7941f24942339e4fafb7a884dc543a74.ppt
- Количество слайдов: 88
 Family Planning & Contraception Manuela Russu MD, Ph D Associate Professor Chief of “Dr I. Cantacuzino” Second Department of Obstetrics & Gynecology “Carol Davila” University of Medicine & Pharmacy MCR, 2010
Family Planning & Contraception Manuela Russu MD, Ph D Associate Professor Chief of “Dr I. Cantacuzino” Second Department of Obstetrics & Gynecology “Carol Davila” University of Medicine & Pharmacy MCR, 2010
 ü Various surveys indicate that 30% to 60% of conceptions worldwide are either unwanted or unplanned ü Even in USA, many pregnancies occur due to: • ignorance, • unavailability, or • unacceptability of the rather limited range of fertility-control methods presently in use Harlap S, et al, 1999
ü Various surveys indicate that 30% to 60% of conceptions worldwide are either unwanted or unplanned ü Even in USA, many pregnancies occur due to: • ignorance, • unavailability, or • unacceptability of the rather limited range of fertility-control methods presently in use Harlap S, et al, 1999
 When to recommend contraception ü Whenever pregnancy is not desired in sexual active women ü From puberty to menopause: from menarche to 50’s ü At least some girls, & perhaps the majority, ovulate before their first menstrual period ü Therefore, older women are probably best advised that regular menstrual periods imply recurrent ovulation irrespective of age. A woman younger < 50 yrs who has not menstruated for 2 ys is very unlikely to ovulate spontaneously and to conceive, although there are reported instances in which conception occurred more than 2 ys after the onset of documented hypergonadotropic, hypoestrogenic amenorrhea Szlachter BN, Nachtigall LE, Epstein J, Young BK, 1979
When to recommend contraception ü Whenever pregnancy is not desired in sexual active women ü From puberty to menopause: from menarche to 50’s ü At least some girls, & perhaps the majority, ovulate before their first menstrual period ü Therefore, older women are probably best advised that regular menstrual periods imply recurrent ovulation irrespective of age. A woman younger < 50 yrs who has not menstruated for 2 ys is very unlikely to ovulate spontaneously and to conceive, although there are reported instances in which conception occurred more than 2 ys after the onset of documented hypergonadotropic, hypoestrogenic amenorrhea Szlachter BN, Nachtigall LE, Epstein J, Young BK, 1979
 Classification Oral: COC, POP ü Injectables (long term) 12 ; 8; 4 weeks ü Transdermal & intravaginal ü Implants ü Hormones: n IUD n Barriers Female’s, male’s ü Natural (Periodic/Rhythmic abstinence) § n n ü ü § § n Definitive sterilization § Inert Medicated Physical Chemicals Physic-chemical Prolonged breast feeding Interrupted intercourse Calendar Rhythm Method Temperature Rhythm Method Cervical Mucus Rhythm Method Symptothermal Method
Classification Oral: COC, POP ü Injectables (long term) 12 ; 8; 4 weeks ü Transdermal & intravaginal ü Implants ü Hormones: n IUD n Barriers Female’s, male’s ü Natural (Periodic/Rhythmic abstinence) § n n ü ü § § n Definitive sterilization § Inert Medicated Physical Chemicals Physic-chemical Prolonged breast feeding Interrupted intercourse Calendar Rhythm Method Temperature Rhythm Method Cervical Mucus Rhythm Method Symptothermal Method
 Contraceptive Choice Factors ü Couple Factors: Motivation & Reliability ü Age ü Life-style ü Sexual frequency ü Family-size desires ü Previous contraceptive experience ü Feminine attitudes & Masculine attitudes ü Health Factors: Absolute or Relative Contraindications ü Health status ü History: menstrual, pregnancy, family ü Physical & Pelvic examination ü Contraceptive Method Factors: Acceptability and Continuing Use ü Effectiveness ü Minor side-effects ü Disadvantages ü Potential complications ü Cost
Contraceptive Choice Factors ü Couple Factors: Motivation & Reliability ü Age ü Life-style ü Sexual frequency ü Family-size desires ü Previous contraceptive experience ü Feminine attitudes & Masculine attitudes ü Health Factors: Absolute or Relative Contraindications ü Health status ü History: menstrual, pregnancy, family ü Physical & Pelvic examination ü Contraceptive Method Factors: Acceptability and Continuing Use ü Effectiveness ü Minor side-effects ü Disadvantages ü Potential complications ü Cost
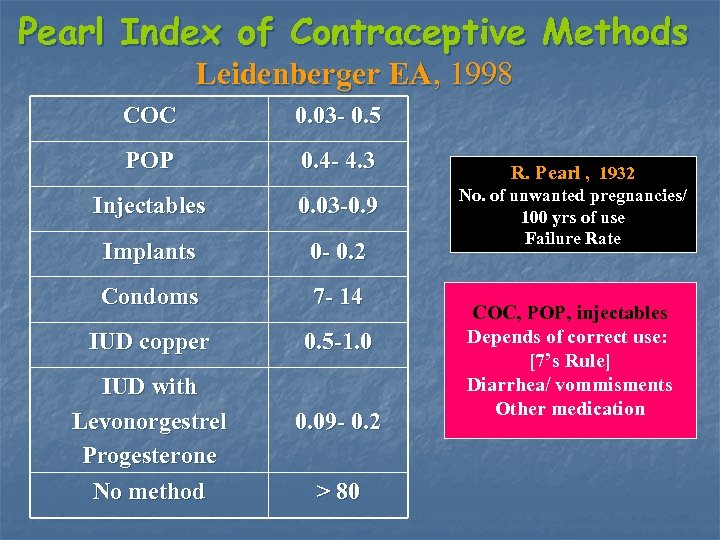 Pearl Index of Contraceptive Methods Leidenberger EA, 1998 COC 0. 03 - 0. 5 POP 0. 4 - 4. 3 Injectables 0. 03 -0. 9 Implants 0 - 0. 2 Condoms 7 - 14 IUD copper 0. 5 -1. 0 IUD with Levonorgestrel Progesterone No method 0. 09 - 0. 2 > 80 R. Pearl , 1932 No. of unwanted pregnancies/ 100 yrs of use Failure Rate COC, POP, injectables Depends of correct use: [7’s Rule] Diarrhea/ vommisments Other medication
Pearl Index of Contraceptive Methods Leidenberger EA, 1998 COC 0. 03 - 0. 5 POP 0. 4 - 4. 3 Injectables 0. 03 -0. 9 Implants 0 - 0. 2 Condoms 7 - 14 IUD copper 0. 5 -1. 0 IUD with Levonorgestrel Progesterone No method 0. 09 - 0. 2 > 80 R. Pearl , 1932 No. of unwanted pregnancies/ 100 yrs of use Failure Rate COC, POP, injectables Depends of correct use: [7’s Rule] Diarrhea/ vommisments Other medication
 Contraceptive Effectiveness Trussel J, Haycher RA, Cates W. Jr, 1990 Method Approximate Use Effectiveness (%) Male sterilization Female sterilization Injectable progestin (Depo-Provera) Implantable progestin capsules (Norplant) Oral steroids: combination IUD: copper IUD: progesterone Oral steroids, progestin only Diaphragm & spermicide Condom only Sponge with spermicide Spermicide only Cervical cap Periodic abstinence Preejaculatory Withdrawal 99+ 99 99 98 98 96 95 90 88 88 85 80 75 70
Contraceptive Effectiveness Trussel J, Haycher RA, Cates W. Jr, 1990 Method Approximate Use Effectiveness (%) Male sterilization Female sterilization Injectable progestin (Depo-Provera) Implantable progestin capsules (Norplant) Oral steroids: combination IUD: copper IUD: progesterone Oral steroids, progestin only Diaphragm & spermicide Condom only Sponge with spermicide Spermicide only Cervical cap Periodic abstinence Preejaculatory Withdrawal 99+ 99 99 98 98 96 95 90 88 88 85 80 75 70
 No contraceptive method is fully effective for preventing pregnancy, nor fully safe from both minor and major side-effects, nor fully acceptable to the couple ü Since each method has its advantages and disadvantages, the method that the couple will use in a consistent manner is the “best” contraceptive method for them ü Counselling woman/man • Advantages, disadvantages • Indications, contraindications, side effects For each method of contraception
