db540ad18830b1b4c1ccf8d68d44157a.ppt
- Количество слайдов: 25
FAT HOPE : BENEFITS FROM HOUSEHOLD WASTES Page • Current Situation 2 • Proposed Changes 6 • Chemistry 7 • Procedures 10 • Equipment Used 22 • Potential Benefits 24 • Process Flow 25 Submitted by : Aow Jie Wei (1101172 C), Gan Shi Yun (1102884 B), Mohd Aidil (1100519 F) Supervisor : Siew Yong Pau (siewyong@tp. edu. sg , hp : 98796781, Lecturer, ASc) 1

1. Current Situation · In this era of healthy living, many households would remove fats and skins from meats before cooking them. The removed fats and skins (from chicken meat, etc. ) are often discarded. This is because they are viewed as of no value or worst, bad for health (Figure 1). Figure 1 : Fats/Skin removed from chicken 2

· Chicken skin is generally made up of fat and protein. 34 g of chicken skin can yield 15 g of fat. The solid fat, found under the chicken skin will yield even more fats. Esters of linoleic acid (C 18 H 32 O 2) constitute about 20% of the chicken fats. 3

· Exhausted alkaline batteries (Figure 2) are often immediately discarded as they are presumed to have no more values. Figure 2 : Example of an alkaline battery · Alkaline batteries contain potassium hydroxide (KOH). If leaked, KOH is a caustic agent that can cause respiratory, eye and skin irritation (Figure 3). Figure 3 : Leaked battery http: //www. wikihow. com/Clean-Battery-Leaks/Spills 4
• Cost of living is increasing at a stupendous pace. Singapore inflation reaches 5. 2% in June July 25, 2011 - SINGAPORE: Singapore's Consumer Price Index (CPI) in June rose 5. 2 per cent year-on-year. This was in line with market expectations, and higher than the 4. 5 per cent rise in May. The upward cost pressures were concentrated in the usual sectors of transport, housing and food. http: //www. channelnewsasia. com Household cost of living has sky-rocketed. • Cost of liquid soap ranges from $5/lt to $64/liter

