of which (subclades 2. 1, 2. 2 and 2. 3) have been responsible for human cases and differ in geographical distribution
 H 5 N 1 Current Status (Late 2005 to the Present) Ø The majority of H 5 N 1 viruses detected in avian species in Africa, Asia and Europe and associated with sporadic human infections are in Clade 2 Ø Clade 2. 1 viruses circulated in poultry and caused human infections in Indonesia; clade 2. 2 viruses caused outbreaks in birds in Africa, Asia and Europe and were most recently associated with human infections in Egypt and Nigeria; viruses in clade 2. 3 caused poultry outbreaks and human cases in China Ø Viruses outside this classification nomenclature were isolated from domestic poultry in Asia – two emerging clades are represented by A/goose/Guiyang/337/06 and A/chicken/Shanxi/2/2006 viruses
H 5 N 1 Current Status (Late 2005 to the Present) Ø The majority of H 5 N 1 viruses detected in avian species in Africa, Asia and Europe and associated with sporadic human infections are in Clade 2 Ø Clade 2. 1 viruses circulated in poultry and caused human infections in Indonesia; clade 2. 2 viruses caused outbreaks in birds in Africa, Asia and Europe and were most recently associated with human infections in Egypt and Nigeria; viruses in clade 2. 3 caused poultry outbreaks and human cases in China Ø Viruses outside this classification nomenclature were isolated from domestic poultry in Asia – two emerging clades are represented by A/goose/Guiyang/337/06 and A/chicken/Shanxi/2/2006 viruses
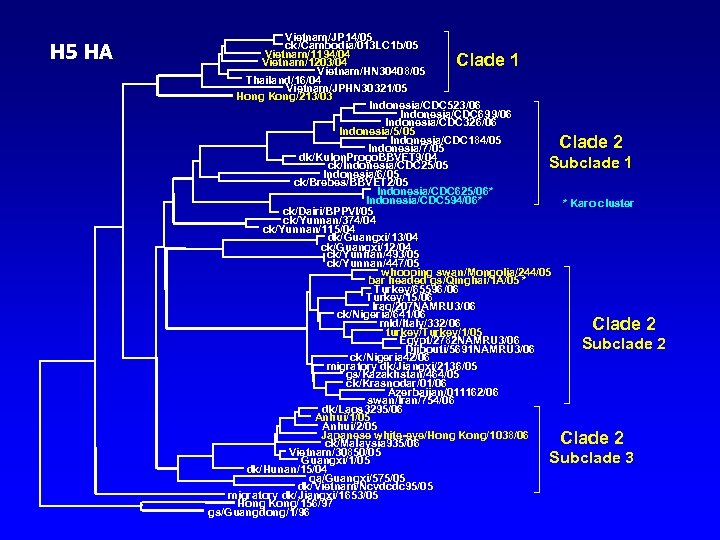 H 5 HA Vietnam/JP 14/05 ck/Cambodia/013 LC 1 b/05 Vietnam/1194/04 Vietnam/1203/04 Vietnam/HN 30408/05 Thailand/16/04 Vietnam/JPHN 30321/05 Hong Kong/213/03 Indonesia/CDC 523/06 Indonesia/CDC 699/06 Indonesia/CDC 326/06 Indonesia/5/05 Indonesia/CDC 184/05 Indonesia/7/05 dk/Kulon. Progo. BBVET 9/04 Subclade 1 ck/Indonesia/CDC 25/05 Indonesia/6/05 ck/Brebes/BBVET 2/05 Indonesia/CDC 625/06* Indonesia/CDC 594/06* * Karo cluster ck/Dairi/BPPVI/05 ck/Yunnan/374/04 ck/Yunnan/115/04 dk/Guangxi/13/04 ck/Guangxi/12/04 ck/Yunnan/493/05 ck/Yunnan/447/05 whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * Turkey/65596/06 Turkey/15/06 Iraq/207 NAMRU 3/06 ck/Nigeria/641/06 mld/Italy/332/06 turkey/Turkey/1/05 Egypt/2782 