No contraceptive method is fully effective for preventing pregnancy, nor fully safe from both minor and major side-effects, nor fully acceptable to the couple ü Since each method has its advantages and disadvantages, the method that the couple will use in a consistent manner is the “best” contraceptive method for them ü Counselling woman/man • Advantages, disadvantages • Indications, contraindications, side effects For each method of contraception
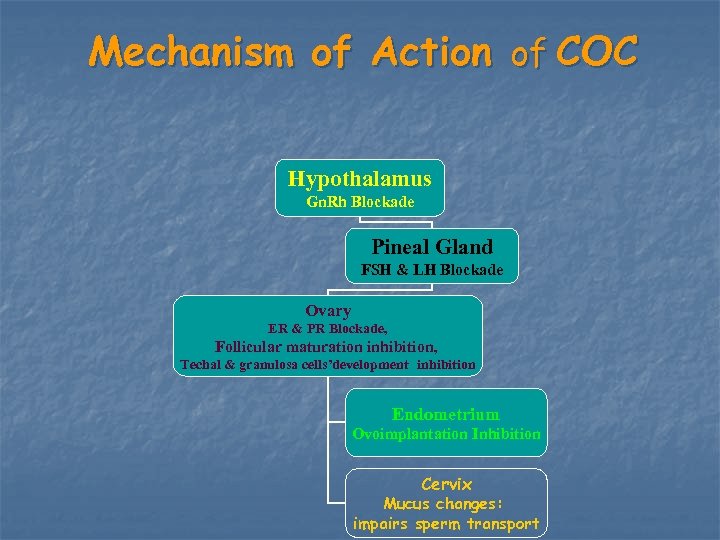 Mechanism of Action of COC Hypothalamus Gn. Rh Blockade Pineal Gland FSH & LH Blockade Ovary ER & PR Blockade, Follicular maturation inhibition, Techal & granulosa cells’development inhibition Endometrium Ovoimplantation Inhibition Cervix Mucus changes: impairs sperm transport
Mechanism of Action of COC Hypothalamus Gn. Rh Blockade Pineal Gland FSH & LH Blockade Ovary ER & PR Blockade, Follicular maturation inhibition, Techal & granulosa cells’development inhibition Endometrium Ovoimplantation Inhibition Cervix Mucus changes: impairs sperm transport
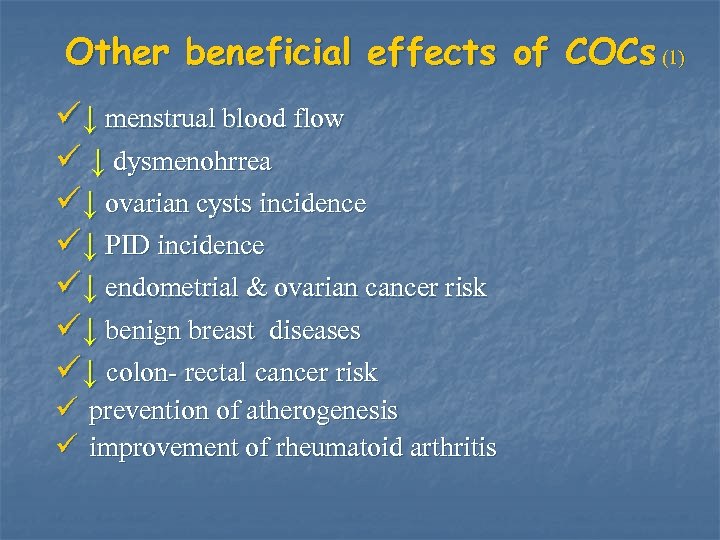 Other beneficial effects of COCs (1) ü↓ menstrual blood flow ü ↓ dysmenohrrea ü↓ ovarian cysts incidence ü↓ PID incidence ü↓ endometrial & ovarian cancer risk ü↓ benign breast diseases ü↓ colon- rectal cancer risk ü prevention of atherogenesis ü improvement of rheumatoid arthritis
Other beneficial effects of COCs (1) ü↓ menstrual blood flow ü ↓ dysmenohrrea ü↓ ovarian cysts incidence ü↓ PID incidence ü↓ endometrial & ovarian cancer risk ü↓ benign breast diseases ü↓ colon- rectal cancer risk ü prevention of atherogenesis ü improvement of rheumatoid arthritis
 Other beneficial effects of COCs (2) A. Therapeutic effects: healing/ alleviation of symptoms of some diseases B. Protective effects : reduction of some diseases’ incidence
Other beneficial effects of COCs (2) A. Therapeutic effects: healing/ alleviation of symptoms of some diseases B. Protective effects : reduction of some diseases’ incidence
 Other beneficial effects of COCs (3) A. Therapeutic effects Ø ü ü ü Menstrual bleeding control: Reducing dysfunctional uterine bleeding (puberty, premenopause), by central- ovarian balance and hormonal endometrial balance, in anovulation, dysovulation and corpus luteum insufficiency Reducing dysmenorrhea: hormonal balance (progestogens act as antiprostaglandins) Reducing the premenstrual syndrome in some cases (±): see Yaz effects of reducing dysforia
Other beneficial effects of COCs (3) A. Therapeutic effects Ø ü ü ü Menstrual bleeding control: Reducing dysfunctional uterine bleeding (puberty, premenopause), by central- ovarian balance and hormonal endometrial balance, in anovulation, dysovulation and corpus luteum insufficiency Reducing dysmenorrhea: hormonal balance (progestogens act as antiprostaglandins) Reducing the premenstrual syndrome in some cases (±): see Yaz effects of reducing dysforia
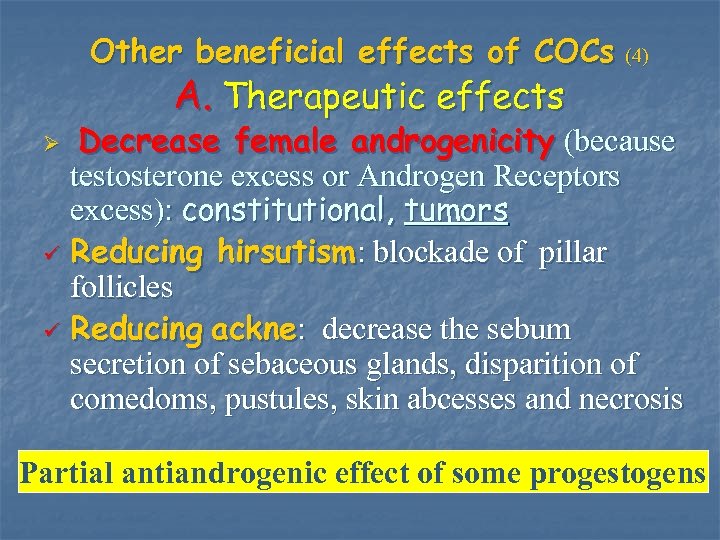 Other beneficial effects of COCs (4) A. Therapeutic effects Decrease female androgenicity (because testosterone excess or Androgen Receptors excess): constitutional, tumors ü Reducing hirsutism: blockade of pillar follicles ü Reducing ackne: decrease the sebum secretion of sebaceous glands, disparition of comedoms, pustules, skin abcesses and necrosis Ø Partial antiandrogenic effect of some progestogens
Other beneficial effects of COCs (4) A. Therapeutic effects Decrease female androgenicity (because testosterone excess or Androgen Receptors excess): constitutional, tumors ü Reducing hirsutism: blockade of pillar follicles ü Reducing ackne: decrease the sebum secretion of sebaceous glands, disparition of comedoms, pustules, skin abcesses and necrosis Ø Partial antiandrogenic effect of some progestogens
 Other beneficial effects of COCs (6) B. Protective Effects ü PID protection (Chlamidya trachomatis/ N. gonorrhoea): ↓ incidence, severity, sequels (infertility, ectopic pregnancy, chronic abdominal pain) ü Protection of ectopic pregnancy ü Protection of myomas, endometriosis ü ↓ ovarian tumors incidence /PCO/ cancer (ovulation blockade) ü ↓ endometrial cancer incidence (daily hormonal balance, antiproliferative effect of progestogens) ü Protection of benign breast tumors ↓ breast hyperplasia ü Protects of colon- rectal cancer
Other beneficial effects of COCs (6) B. Protective Effects ü PID protection (Chlamidya trachomatis/ N. gonorrhoea): ↓ incidence, severity, sequels (infertility, ectopic pregnancy, chronic abdominal pain) ü Protection of ectopic pregnancy ü Protection of myomas, endometriosis ü ↓ ovarian tumors incidence /PCO/ cancer (ovulation blockade) ü ↓ endometrial cancer incidence (daily hormonal balance, antiproliferative effect of progestogens) ü Protection of benign breast tumors ↓ breast hyperplasia ü Protects of colon- rectal cancer
 Drugs Interactions Ø phenytoin, phenobarbital, primidone, diazepam, alprazolam ↑ liver metabolism Ø rifampin, penicillins, tetracycline/doxycicline, griseofulvine ↓ absorbtion Ø Ø folic acid, ascorbic acid, Zn, B 2 vit, B 12 vit phenylbutazone ↓ contraceptive effect
Drugs Interactions Ø phenytoin, phenobarbital, primidone, diazepam, alprazolam ↑ liver metabolism Ø rifampin, penicillins, tetracycline/doxycicline, griseofulvine ↓ absorbtion Ø Ø folic acid, ascorbic acid, Zn, B 2 vit, B 12 vit phenylbutazone ↓ contraceptive effect
 Possible Adverse Events (1) I. § § II. Pregnancy like changes ↑T 4, TBG, ↓ T 3 ↑ transcortin, cortisol Metabolic changes Lipoproteins: LDL/HDL: HDL 2, HDL 3; but COC are not atherogenic : Wallach M, Grimes DA, 2000 § Carbohydrate: diabetes effect was real with higher formulations; no concern with actual formulation: Speroff L, Darney PD, 2001 § Proteins: ü ↑ angiotensinogen: “pill- induced hypertension” ü ↑ fibrinogen; clotting factors: II, VII, IX, X, XII: direct connection to estrogen dose §
Possible Adverse Events (1) I. § § II. Pregnancy like changes ↑T 4, TBG, ↓ T 3 ↑ transcortin, cortisol Metabolic changes Lipoproteins: LDL/HDL: HDL 2, HDL 3; but COC are not atherogenic : Wallach M, Grimes DA, 2000 § Carbohydrate: diabetes effect was real with higher formulations; no concern with actual formulation: Speroff L, Darney PD, 2001 § Proteins: ü ↑ angiotensinogen: “pill- induced hypertension” ü ↑ fibrinogen; clotting factors: II, VII, IX, X, XII: direct connection to estrogen dose §