2. Proposed Changes · Make use of discarded chicken skins/fats and potassium hydroxide (KOH) electrolyte from exhausted batteries to manufacture home-made soap. + → Figure 4 : Soap from waste materials 6
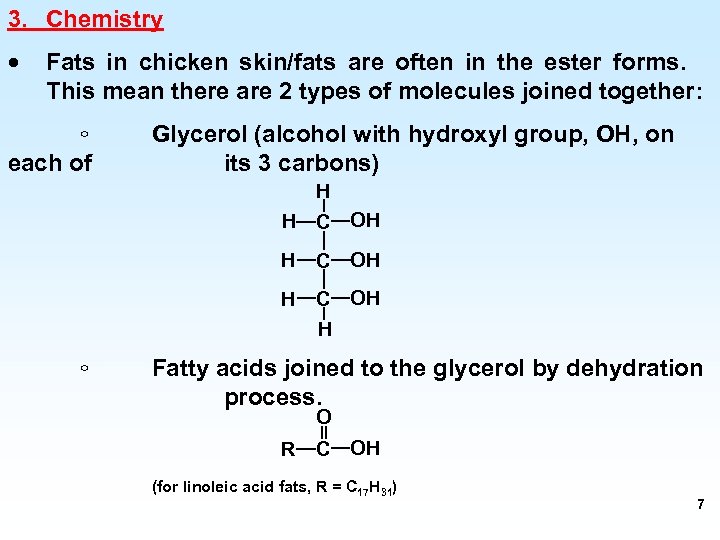
3. Chemistry · Fats in chicken skin/fats are often in the ester forms. This mean there are 2 types of molecules joined together: ◦ each of Glycerol (alcohol with hydroxyl group, OH, on its 3 carbons) H H C OH H ◦ Fatty acids joined to the glycerol by dehydration process. O R C OH (for linoleic acid fats, R = C 17 H 31) 7
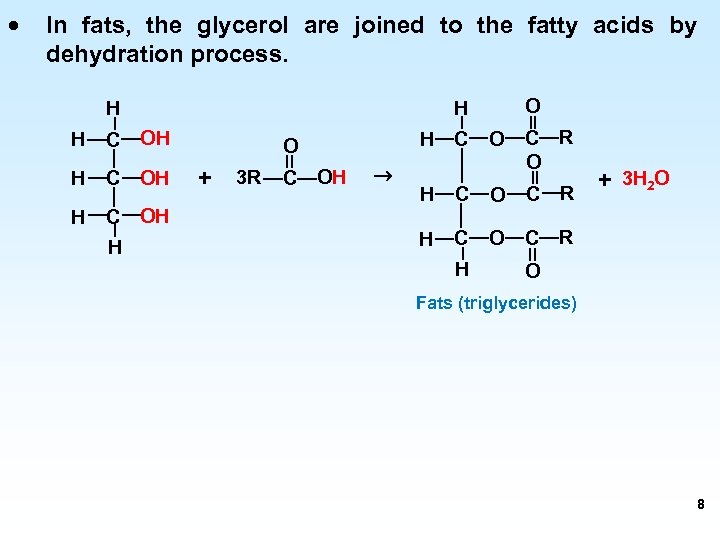
· In fats, the glycerol are joined to the fatty acids by dehydration process. H C OH H O H H H C O + 3 R C OH → O C R H C O C R H + 3 H 2 O O Fats (triglycerides) 8
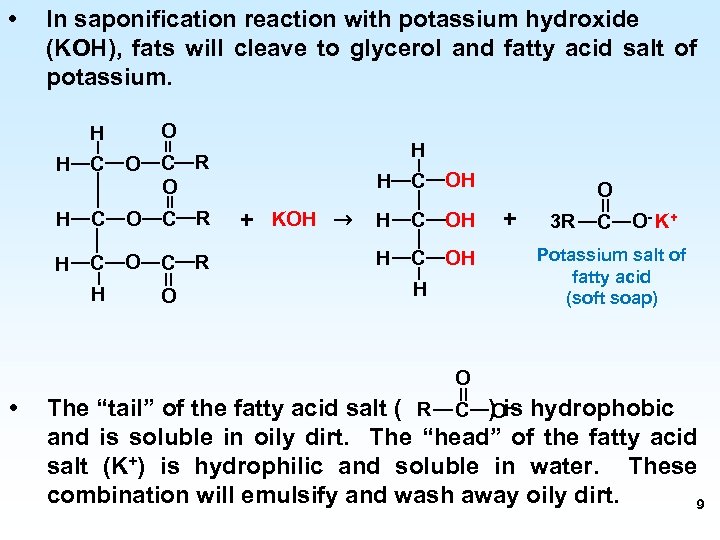
• In saponification reaction with potassium hydroxide (KOH), fats will cleave to glycerol and fatty acid salt of potassium. O H H C O C R H O H H C OH + KOH → H C OH H O + 3 R C O - K+ Potassium salt of fatty acid (soft soap) O • The “tail” of the fatty acid salt ( R C ) is hydrophobic Oand is soluble in oily dirt. The “head” of the fatty acid salt (K+) is hydrophilic and soluble in water. These combination will emulsify and wash away oily dirt. 9

4. Procedures to Obtain Oils from Waste Skins/Fats • Heat the animal fat/skin with water. This will be enough to dissolve the fats. Figure 5 : Heating the skin/fats • Squeeze the oil out by using spatula, etc. This will expedite the process. Figure 6 : Squeezing out the oil 10