NAMRU 3/06 Subclade Djibouti/5691 NAMRU 3/06 ck/Nigeria 42/06 migratory dk/Jiangxi/2136/05 gs/Kazakhstan/464/05 ck/Krasnodar/01/06 Azerbaijan/011162/06 swan/Iran/754/06 dk/Laos 3295/06 Anhui/1/05 Anhui/2/05 Japanese white-eye/Hong Kong/1038/06 ck/Malaysia 935/06 Vietnam/30850/05 Subclade 3 Guangxi/1/05 dk/Hunan/15/04 qa/Guangxi/575/05 dk/Vietnam/Ncvdcdc 95/05 migratory dk/Jiangxi/1653/05 Hong Kong/156/97 gs/Guangdong/1/96 Clade 1 Clade 2 2
H 5 HA Vietnam/JP 14/05 ck/Cambodia/013 LC 1 b/05 Vietnam/1194/04 Vietnam/1203/04 Vietnam/HN 30408/05 Thailand/16/04 Vietnam/JPHN 30321/05 Hong Kong/213/03 Indonesia/CDC 523/06 Indonesia/CDC 699/06 Indonesia/CDC 326/06 Indonesia/5/05 Indonesia/CDC 184/05 Indonesia/7/05 dk/Kulon. Progo. BBVET 9/04 Subclade 1 ck/Indonesia/CDC 25/05 Indonesia/6/05 ck/Brebes/BBVET 2/05 Indonesia/CDC 625/06* Indonesia/CDC 594/06* * Karo cluster ck/Dairi/BPPVI/05 ck/Yunnan/374/04 ck/Yunnan/115/04 dk/Guangxi/13/04 ck/Guangxi/12/04 ck/Yunnan/493/05 ck/Yunnan/447/05 whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * Turkey/65596/06 Turkey/15/06 Iraq/207 NAMRU 3/06 ck/Nigeria/641/06 mld/Italy/332/06 turkey/Turkey/1/05 Egypt/2782 NAMRU 3/06 Subclade Djibouti/5691 NAMRU 3/06 ck/Nigeria 42/06 migratory dk/Jiangxi/2136/05 gs/Kazakhstan/464/05 ck/Krasnodar/01/06 Azerbaijan/011162/06 swan/Iran/754/06 dk/Laos 3295/06 Anhui/1/05 Anhui/2/05 Japanese white-eye/Hong Kong/1038/06 ck/Malaysia 935/06 Vietnam/30850/05 Subclade 3 Guangxi/1/05 dk/Hunan/15/04 qa/Guangxi/575/05 dk/Vietnam/Ncvdcdc 95/05 migratory dk/Jiangxi/1653/05 Hong Kong/156/97 gs/Guangdong/1/96 Clade 1 Clade 2 2
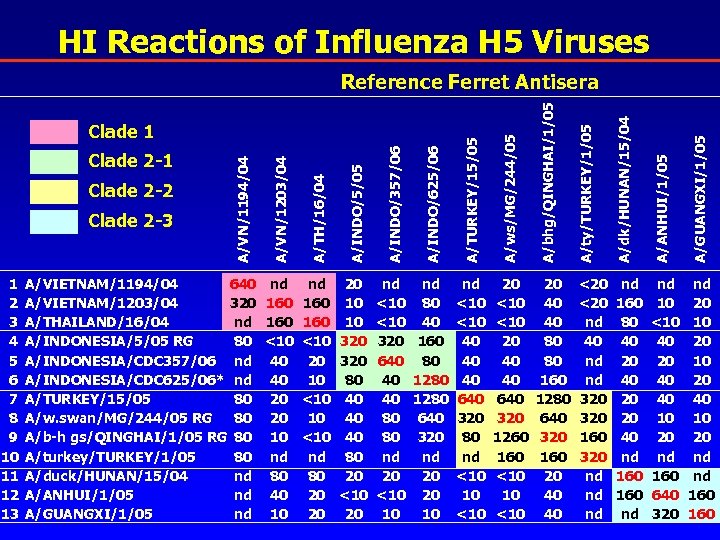 HI Reactions of Influenza H 5 Viruses nd <10 320 640 40 40 80 80 nd 20 <10 10 nd 80 40 160 80 1280 640 320 nd 20 20 10 nd <10 40 40 40 640 320 80 nd <10 10 <10 20 40 40 640 320 1260 160 <10 10 <10 A/GUANGXI/1/05 20 10 10 320 80 40 40 40 80 20 <10 20 A/ANHUI/1/05 A/ws/MG/244/05 nd 160 <10 20 10 <10 nd 80 20 20 A/dk/HUNAN/15/04 A/TURKEY/15/05 nd 160 <10 40 40 20 20 10 nd 80 40 10 A/ty/TURKEY/1/05 A/INDO/625/06 A/VIETNAM/1194/04 640 A/VIETNAM/1203/04 320 nd A/THAILAND/16/04 80 A/INDONESIA/5/05 RG A/INDONESIA/CDC 357/06 nd A/INDONESIA/CDC 625/06* nd A/TURKEY/15/05 80 A/w. swan/MG/244/05 RG 80 A/b-h gs/QINGHAI/1/05 RG 