 Possible Adverse Events (2) III. Cardiovascular effects Vasoconstriction ↑, platelets aggregation ↑ deep vein thrombosis, pulmonary embolism myocardial infarction ( ) + ischemic & hemorrhagic stroke hypertension § IV. Liver Disease § § cholestasis, cholestatic jaundice gallbladder lithiasis
Possible Adverse Events (2) III. Cardiovascular effects Vasoconstriction ↑, platelets aggregation ↑ deep vein thrombosis, pulmonary embolism myocardial infarction ( ) + ischemic & hemorrhagic stroke hypertension § IV. Liver Disease § § cholestasis, cholestatic jaundice gallbladder lithiasis
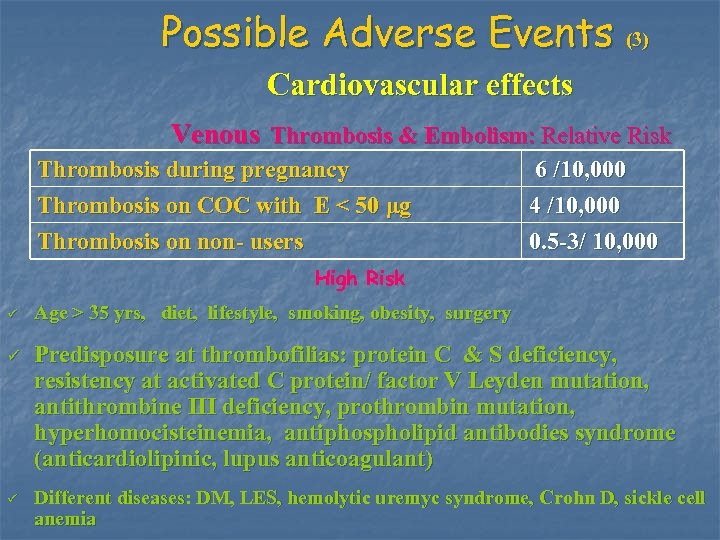 Possible Adverse Events (3) Cardiovascular effects Venous Thrombosis & Embolism: Relative Risk Thrombosis during pregnancy Thrombosis on COC with E < 50 μg 6 /10, 000 4 /10, 000 Thrombosis on non- users 0. 5 -3/ 10, 000 High Risk ü ü ü Age > 35 yrs, diet, lifestyle, smoking, obesity, surgery Predisposure at thrombofilias: protein C & S deficiency, resistency at activated C protein/ factor V Leyden mutation, antithrombine III deficiency, prothrombin mutation, hyperhomocisteinemia, antiphospholipid antibodies syndrome (anticardiolipinic, lupus anticoagulant) Different diseases: DM, LES, hemolytic uremyc syndrome, Crohn D, sickle cell anemia
Possible Adverse Events (3) Cardiovascular effects Venous Thrombosis & Embolism: Relative Risk Thrombosis during pregnancy Thrombosis on COC with E < 50 μg 6 /10, 000 4 /10, 000 Thrombosis on non- users 0. 5 -3/ 10, 000 High Risk ü ü ü Age > 35 yrs, diet, lifestyle, smoking, obesity, surgery Predisposure at thrombofilias: protein C & S deficiency, resistency at activated C protein/ factor V Leyden mutation, antithrombine III deficiency, prothrombin mutation, hyperhomocisteinemia, antiphospholipid antibodies syndrome (anticardiolipinic, lupus anticoagulant) Different diseases: DM, LES, hemolytic uremyc syndrome, Crohn D, sickle cell anemia
 Possible Adverse Events (4) V. Some neoplasias stimulation Preinvasive and invasive Cervical Cancer after 5 yrs of use: HPV Thomas DB, 1996 § Breast Cancer (after 12 yrs of use): RR 1. 24; lower stadies gradually after 10 yrs from interruption (because of progestogens): The Collaborative Group on Hormonal Factors in Breast Cancer, 1996 § Liver Nodular Hyperplasia & Cancer § Pineal Gland Adenomas (specially prolactinoma) § VI. Effects on reproduction § § § Postpill Amenorrhea: is likely reflective of preexisting problem Wallach M, Grimes DA, 2000 Congenital Defects: no association: Lammer EJ , Cordero FJ, 1986 Lactation Decrease: actually ambiguous resultats: to be started after 3 weeks postpartum: Kaunitz A, 1997
Possible Adverse Events (4) V. Some neoplasias stimulation Preinvasive and invasive Cervical Cancer after 5 yrs of use: HPV Thomas DB, 1996 § Breast Cancer (after 12 yrs of use): RR 1. 24; lower stadies gradually after 10 yrs from interruption (because of progestogens): The Collaborative Group on Hormonal Factors in Breast Cancer, 1996 § Liver Nodular Hyperplasia & Cancer § Pineal Gland Adenomas (specially prolactinoma) § VI. Effects on reproduction § § § Postpill Amenorrhea: is likely reflective of preexisting problem Wallach M, Grimes DA, 2000 Congenital Defects: no association: Lammer EJ , Cordero FJ, 1986 Lactation Decrease: actually ambiguous resultats: to be started after 3 weeks postpartum: Kaunitz A, 1997
 Adverse Events (5) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general: commune (1) P Headache P Mastalgia; breast enlargement P Nausea P Nervousness P Irritability P Oedema/ Weight gain Variable: patient, formula Diminish/ Increase with time
Adverse Events (5) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general: commune (1) P Headache P Mastalgia; breast enlargement P Nausea P Nervousness P Irritability P Oedema/ Weight gain Variable: patient, formula Diminish/ Increase with time
 Possible Adverse Events (6) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general : rare events (2) P P P P Migraine headaches Dizziness Visual disturbances & eyes irritation (contact lens) Libido changes Different skin changes: hyperpigmentation/chloasma – less with low dose of E, nodos erytema, hair loss Mood changes: emotional lability, hyperreactvity/depression: reduced by low dose E Vommisments
Possible Adverse Events (6) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general : rare events (2) P P P P Migraine headaches Dizziness Visual disturbances & eyes irritation (contact lens) Libido changes Different skin changes: hyperpigmentation/chloasma – less with low dose of E, nodos erytema, hair loss Mood changes: emotional lability, hyperreactvity/depression: reduced by low dose E Vommisments
 Possible Adverse Events (7) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general : very rare events (3) P P P deep vein thrombosis & pulmonary embolism arterial thrombosis [myocardial infarction ( ) , ischemic & hemorrhagic stroke] hypertension coronary heart disease liver adenomas cholestasis jaundice
Possible Adverse Events (7) Oxford Family Planning, 1995 Royal College of General Practitioners, 1999 A. general : very rare events (3) P P P deep vein thrombosis & pulmonary embolism arterial thrombosis [myocardial infarction ( ) , ischemic & hemorrhagic stroke] hypertension coronary heart disease liver adenomas cholestasis jaundice
 Possible Adverse Events (8) Effects on Reproduction Amenorrhea: Usually in cases on psihiatric therapy, which ü influences hypothalamo- pituitary-ovarian axis together with COC & ü Interacts to mono-aminergic neurotransmitters metabolism Conclusions : If the patient on psyhotropic drugs has menstrual irregularities the collaboration between gyncologist & neuropsyhiatrist is mandatory 2. Any drug influencing hypothalamic system must atentivelly follow –up in women on COC 1.
Possible Adverse Events (8) Effects on Reproduction Amenorrhea: Usually in cases on psihiatric therapy, which ü influences hypothalamo- pituitary-ovarian axis together with COC & ü Interacts to mono-aminergic neurotransmitters metabolism Conclusions : If the patient on psyhotropic drugs has menstrual irregularities the collaboration between gyncologist & neuropsyhiatrist is mandatory 2. Any drug influencing hypothalamic system must atentivelly follow –up in women on COC 1.