• Released oils, being lower in density, will float to the top. Water and pieces of skin/fats will settle at the bottom. Figure 7 : Oil extracted from the skin/fats • Pour the oils into a separation funnel using a mesh filter. Remnants of skins/fats will be trapped by the mesh filter. 11 Figure 8 : Filtering out skins/fats
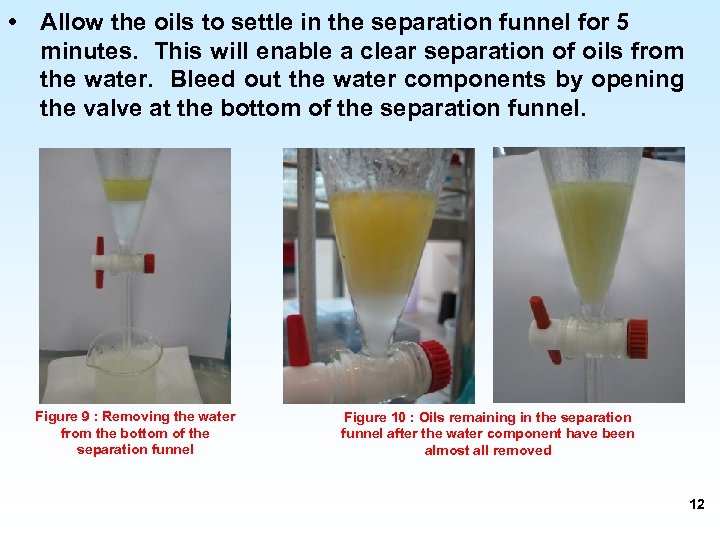
• Allow the oils to settle in the separation funnel for 5 minutes. This will enable a clear separation of oils from the water. Bleed out the water components by opening the valve at the bottom of the separation funnel. Figure 9 : Removing the water from the bottom of the separation funnel Figure 10 : Oils remaining in the separation funnel after the water component have been almost all removed 12

• Using water, wash the oils sticking on the funnel and the wall of the separation funnel. This process will also clean the oil of the residue chicken smell. Figure 11 : Washing the wall of the separation funnel • After the oil is well washed, collect it using a beaker. Weight the mass of oil collected. 13 Figure 12 : Collected clean oils

Procedures to Obtain KOH from Exhausted Batteries • Use a simple saw to cut off the base of the exhausted batteries. Figure 13 : Cutting the exhausted batteries • Remove the electrolyte (with zinc powder). Figure 14 : Removing the KOH electrolyte 14

• Add some (≈ 100 ml) water to dissolve the potassium hydroxide (KOH). Zinc powder does not dissolve. Figure 15 : Dissolving the KOH electrolyte Figure 16 : Undissolved zinc powder collected at bottom 15
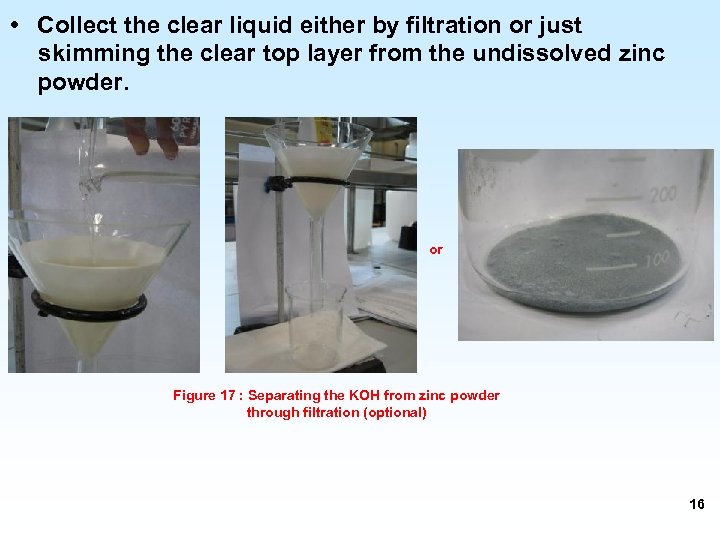
• Collect the clear liquid either by filtration or just skimming the clear top layer from the undissolved zinc powder. or Figure 17 : Separating the KOH from zinc powder through filtration (optional) 16