80 A/turkey/TURKEY/1/05 80 A/duck/HUNAN/15/04 nd A/ANHUI/1/05 nd A/GUANGXI/1/05 nd A/INDO/357/06 1 2 3 4 5 6 7 8 9 10 11 12 13 A/INDO/5/05 Clade 2 -3 A/TH/16/04 Clade 2 -2 A/VN/1203/04 Clade 2 -1 A/VN/1194/04 Clade 1 A/bhg/QINGHAI/1/05 Reference Ferret Antisera 20 40 40 80 80 160 1280 640 320 160 20 40 40 <20 nd 40 nd nd 320 160 320 nd nd 160 80 40 20 20 40 nd 160 nd nd 10 <10 40 20 40 40 10 20 nd 160 640 320 nd 20 10 20 40 10 20 nd nd 160
HI Reactions of Influenza H 5 Viruses nd <10 320 640 40 40 80 80 nd 20 <10 10 nd 80 40 160 80 1280 640 320 nd 20 20 10 nd <10 40 40 40 640 320 80 nd <10 10 <10 20 40 40 640 320 1260 160 <10 10 <10 A/GUANGXI/1/05 20 10 10 320 80 40 40 40 80 20 <10 20 A/ANHUI/1/05 A/ws/MG/244/05 nd 160 <10 20 10 <10 nd 80 20 20 A/dk/HUNAN/15/04 A/TURKEY/15/05 nd 160 <10 40 40 20 20 10 nd 80 40 10 A/ty/TURKEY/1/05 A/INDO/625/06 A/VIETNAM/1194/04 640 A/VIETNAM/1203/04 320 nd A/THAILAND/16/04 80 A/INDONESIA/5/05 RG A/INDONESIA/CDC 357/06 nd A/INDONESIA/CDC 625/06* nd A/TURKEY/15/05 80 A/w. swan/MG/244/05 RG 80 A/b-h gs/QINGHAI/1/05 RG 80 A/turkey/TURKEY/1/05 80 A/duck/HUNAN/15/04 nd A/ANHUI/1/05 nd A/GUANGXI/1/05 nd A/INDO/357/06 1 2 3 4 5 6 7 8 9 10 11 12 13 A/INDO/5/05 Clade 2 -3 A/TH/16/04 Clade 2 -2 A/VN/1203/04 Clade 2 -1 A/VN/1194/04 Clade 1 A/bhg/QINGHAI/1/05 Reference Ferret Antisera 20 40 40 80 80 160 1280 640 320 160 20 40 40 <20 nd 40 nd nd 320 160 320 nd nd 160 80 40 20 20 40 nd 160 nd nd 10 <10 40 20 40 40 10 20 nd 160 640 320 nd 20 10 20 40 10 20 nd nd 160
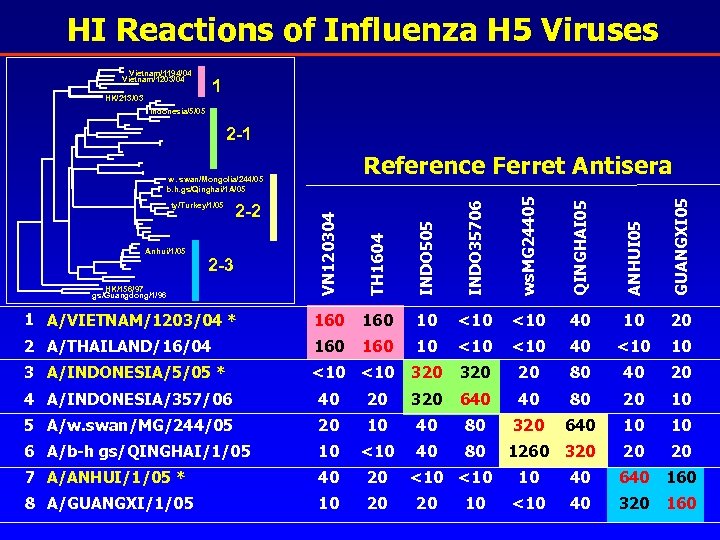 HI Reactions of Influenza H 5 Viruses Vietnam/1194/04 Vietnam/1203/04 1 HK/213/03 Indonesia/5/05 2 -1 Reference Ferret Antisera HK/156/97 gs/Guangdong/1/96 VN 120304 TH 1604 INDO 505 INDO 35706 ws. MG 24405 QINGHAI 05 ANHUI 05 GUANGXI 05 w. swan/Mongolia/244/05 b. h. gs/Qinghai/1 A/05 1 A/VIETNAM/1203/04 * 160 10 <10 40 10 20 2 A/THAILAND/16/04 160 10 <10 40 <10 10 3 A/INDONESIA/5/05 * <10 320 20 80 40 20 ty/Turkey/1/05 Anhui/1/05 2 -2 2 -3 4 A/INDONESIA/357/06 40 20 320 640 40 80 20 10 5 A/w. swan/MG/244/05 20 10 40 80 320 640 10 10 6 A/b-h gs/QINGHAI/1/05 10 <10 40 80 1260 320 20 20 7 A/ANHUI/1/05 * 40 20 8 A/GUANGXI/1/05 10 20 <10 20 10 10 40 640 160 <10 40 320 160