 Possible Adverse Events (9) Effects on Reproduction Ovulation recovery after COC ü First cycles after COC become biphasic, with some prolongation of proliferative phase and menstrual delay of 6 -8 days ü After long term use of COCs amenorrhea may appear, with high frecquence at adolescents and some delay in ovulation recovery ü Short term COC administration may stimulate fertility and if pregnancy is unwanted, is not recommended to stop COC
Possible Adverse Events (9) Effects on Reproduction Ovulation recovery after COC ü First cycles after COC become biphasic, with some prolongation of proliferative phase and menstrual delay of 6 -8 days ü After long term use of COCs amenorrhea may appear, with high frecquence at adolescents and some delay in ovulation recovery ü Short term COC administration may stimulate fertility and if pregnancy is unwanted, is not recommended to stop COC
 Possible Adverse Events (10) Effects on Reproduction Interference with Pregnancy ü Administration during pregnancy was considered an absolute contraindication, because interferention with organogenesis dependent on steroid hormones: external genitalia, but a recent meta- analysis (Raman –Wilms L, et al, 1995) did not confirm any congenital malformation ü For pregnancy safety is not imposed a longer break than 7 days (after the last pill from the blister) ü Usually COC are not recommanded during lactation: because excretion in milk
Possible Adverse Events (10) Effects on Reproduction Interference with Pregnancy ü Administration during pregnancy was considered an absolute contraindication, because interferention with organogenesis dependent on steroid hormones: external genitalia, but a recent meta- analysis (Raman –Wilms L, et al, 1995) did not confirm any congenital malformation ü For pregnancy safety is not imposed a longer break than 7 days (after the last pill from the blister) ü Usually COC are not recommanded during lactation: because excretion in milk
 COCs’Adverse Efecte : Breast Cancer Magnusson CM, et al; Sweden (Int J Cancer, 1999) Case- control population epidemiological study based on phone administered questionnaires: • compairs 3016 women (50 -74 yrs) with invasive breast cancer to 3263 (same ages) as control group Ø No risk of breast cancer when on COC This large population study brings evidences on environment factors which act in the first years after menarche and emphasizes the births’ importance at younger ages to prevent breast cancer
COCs’Adverse Efecte : Breast Cancer Magnusson CM, et al; Sweden (Int J Cancer, 1999) Case- control population epidemiological study based on phone administered questionnaires: • compairs 3016 women (50 -74 yrs) with invasive breast cancer to 3263 (same ages) as control group Ø No risk of breast cancer when on COC This large population study brings evidences on environment factors which act in the first years after menarche and emphasizes the births’ importance at younger ages to prevent breast cancer
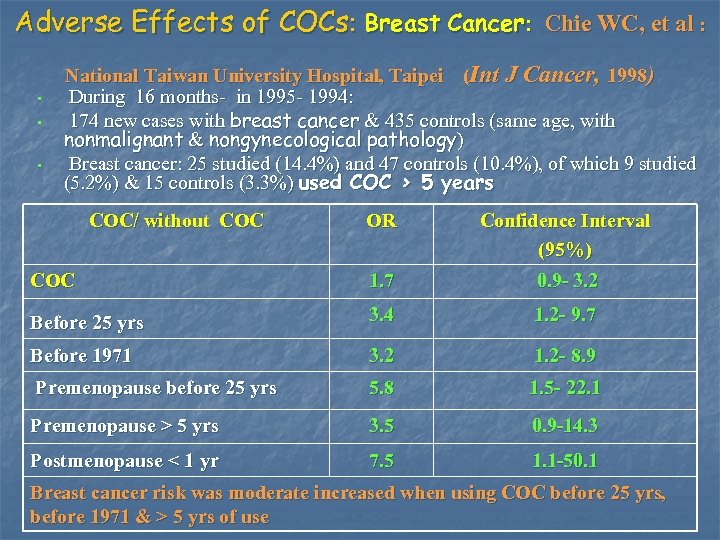 Adverse Effects of COCs: Breast Cancer: Chie WC, et al : • • • National Taiwan University Hospital, Taipei (Int J Cancer, 1998) During 16 months- in 1995 - 1994: 174 new cases with breast cancer & 435 controls (same age, with nonmalignant & nongynecological pathology) Breast cancer: 25 studied (14. 4%) and 47 controls (10. 4%), of which 9 studied (5. 2%) & 15 controls (3. 3%) used COC > 5 years COC/ without COC OR Confidence Interval (95%) COC 1. 7 0. 9 - 3. 2 Before 25 yrs 3. 4 1. 2 - 9. 7 Before 1971 3. 2 1. 2 - 8. 9 Premenopause before 25 yrs 5. 8 1. 5 - 22. 1 Premenopause > 5 yrs 3. 5 0. 9 -14. 3 Postmenopause < 1 yr 7. 5 1. 1 -50. 1 Breast cancer risk was moderate increased when using COC before 25 yrs, before 1971 & > 5 yrs of use
Adverse Effects of COCs: Breast Cancer: Chie WC, et al : • • • National Taiwan University Hospital, Taipei (Int J Cancer, 1998) During 16 months- in 1995 - 1994: 174 new cases with breast cancer & 435 controls (same age, with nonmalignant & nongynecological pathology) Breast cancer: 25 studied (14. 4%) and 47 controls (10. 4%), of which 9 studied (5. 2%) & 15 controls (3. 3%) used COC > 5 years COC/ without COC OR Confidence Interval (95%) COC 1. 7 0. 9 - 3. 2 Before 25 yrs 3. 4 1. 2 - 9. 7 Before 1971 3. 2 1. 2 - 8. 9 Premenopause before 25 yrs 5. 8 1. 5 - 22. 1 Premenopause > 5 yrs 3. 5 0. 9 -14. 3 Postmenopause < 1 yr 7. 5 1. 1 -50. 1 Breast cancer risk was moderate increased when using COC before 25 yrs, before 1971 & > 5 yrs of use
 Risk of death Mortality associated to COC use is very low if: • woman is younger than 35 yrs, • has no systemic illness • does not smoke 1 death/ 55, 000 woman-years from the Group Health Cooperative of Puget Sound: Porter JB, et all, 1987