• The clear liquid (KOH) can be analyzed for its strength (titration with acid) and then used in the saponification reaction with the oils. Figure 18 : Clear KOH solution minus the zinc powder 17
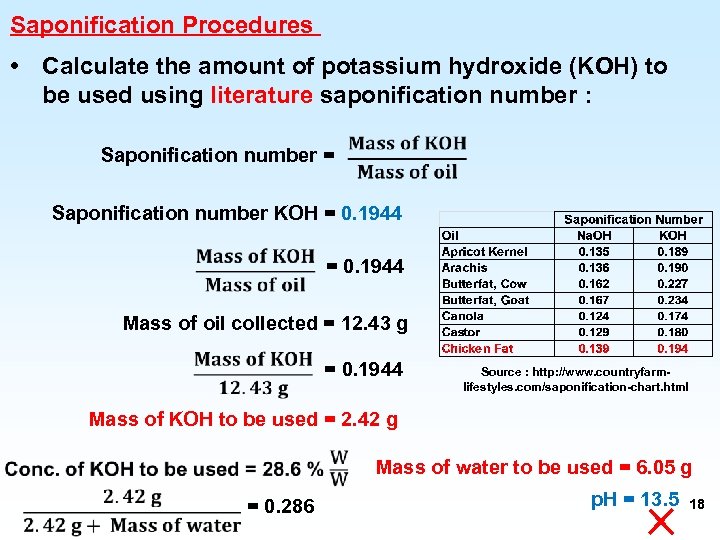
Saponification Procedures • Calculate the amount of potassium hydroxide (KOH) to be used using literature saponification number : Saponification number = Saponification number KOH = 0. 1944 Mass of oil collected = 12. 43 g = 0. 1944 Source : http: //www. countryfarmlifestyles. com/saponification-chart. html Mass of KOH to be used = 2. 42 g Mass of water to be used = 6. 05 g = 0. 286 p. H = 13. 5 18
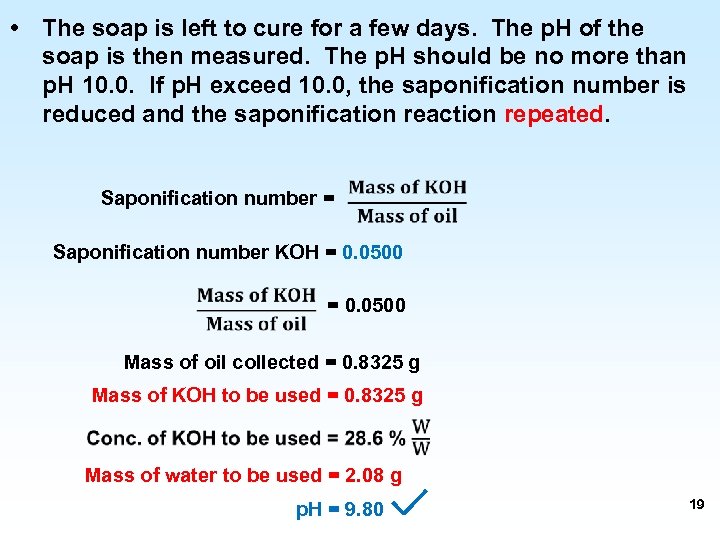
• The soap is left to cure for a few days. The p. H of the soap is then measured. The p. H should be no more than p. H 10. 0. If p. H exceed 10. 0, the saponification number is reduced and the saponification reaction repeated. Saponification number = Saponification number KOH = 0. 0500 Mass of oil collected = 0. 8325 g Mass of KOH to be used = 0. 8325 g Mass of water to be used = 2. 08 g p. H = 9. 80 19

• React the oil with the potassium hydroxide (KOH) obtained from the used battery. You will be able to catch the scented smell as reaction produces soap. Heat will help speed up the reaction. Figure 19 : Oil / KOH Reaction Figure 20 : Foam forming indicating soap formation 20
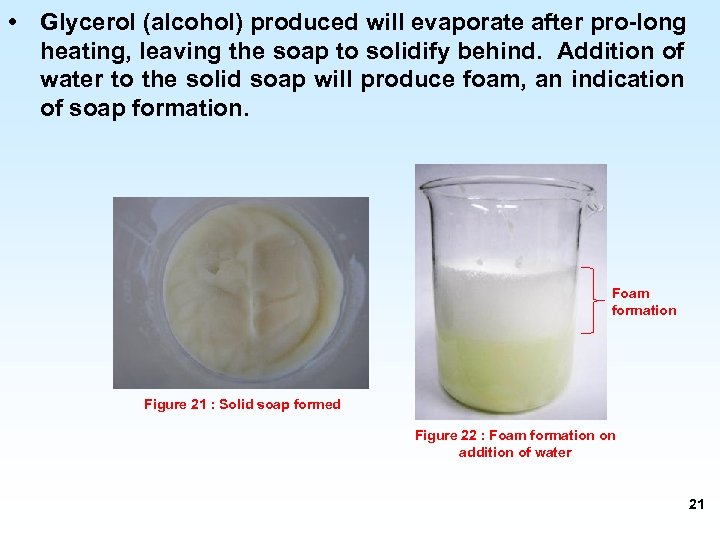
• Glycerol (alcohol) produced will evaporate after pro-long heating, leaving the soap to solidify behind. Addition of water to the solid soap will produce foam, an indication of soap formation. Foam formation Figure 21 : Solid soap formed Figure 22 : Foam formation on addition of water 21