HI Reactions of Influenza H 5 Viruses Vietnam/1194/04 Vietnam/1203/04 1 HK/213/03 Indonesia/5/05 2 -1 Reference Ferret Antisera HK/156/97 gs/Guangdong/1/96 VN 120304 TH 1604 INDO 505 INDO 35706 ws. MG 24405 QINGHAI 05 ANHUI 05 GUANGXI 05 w. swan/Mongolia/244/05 b. h. gs/Qinghai/1 A/05 1 A/VIETNAM/1203/04 * 160 10 <10 40 10 20 2 A/THAILAND/16/04 160 10 <10 40 <10 10 3 A/INDONESIA/5/05 * <10 320 20 80 40 20 ty/Turkey/1/05 Anhui/1/05 2 -2 2 -3 4 A/INDONESIA/357/06 40 20 320 640 40 80 20 10 5 A/w. swan/MG/244/05 20 10 40 80 320 640 10 10 6 A/b-h gs/QINGHAI/1/05 10 <10 40 80 1260 320 20 20 7 A/ANHUI/1/05 * 40 20 8 A/GUANGXI/1/05 10 20 <10 20 10 10 40 640 160 <10 40 320 160
 H 5 N 1 Virus Resistance to M 2 Channel Blockers Clade 1 Resistant Asn-31 Vietnam/1194/04 Vietnam/1203/04 Hong Kong/213/03 Indonesia/5/05 Clade 2 -1 ~80% resistant Asn-31 or Ala-27 whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * turkey/Turkey/1/05 Anhui/1/05 Hong Kong/156/97 gs/Guangdong/1/96 Clade 2 -2 Sensitive Ser-31 Clade 2 -3 Sensitive Ser-31
H 5 N 1 Virus Resistance to M 2 Channel Blockers Clade 1 Resistant Asn-31 Vietnam/1194/04 Vietnam/1203/04 Hong Kong/213/03 Indonesia/5/05 Clade 2 -1 ~80% resistant Asn-31 or Ala-27 whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * turkey/Turkey/1/05 Anhui/1/05 Hong Kong/156/97 gs/Guangdong/1/96 Clade 2 -2 Sensitive Ser-31 Clade 2 -3 Sensitive Ser-31
 H 5 N 1 Resistance to Neuraminidase Inhibitors Vietnam/1194/04 Vietnam/1203/04 Hong Kong/213/03 Clade 1 Sensitive Several resistant mutants isolated from treated patients Indonesia/5/05 Clade 2 -1 Sensitive whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * Clade 2 -2 turkey/Turkey/1/05 Anhui/1/05 Hong Kong/156/97 gs/Guangdong/1/96 Generally Sensitive - but moderately resistant viruses detected Clade 2 -3 Sensitive
H 5 N 1 Resistance to Neuraminidase Inhibitors Vietnam/1194/04 Vietnam/1203/04 Hong Kong/213/03 Clade 1 Sensitive Several resistant mutants isolated from treated patients Indonesia/5/05 Clade 2 -1 Sensitive whooping swan/Mongolia/244/05 bar headed gs/Qinghai/1 A/05 * Clade 2 -2 turkey/Turkey/1/05 Anhui/1/05 Hong Kong/156/97 gs/Guangdong/1/96 Generally Sensitive - but moderately resistant viruses detected Clade 2 -3 Sensitive
 HI REACTIONS OF INFLUENZA H 5 VIRUSES
HI REACTIONS OF INFLUENZA H 5 VIRUSES