Risk of death Mortality associated to COC use is very low if: • woman is younger than 35 yrs, • has no systemic illness • does not smoke 1 death/ 55, 000 woman-years from the Group Health Cooperative of Puget Sound: Porter JB, et all, 1987
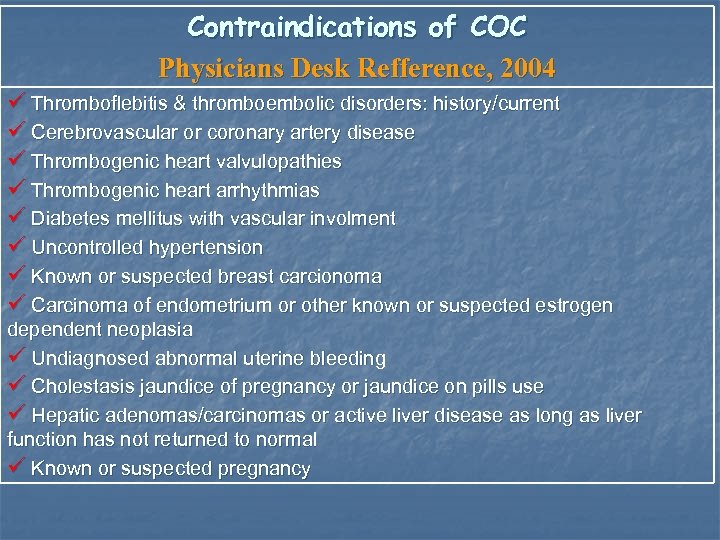 Contraindications of COC Physicians Desk Refference, 2004 ü Thromboflebitis & thromboembolic disorders: history/current ü Cerebrovascular or coronary artery disease ü Thrombogenic heart valvulopathies ü Thrombogenic heart arrhythmias ü Diabetes mellitus with vascular involment ü Uncontrolled hypertension ü Known or suspected breast carcionoma ü Carcinoma of endometrium or other known or suspected estrogen dependent neoplasia ü Undiagnosed abnormal uterine bleeding ü Cholestasis jaundice of pregnancy or jaundice on pills use ü Hepatic adenomas/carcinomas or active liver disease as long as liver function has not returned to normal ü Known or suspected pregnancy
Contraindications of COC Physicians Desk Refference, 2004 ü Thromboflebitis & thromboembolic disorders: history/current ü Cerebrovascular or coronary artery disease ü Thrombogenic heart valvulopathies ü Thrombogenic heart arrhythmias ü Diabetes mellitus with vascular involment ü Uncontrolled hypertension ü Known or suspected breast carcionoma ü Carcinoma of endometrium or other known or suspected estrogen dependent neoplasia ü Undiagnosed abnormal uterine bleeding ü Cholestasis jaundice of pregnancy or jaundice on pills use ü Hepatic adenomas/carcinomas or active liver disease as long as liver function has not returned to normal ü Known or suspected pregnancy
 Warnings for COC Physicians Desk Refference, 2004 ü Cigarette smoking increases the risk of serious cardiovascular side effects from COC. ü This risk increases with age and with extent of smoking (in epidemiological studies: smoking 15 or more cigarettes per day was associated with a significantly increased risk) and is quite marked in women older than 35 yrs of age ü Women on COC should be strongly advised not to smoke
Warnings for COC Physicians Desk Refference, 2004 ü Cigarette smoking increases the risk of serious cardiovascular side effects from COC. ü This risk increases with age and with extent of smoking (in epidemiological studies: smoking 15 or more cigarettes per day was associated with a significantly increased risk) and is quite marked in women older than 35 yrs of age ü Women on COC should be strongly advised not to smoke
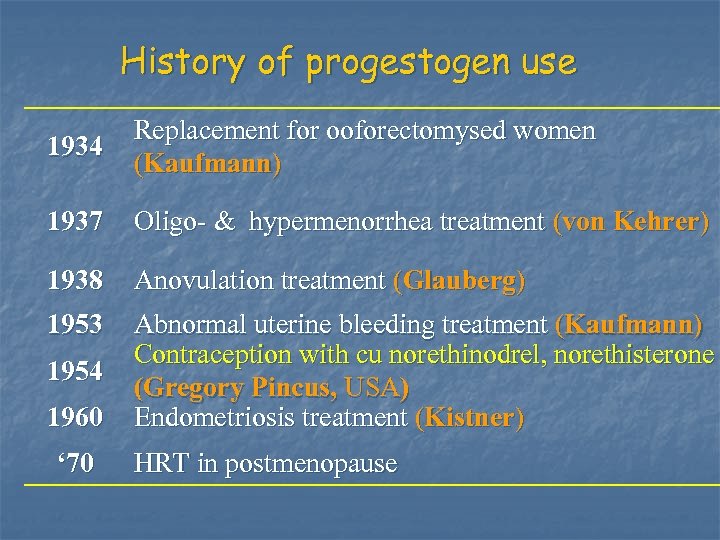 History of progestogen use 1934 Replacement for ooforectomysed women (Kaufmann) 1937 Oligo- & hypermenorrhea treatment (von Kehrer) 1938 Anovulation treatment (Glauberg) 1953 Abnormal uterine bleeding treatment (Kaufmann) Contraception with cu norethinodrel, norethisterone (Gregory Pincus, USA) Endometriosis treatment (Kistner) 1954 1960 ‘ 70 HRT in postmenopause
History of progestogen use 1934 Replacement for ooforectomysed women (Kaufmann) 1937 Oligo- & hypermenorrhea treatment (von Kehrer) 1938 Anovulation treatment (Glauberg) 1953 Abnormal uterine bleeding treatment (Kaufmann) Contraception with cu norethinodrel, norethisterone (Gregory Pincus, USA) Endometriosis treatment (Kistner) 1954 1960 ‘ 70 HRT in postmenopause
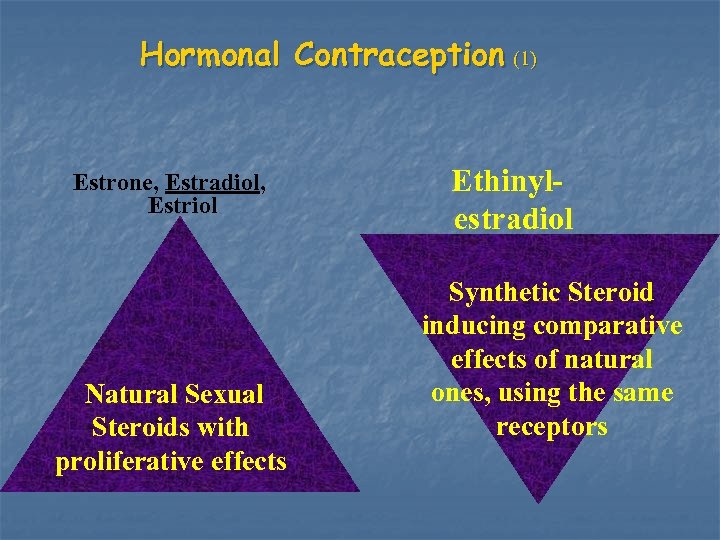 Hormonal Contraception (1) Estrone, Estradiol, Estriol Natural Sexual Steroids with proliferative effects Ethinylestradiol Synthetic Steroid inducing comparative effects of natural ones, using the same receptors
Hormonal Contraception (1) Estrone, Estradiol, Estriol Natural Sexual Steroids with proliferative effects Ethinylestradiol Synthetic Steroid inducing comparative effects of natural ones, using the same receptors
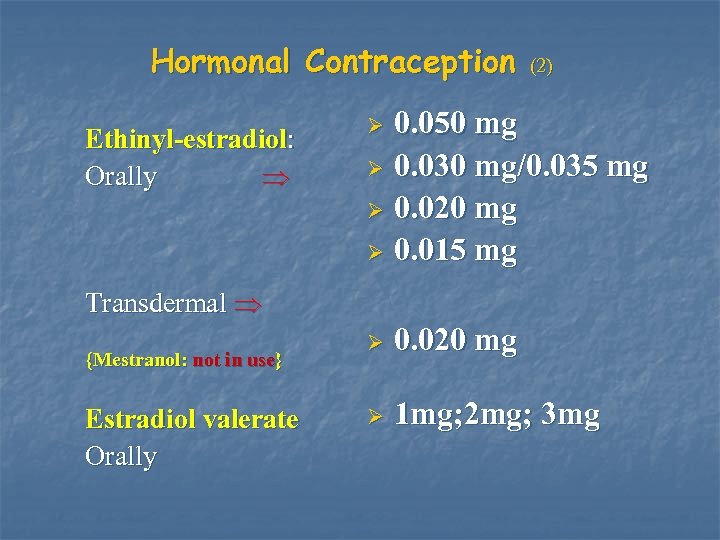 Hormonal Contraception Ethinyl-estradiol: Orally (2) 0. 050 mg Ø 0. 030 mg/0. 035 mg Ø 0. 020 mg Ø 0. 015 mg Ø Transdermal {Mestranol: not in use} Estradiol valerate Orally Ø 0. 020 mg Ø 1 mg; 2 mg; 3 mg
Hormonal Contraception Ethinyl-estradiol: Orally (2) 0. 050 mg Ø 0. 030 mg/0. 035 mg Ø 0. 020 mg Ø 0. 015 mg Ø Transdermal {Mestranol: not in use} Estradiol valerate Orally Ø 0. 020 mg Ø 1 mg; 2 mg; 3 mg
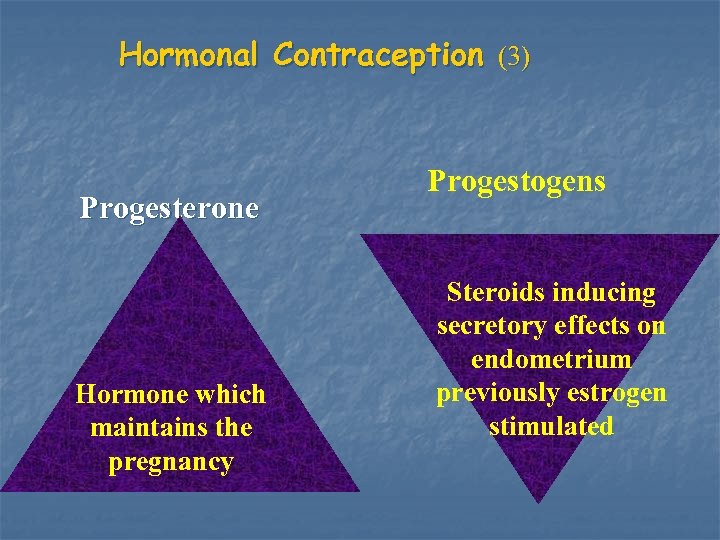 Hormonal Contraception (3) Progesterone Hormone which maintains the pregnancy Progestogens Steroids inducing secretory effects on endometrium previously estrogen stimulated
Hormonal Contraception (3) Progesterone Hormone which maintains the pregnancy Progestogens Steroids inducing secretory effects on endometrium previously estrogen stimulated
 Hystory of use of estrogens and progestogens in contraception 1938: Ethinyl-estradiol introduced in contraception: oral/ transdermal 2000: Estradiol valerate: introduced in contraception: oral B. Injectable progestogens A. Oral progestogens Medroxy- progesterone: DMPA * Norethisterone: Noristerat* C. Transdermal Progestogens : Norelgestromin : Ortho Evra/Evra* 5. Norethisterone Levonorgestrel Cyproterone acetate Desogestrel, gestodene, norgestimate Dienogest: summarises the effects of 19 - 6. Drospirenone 1. 2. 3. 4. norproprogestogens and of progesterone derivates, without their negative effects