5. Equipment Used • Vacuum flask is used to liquefy the skins/fats to oils. Vacuum flask is able to retain the heat of hot water poured into it, reducing the energy cost of producing the soap. Figure 23 : Common vacuum flask to hold heat in while fats are dissolved 22
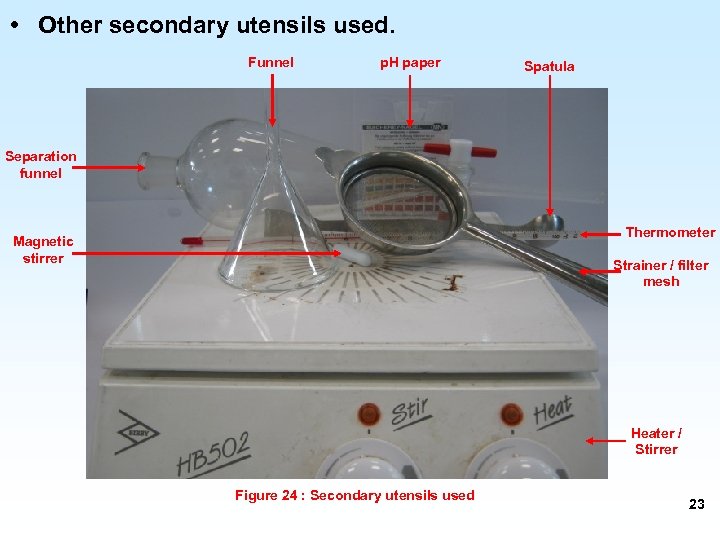
• Other secondary utensils used. Funnel p. H paper Spatula Separation funnel Thermometer Magnetic stirrer Strainer / filter mesh Heater / Stirrer Figure 24 : Secondary utensils used 23

6. Potential Benefits ● Reduce pollution due to leaching of electrolyte (KOH) into the environment when exhausted batteries are discarded. ● Reuse waste materials often deemed of little value such as chicken skin/fats. ● Promoting healthy living by encouraging removal of skins/fats from meat before they are consumed. ● Produce useful daily household-used items such as soap from wastes. This helps to reduce household spending ; appropriate at this time when cost of living is sky-rocketing. 24
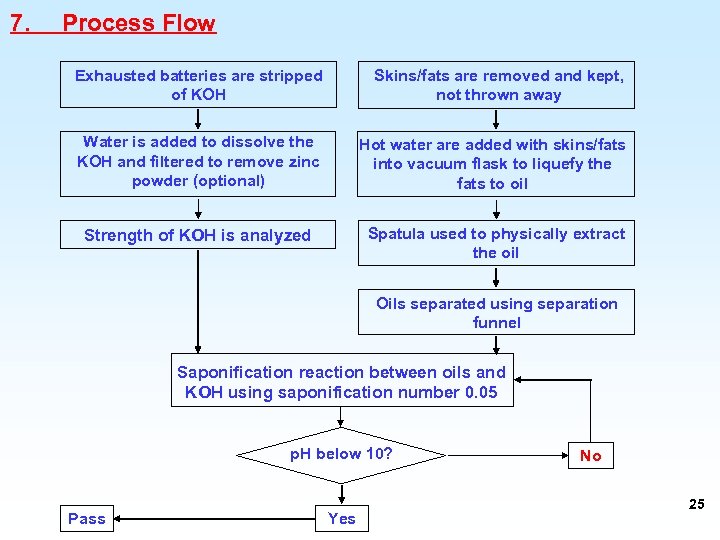
7. Process Flow Exhausted batteries are stripped of KOH Skins/fats are removed and kept, not thrown away Water is added to dissolve the KOH and filtered to remove zinc powder (optional) Hot water are added with skins/fats into vacuum flask to liquefy the fats to oil Strength of KOH is analyzed Spatula used to physically extract the oil Oils separated using separation funnel Saponification reaction between oils and KOH using saponification number 0. 05 p. H below 10? Pass Yes No 25
db540ad18830b1b4c1ccf8d68d44157a.ppt