 Evolution of the H 5 N 1 Hemagglutinin Gene Candidate Vaccine Reference Viruses ck/Shannxi/62/04 ck/Yunnan/447/05|ck/Yunnan/493/05 dk/Guangxi/13/04 ck/Yunnan/30/04 ck/Yunnan/115/04 ck/Yunnan/374/04 Indonesia/7/05 Indonesia/5/05 Indonesia/CDC 742/06 Indonesia/CDC 940/06 Indonesia/CDC 1031/07 Clade 2. 1 Indonesia/CDC 1047/07 Indonesia/CDC 887/06 Indonesia/CDC 938/06 Indonesia/CDC 1032/07 Indonesia/CDC 1046/07 Barhdgs/Qinghai/1 A/05 ck/Liaoning/23/05 Barhdgs/Qinghai/12/05 ck/Krasnodar/01/06 Azerbaijan/001161/06 swan/Iran/754/06 Turkey/15/06 Iraq/207 NAMRU-3/06 Clade 2. 2 ck/Nigeria/641/06 whswan/Mongolia/244/05 tky/Turkey/1/05 Egypt/14724 NAMRU-3/06 Djibouti/5691 NAMRU-3/06 dk/Egypt/22533/06 Egypt/0636 NAMRU-3/07 egret/Egypt/1162 NAMRU-3/06 dk/Hunan/15/04 scalybreasted. Munia/Hong. Kong/45/07 Japanese. White. Eye/Hong. Kong/1038/06 Anhui/1/05 dk/Laos/3295/06 ck/Malaysia/935/06 Clade 2. 3 common magpie/Hong Kong/645/06 Guangxi/1/05 House Crow/Hong Kong/719/07 Japanese. White. Eye/Hong Kong/737/07 White. Backed. Munia/Hong. Kong/828/07 Thailand/676/05 Vietnam/JP 14/05 ck/Cambodia/013 LC 1 b/05 Vietnam/JPHN 30321/05 Clade 1 Vietnam/1203/04 Vietnam/1194/04 Vietnam/HN 30408/05 Thailand/16/04 Hong Kong/213/03 migdk/Jiangxi/1653/05 ck/Hunan/41/04 ck/Hunan/2292/06 New subgroup ck/Shanxi/2/06 ck/Myanmar/06010011 B/06 ck/Yunnan/71/05 ck/Guiyang/237/06 gs/Guiyang/1325/06 dk/Guiyang/50406 New subgroup gs/Fujian/bb/03 gs/Vietnam/GZ-3/05 Hong Kong/156/97 gs/Guangdong/1/96
Evolution of the H 5 N 1 Hemagglutinin Gene Candidate Vaccine Reference Viruses ck/Shannxi/62/04 ck/Yunnan/447/05|ck/Yunnan/493/05 dk/Guangxi/13/04 ck/Yunnan/30/04 ck/Yunnan/115/04 ck/Yunnan/374/04 Indonesia/7/05 Indonesia/5/05 Indonesia/CDC 742/06 Indonesia/CDC 940/06 Indonesia/CDC 1031/07 Clade 2. 1 Indonesia/CDC 1047/07 Indonesia/CDC 887/06 Indonesia/CDC 938/06 Indonesia/CDC 1032/07 Indonesia/CDC 1046/07 Barhdgs/Qinghai/1 A/05 ck/Liaoning/23/05 Barhdgs/Qinghai/12/05 ck/Krasnodar/01/06 Azerbaijan/001161/06 swan/Iran/754/06 Turkey/15/06 Iraq/207 NAMRU-3/06 Clade 2. 2 ck/Nigeria/641/06 whswan/Mongolia/244/05 tky/Turkey/1/05 Egypt/14724 NAMRU-3/06 Djibouti/5691 NAMRU-3/06 dk/Egypt/22533/06 Egypt/0636 NAMRU-3/07 egret/Egypt/1162 NAMRU-3/06 dk/Hunan/15/04 scalybreasted. Munia/Hong. Kong/45/07 Japanese. White. Eye/Hong. Kong/1038/06 Anhui/1/05 dk/Laos/3295/06 ck/Malaysia/935/06 Clade 2. 