Hystory of use of estrogens and progestogens in contraception 1938: Ethinyl-estradiol introduced in contraception: oral/ transdermal 2000: Estradiol valerate: introduced in contraception: oral B. Injectable progestogens A. Oral progestogens Medroxy- progesterone: DMPA * Norethisterone: Noristerat* C. Transdermal Progestogens : Norelgestromin : Ortho Evra/Evra* 5. Norethisterone Levonorgestrel Cyproterone acetate Desogestrel, gestodene, norgestimate Dienogest: summarises the effects of 19 - 6. Drospirenone 1. 2. 3. 4. norproprogestogens and of progesterone derivates, without their negative effects
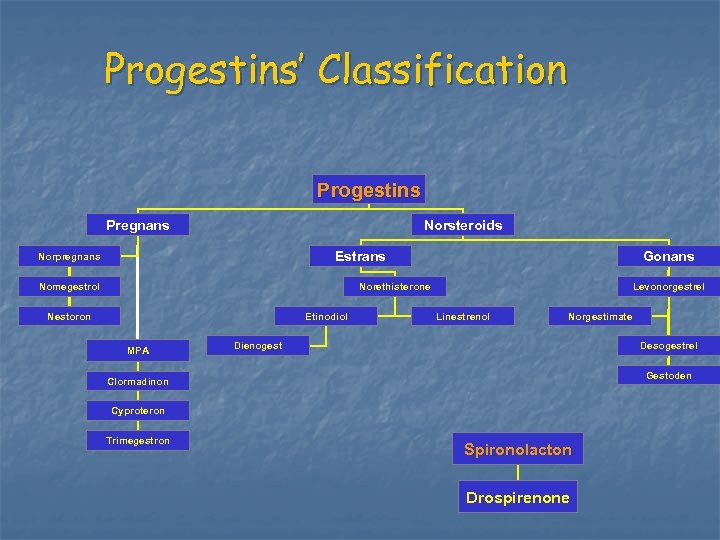 Progestins’ Classification Progestins Pregnans Norsteroids Estrans Norpregnans Nomegestrol Gonans Norethisterone Nestoron Etinodiol MPA Levonorgestrel Linestrenol Norgestimate Dienogest Desogestrel Gestoden Clormadinon Cyproteron Trimegestron Spironolacton Drospirenone
Progestins’ Classification Progestins Pregnans Norsteroids Estrans Norpregnans Nomegestrol Gonans Norethisterone Nestoron Etinodiol MPA Levonorgestrel Linestrenol Norgestimate Dienogest Desogestrel Gestoden Clormadinon Cyproteron Trimegestron Spironolacton Drospirenone
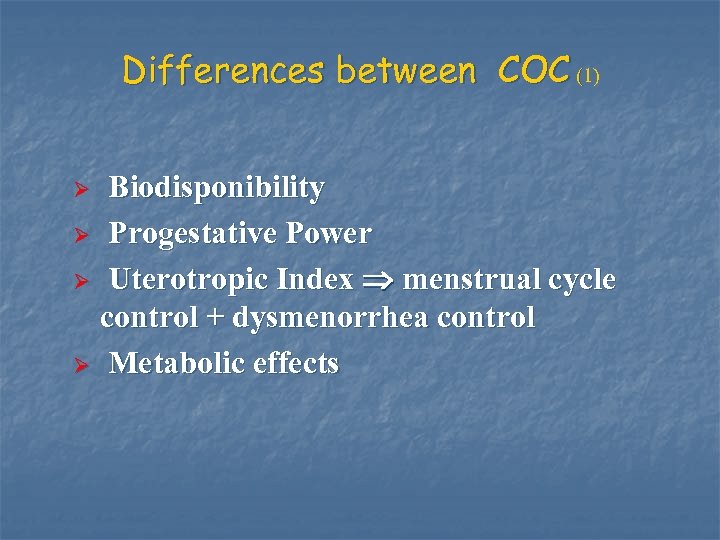 Differences between COC (1) Biodisponibility Ø Progestative Power Ø Uterotropic Index menstrual cycle control + dysmenorrhea control Ø Metabolic effects Ø
Differences between COC (1) Biodisponibility Ø Progestative Power Ø Uterotropic Index menstrual cycle control + dysmenorrhea control Ø Metabolic effects Ø
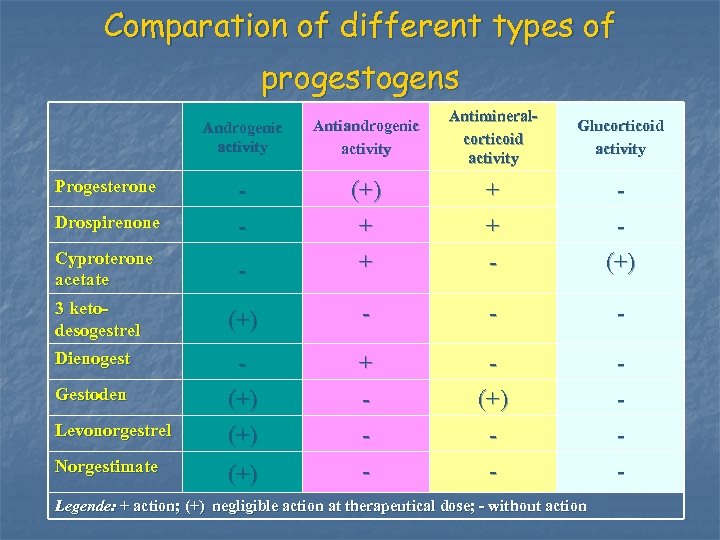 Comparation of different types of progestogens Androgenic activity Antiandrogenic activity Antimineralcorticoid activity Glucorticoid activity Drospirenone - (+) + + + - Cyproterone acetate - + - (+) 3 ketodesogestrel (+) - - - Dienogest (+) + - (+) - - Progesterone Gestoden Levonorgestrel Norgestimate (+) Legende: + action; (+) negligible action at therapeutical dose; - without action
Comparation of different types of progestogens Androgenic activity Antiandrogenic activity Antimineralcorticoid activity Glucorticoid activity Drospirenone - (+) + + + - Cyproterone acetate - + - (+) 3 ketodesogestrel (+) - - - Dienogest (+) + - (+) - - Progesterone Gestoden Levonorgestrel Norgestimate (+) Legende: + action; (+) negligible action at therapeutical dose; - without action
 Differences between COC (2) ü ü - COC Structure differs the old formulation from the new ones, in which estrogen & progestogen doses are less, with wish to reduce adverse events. There are limits to which compounds doses can be reduced: Maintenance of contraceptive efficacy with an satisfactory Pearl index This necessity can be obtain on pills with small doses in a regimen of 24 days: example: 15 µg ethinyl estradiol + 60 µg gestoden Brincat M, Muscat Baron Y, Galea R, 2003
Differences between COC (2) ü ü - COC Structure differs the old formulation from the new ones, in which estrogen & progestogen doses are less, with wish to reduce adverse events. There are limits to which compounds doses can be reduced: Maintenance of contraceptive efficacy with an satisfactory Pearl index This necessity can be obtain on pills with small doses in a regimen of 24 days: example: 15 µg ethinyl estradiol + 60 µg gestoden Brincat M, Muscat Baron Y, Galea R, 2003
 Criteria in choosing a contraceptive pill ü Good control of menstrual cycle ü Low dose of estrogens ü High tolerance
Criteria in choosing a contraceptive pill ü Good control of menstrual cycle ü Low dose of estrogens ü High tolerance
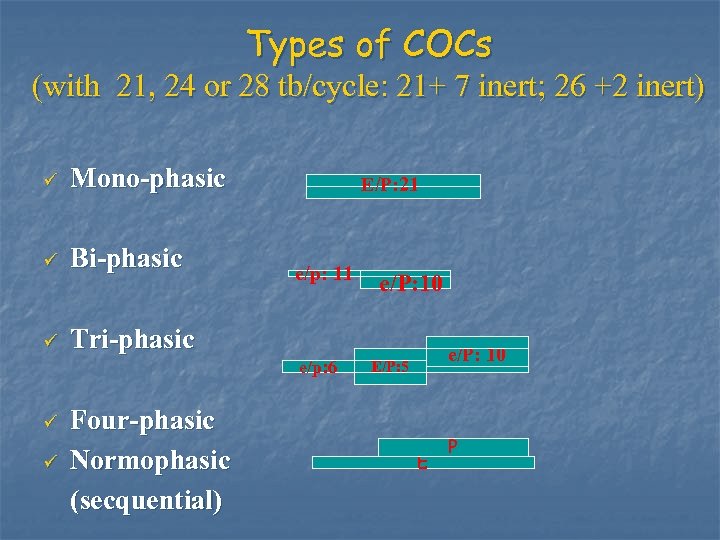 Types of COCs (with 21, 24 or 28 tb/cycle: 21+ 7 inert; 26 +2 inert) ü Mono-phasic ü Bi-phasic ü Tri-phasic E/P: 21 e/p: 11 e/p: 6 ü ü Four-phasic Normophasic (secquential) e/P: 10 E/P: 5 E P
Types of COCs (with 21, 24 or 28 tb/cycle: 21+ 7 inert; 26 +2 inert) ü Mono-phasic ü Bi-phasic ü Tri-phasic E/P: 21 e/p: 11 e/p: 6 ü ü Four-phasic Normophasic (secquential) e/P: 10 E/P: 5 E P
 A Novel four phasic COC An investigational, four-phasic oral contraceptive (OC) containing natural estradiol (E 2) valerate + dienogest, a progesterone formulation commonly used in Europe but not in the United States, "is expected to become the first globally available E 2 -containing OC”American College of Obstetricians and Gynecologists 56 th Annual ü ü • • • Clinical Meeting- 8 May 2008 Women received 20 cycles of 28 days of the four-phasic OC. Each cycle contained: 2 days: E 2 valerate 3 mg, 5 days: E 2 valerate 2 mg/dienogest 2 mg, 17 days: E 2 valerate 2 mg/dienogest 3 mg, and 2 days: E 2 valerate 1 mg, and 2 days: placebo.