3 common magpie/Hong Kong/645/06 Guangxi/1/05 House Crow/Hong Kong/719/07 Japanese. White. Eye/Hong Kong/737/07 White. Backed. Munia/Hong. Kong/828/07 Thailand/676/05 Vietnam/JP 14/05 ck/Cambodia/013 LC 1 b/05 Vietnam/JPHN 30321/05 Clade 1 Vietnam/1203/04 Vietnam/1194/04 Vietnam/HN 30408/05 Thailand/16/04 Hong Kong/213/03 migdk/Jiangxi/1653/05 ck/Hunan/41/04 ck/Hunan/2292/06 New subgroup ck/Shanxi/2/06 ck/Myanmar/06010011 B/06 ck/Yunnan/71/05 ck/Guiyang/237/06 gs/Guiyang/1325/06 dk/Guiyang/50406 New subgroup gs/Fujian/bb/03 gs/Vietnam/GZ-3/05 Hong Kong/156/97 gs/Guangdong/1/96
 Conclusions Ø H 5 N 1 viruses remain a pandemic threat but have not yet developed to the ability to be transmitted efficiently from person-to-person Ø It is not able to predict which if any of the H 5 N 1 antigenic/genetic variants might acquire the ability to be transmitted efficiently Ø Distinct geographical distribution of H 5 N 1 genetic and antigenic variants have been identified Ø In this instance, specific WHO pre-pandemic vaccine recommendations are not appropriate because it is not possible to predict which of the viruses in the distinct antigenic/genetic groups might acquire the ability to become efficiently transmissible
Conclusions Ø H 5 N 1 viruses remain a pandemic threat but have not yet developed to the ability to be transmitted efficiently from person-to-person Ø It is not able to predict which if any of the H 5 N 1 antigenic/genetic variants might acquire the ability to be transmitted efficiently Ø Distinct geographical distribution of H 5 N 1 genetic and antigenic variants have been identified Ø In this instance, specific WHO pre-pandemic vaccine recommendations are not appropriate because it is not possible to predict which of the viruses in the distinct antigenic/genetic groups might acquire the ability to become efficiently transmissible
 Availability of Vaccine Viruses and Reagents Ø rg A/Viet Nam/1203/2004 (Clade 1) – available from St. Jude CRH • Antigen and sheep serum available from CBER Ø rg A/Viet Nam/1194/2004 (Clade 1) – available from NIBSC • Antigen and sheep serum available from NIBSC Ø rg A/Indonesia/05/2005 (Clade 2. 1) – available from CDC • Antigen and sheep serum - available soon from CBER Ø rg A/Turkey/turkey/1/2005 (Clade 2. 2) – available from NIBSC • Antigen and sheep serum available from NIBSC Ø rg A/BHG/Qinghai Lake/1 A/05 (Clade 2. 2) – available from St. Jude • Reagents not yet in production Ø rg A/whooper swan/Mongolia/244/2005 (Clade 2. 2) – available for research • Reagents not in production Ø rg A/Anhui/1/05 (Clade 2. 3) – available from CDC • Reagents not yet in production
Availability of Vaccine Viruses and Reagents Ø rg A/Viet Nam/1203/2004 (Clade 1) – available from St. Jude CRH • Antigen and sheep serum available from CBER Ø rg A/Viet Nam/1194/2004 (Clade 1) – available from NIBSC • Antigen and sheep serum available from NIBSC Ø rg A/Indonesia/05/2005 (Clade 2. 1) – available from CDC • Antigen and sheep serum - available soon from CBER Ø rg A/Turkey/turkey/1/2005 (Clade 2. 2) – available from NIBSC • Antigen and sheep serum available from NIBSC Ø rg A/BHG/Qinghai Lake/1 A/05 (Clade 2. 2) – available from St. Jude • Reagents not yet in production Ø rg A/whooper swan/Mongolia/244/2005 (Clade 2. 2) – available for research • Reagents not in production Ø rg A/Anhui/1/05 (Clade 2. 3) – available from CDC • Reagents not yet in production
 Acknowledgements Ø Ø Ø Ø WHO National Influenza Centers WHO Collaborating Centers and Regulatory Authorities in London, Tokyo, Melbourne, Canberra, and Memphis WHO H 5 Reference Laboratories WHO Regional Offices WHO Headquarters in Geneva Many colleagues in Azerbaijan, Cambodia, China, Djibouti, Egypt, Indonesia, Iraq, Nigeria, Thailand, Turkey, Vietnam and other affected countries Ministries of Agriculture in affected countries and FAO and OIE members Members of the Influenza Division, CDC
Acknowledgements Ø Ø Ø Ø WHO National Influenza Centers WHO Collaborating Centers and Regulatory Authorities in London, Tokyo, Melbourne, Canberra, and Memphis WHO H 5 Reference Laboratories WHO Regional Offices WHO Headquarters in Geneva Many colleagues in Azerbaijan, Cambodia, China, Djibouti, Egypt, Indonesia, Iraq, Nigeria, Thailand, Turkey, Vietnam and other affected countries Ministries of Agriculture in affected countries and FAO and OIE members Members of the Influenza Division, CDC
 Thank You
Thank You