A Novel four phasic COC An investigational, four-phasic oral contraceptive (OC) containing natural estradiol (E 2) valerate + dienogest, a progesterone formulation commonly used in Europe but not in the United States, "is expected to become the first globally available E 2 -containing OC”American College of Obstetricians and Gynecologists 56 th Annual ü ü • • • Clinical Meeting- 8 May 2008 Women received 20 cycles of 28 days of the four-phasic OC. Each cycle contained: 2 days: E 2 valerate 3 mg, 5 days: E 2 valerate 2 mg/dienogest 2 mg, 17 days: E 2 valerate 2 mg/dienogest 3 mg, and 2 days: E 2 valerate 1 mg, and 2 days: placebo.
 New revolution in contraception Ø Ø Quilara the four phasic contraceptive : 55 th Annual Meeting of ACOG (San-Diego, May 2007) Congress of Contraception and Health Reproduction Care (Praga, May 2008) World Congress of Controversis in Obst- Gynecol & Infertility (Paris, Nov. 2008) Congress of the European Society of Gynecology (Rome, Sept. 2009)
New revolution in contraception Ø Ø Quilara the four phasic contraceptive : 55 th Annual Meeting of ACOG (San-Diego, May 2007) Congress of Contraception and Health Reproduction Care (Praga, May 2008) World Congress of Controversis in Obst- Gynecol & Infertility (Paris, Nov. 2008) Congress of the European Society of Gynecology (Rome, Sept. 2009)
 Novel approaches to avoid bleeding (1) Loudon NB, 1977: tried the approach that gained interest around the year 2000 when surveys of women's attitudes toward monthly menstrual bleeding started to show a major change: ü more and more women declared that they would welcome a hormonal contraceptive method that reduced bleeding episodes to 4, 2 or even 1 per year.
Novel approaches to avoid bleeding (1) Loudon NB, 1977: tried the approach that gained interest around the year 2000 when surveys of women's attitudes toward monthly menstrual bleeding started to show a major change: ü more and more women declared that they would welcome a hormonal contraceptive method that reduced bleeding episodes to 4, 2 or even 1 per year.
 Novel approaches to avoid bleeding Ø Ø Ø (2) The first new modality consisted of changing the 7 day medication-free interval, either shortening it to fewer than 7 days, or by the administration of low-dose estrogens during the interval between packages. continuous administration regimens started to be investigated. Doctors had for years empirically utilized various continuous administration regimens. Amenorrhoea and absence of breakthrough bleeding increase in incidence with extended administration.
Novel approaches to avoid bleeding Ø Ø Ø (2) The first new modality consisted of changing the 7 day medication-free interval, either shortening it to fewer than 7 days, or by the administration of low-dose estrogens during the interval between packages. continuous administration regimens started to be investigated. Doctors had for years empirically utilized various continuous administration regimens. Amenorrhoea and absence of breakthrough bleeding increase in incidence with extended administration.
 Novel approaches to avoid bleeding ü ü ü (3) The first extended-cycle oral contraceptive regimen introduced in clinical practice is an 84 -day regimen that results in bleeding only 4 times a year. A commercial product specifically packed for continuous use is now available in Europe and contains 30 μg EE + 150 μg LNG. In a variation of this regimen, after administration of the same combination for 84 days, women are given 7 pills containing 10 μg EE -Kroll R, Reape KZ, Margolis M, 2010; Seatle, USA
Novel approaches to avoid bleeding ü ü ü (3) The first extended-cycle oral contraceptive regimen introduced in clinical practice is an 84 -day regimen that results in bleeding only 4 times a year. A commercial product specifically packed for continuous use is now available in Europe and contains 30 μg EE + 150 μg LNG. In a variation of this regimen, after administration of the same combination for 84 days, women are given 7 pills containing 10 μg EE -Kroll R, Reape KZ, Margolis M, 2010; Seatle, USA
 Novel approaches to avoid bleeding (4) ü A 6 -monthly regimen has also been tested: EE 20μg + LNG 100 μg taken with and without a hormone- free interval- Benagiano G, Carrara S, Fillippi V, 2009 – Sapienza University, Rome, Italy ü Women in the continuous group reported significantly fewer bleeding days requiring protection and were more likely to have amenorrhea; in addition they also reported significantly fewer days of bloating and menstrual pain.
Novel approaches to avoid bleeding (4) ü A 6 -monthly regimen has also been tested: EE 20μg + LNG 100 μg taken with and without a hormone- free interval- Benagiano G, Carrara S, Fillippi V, 2009 – Sapienza University, Rome, Italy ü Women in the continuous group reported significantly fewer bleeding days requiring protection and were more likely to have amenorrhea; in addition they also reported significantly fewer days of bloating and menstrual pain.
 Novel approaches to avoid bleeding (5) A yearly regimen is now developed: ü Each pill of this novel formulation contains EE 20 μg + LNG 90 μg to be taken continuously (364 days; 13 cycles) per year. ü A phase III trial has now evaluated safety, efficacy and menses inhibition. ü At the end of the 1 -year trial amenorrhea was present in 58. 7% cases and a complete absence of bleeding in 79. 0%. Overall, the number of bleeding and spotting days per pill pack declined with time and adverse events and discontinuations were comparable to those reported for cyclic oral contraceptive regimens Archer DF, Jensen JT, et al, 2006 - CONRAD Clinical Research Center, Eastern Virginia Medical School, Norfolk (USA)
Novel approaches to avoid bleeding (5) A yearly regimen is now developed: ü Each pill of this novel formulation contains EE 20 μg + LNG 90 μg to be taken continuously (364 days; 13 cycles) per year. ü A phase III trial has now evaluated safety, efficacy and menses inhibition. ü At the end of the 1 -year trial amenorrhea was present in 58. 7% cases and a complete absence of bleeding in 79. 0%. Overall, the number of bleeding and spotting days per pill pack declined with time and adverse events and discontinuations were comparable to those reported for cyclic oral contraceptive regimens Archer DF, Jensen JT, et al, 2006 - CONRAD Clinical Research Center, Eastern Virginia Medical School, Norfolk (USA)
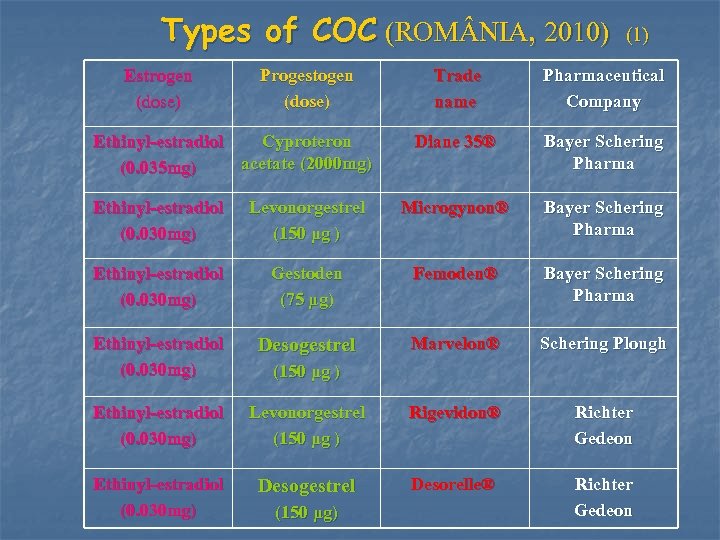 Types of COC (ROM NIA, 2010) Estrogen (dose) Progestogen (dose) Ethinyl-estradiol Cyproteron acetate (2000 mg) (0. 035 mg) (1) Trade name Pharmaceutical Company Diane 35® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Levonorgestrel (150 μg ) Microgynon® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Gestoden (75 μg) Femoden® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Desogestrel Marvelon® Schering Plough Ethinyl-estradiol (0. 030 mg) Levonorgestrel (150 μg ) Rigevidon® Richter Gedeon Ethinyl-estradiol (0. 030 mg) Desogestrel (150 μg ) (150 μg) Desorelle® Richter Gedeon
Types of COC (ROM NIA, 2010) Estrogen (dose) Progestogen (dose) Ethinyl-estradiol Cyproteron acetate (2000 mg) (0. 035 mg) (1) Trade name Pharmaceutical Company Diane 35® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Levonorgestrel (150 μg ) Microgynon® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Gestoden (75 μg) Femoden® Bayer Schering Pharma Ethinyl-estradiol (0. 030 mg) Desogestrel Marvelon® Schering Plough Ethinyl-estradiol (0. 030 mg) Levonorgestrel (150 μg ) Rigevidon® Richter Gedeon Ethinyl-estradiol (0. 030 mg) Desogestrel (150 μg ) (150 μg) Desorelle® Richter Gedeon
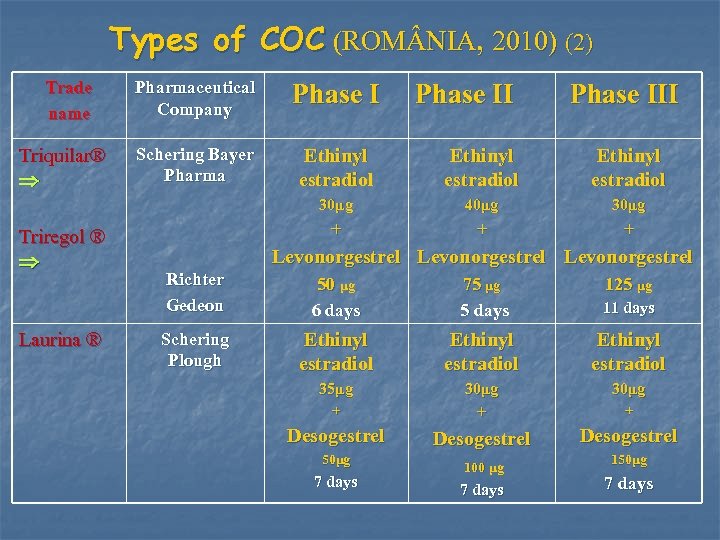 Types of COC (ROM NIA, 2010) (2) Trade name Triregol ® Laurina ® Phase I Schering Bayer Pharma Ethinyl estradiol 30μg Triquilar® Pharmaceutical Company 40μg 30μg + + + Phase III Levonorgestrel Richter Gedeon 50 μg 6 days Schering Plough Ethinyl estradiol 35μg 30μg + Desogestrel 50μg 100 μg 150μg 7 days 75 μg 5 days 7 days 125 μg 11 days + 7 days
Types of COC (ROM NIA, 2010) (2) Trade name Triregol ® Laurina ® Phase I Schering Bayer Pharma Ethinyl estradiol 30μg Triquilar® Pharmaceutical Company 40μg 30μg + + + Phase III Levonorgestrel Richter Gedeon 50 μg 6 days Schering Plough Ethinyl estradiol 35μg 30μg + Desogestrel 50μg 100 μg 150μg 7 days 75 μg 5 days 7 days 125 μg 11 days + 7 days
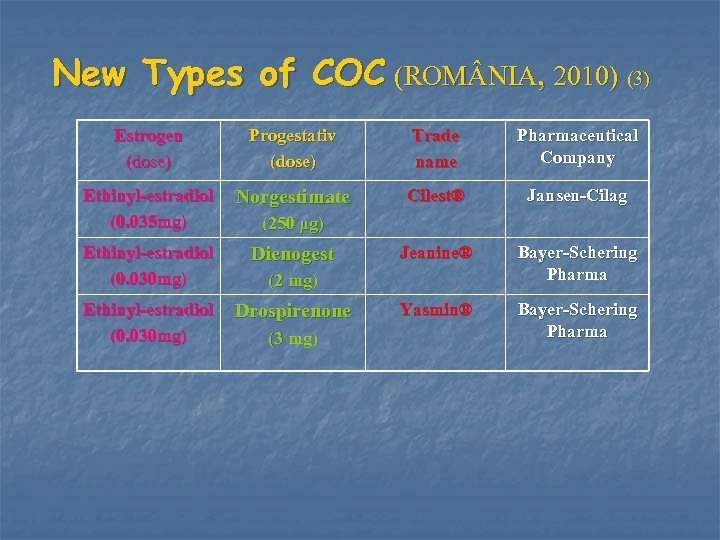 New Types of COC (ROM NIA, 2010) (3) Estrogen (dose) Progestativ (dose) Trade name Pharmaceutical Company Ethinyl-estradiol (0. 035 mg) Norgestimate Cilest® Jansen-Cilag Ethinyl-estradiol (0. 030 mg) Dienogest Jeanine® Bayer-Schering Pharma Ethinyl-estradiol (0. 030 mg) Drospirenone Yasmin® Bayer-Schering Pharma (250 μg) (2 mg) (3 mg)
New Types of COC (ROM NIA, 2010) (3) Estrogen (dose) Progestativ (dose) Trade name Pharmaceutical Company Ethinyl-estradiol (0. 035 mg) Norgestimate Cilest® Jansen-Cilag Ethinyl-estradiol (0. 030 mg) Dienogest Jeanine® Bayer-Schering Pharma Ethinyl-estradiol (0. 030 mg) Drospirenone Yasmin® Bayer-Schering Pharma (250 μg) (2 mg) (3 mg)
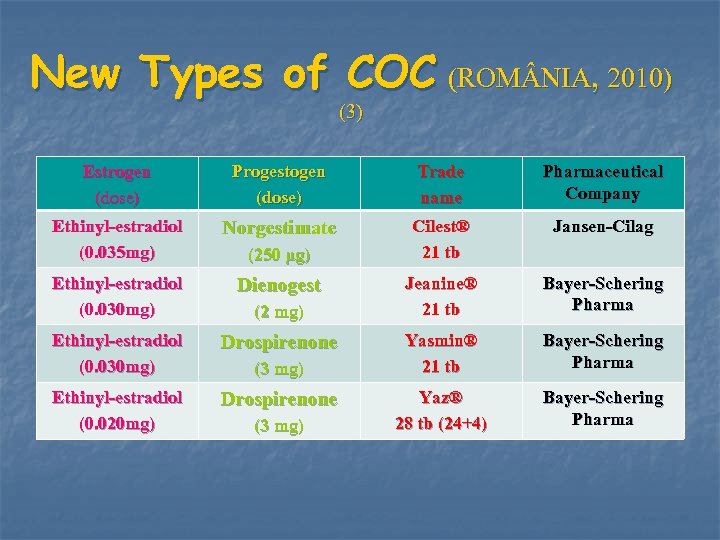 New Types of COC (ROM NIA, 2010) (3) Estrogen (dose) Progestogen (dose) Trade name Ethinyl-estradiol (0. 035 mg) Norgestimate Cilest® 21 tb Jansen-Cilag Ethinyl-estradiol (0. 030 mg) Dienogest Jeanine® 21 tb Bayer-Schering Pharma Ethinyl-estradiol (0. 030 mg) Drospirenone Yasmin® 21 tb Bayer-Schering Pharma Ethinyl-estradiol (0. 020 mg) Drospirenone Yaz® 28 tb (24+4) Bayer-Schering Pharma (250 μg) (2 mg) (3 mg) Pharmaceutical Company
New Types of COC (ROM NIA, 2010) (3) Estrogen (dose) Progestogen (dose) Trade name Ethinyl-estradiol (0. 035 mg) Norgestimate Cilest® 21 tb Jansen-Cilag Ethinyl-estradiol (0. 030 mg) Dienogest Jeanine® 21 tb Bayer-Schering Pharma Ethinyl-estradiol (0. 030 mg) Drospirenone Yasmin® 21 tb Bayer-Schering Pharma Ethinyl-estradiol (0. 020 mg) Drospirenone Yaz® 28 tb (24+4) Bayer-Schering Pharma (250 μg) (2 mg) (3 mg) Pharmaceutical Company
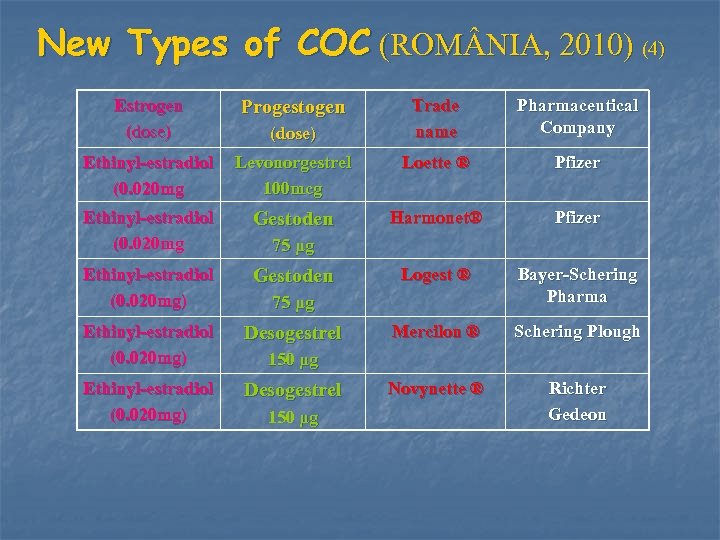 New Types of COC (ROM NIA, 2010) (4) Estrogen (dose) Progestogen Ethinyl-estradiol (0. 020 mg Levonorgestrel 100 mcg Loette ® Pfizer Ethinyl-estradiol (0. 020 mg Gestoden Harmonet® Pfizer Ethinyl-estradiol (0. 020 mg) Gestoden Logest ® Bayer-Schering Pharma Ethinyl-estradiol (0. 020 mg) Desogestrel Mercilon ® Schering Plough Ethinyl-estradiol (0. 020 mg) Desogestrel Novynette ® Richter Gedeon (dose) Trade name Pharmaceutical Company 75 μg 150 μg
New Types of COC (ROM NIA, 2010) (4) Estrogen (dose) Progestogen Ethinyl-estradiol (0. 020 mg Levonorgestrel 100 mcg Loette ® Pfizer Ethinyl-estradiol (0. 020 mg Gestoden Harmonet® Pfizer Ethinyl-estradiol (0. 020 mg) Gestoden Logest ® Bayer-Schering Pharma Ethinyl-estradiol (0. 020 mg) Desogestrel Mercilon ® Schering Plough Ethinyl-estradiol (0. 020 mg) Desogestrel Novynette ® Richter Gedeon (dose) Trade name Pharmaceutical Company 75 μg 150 μg
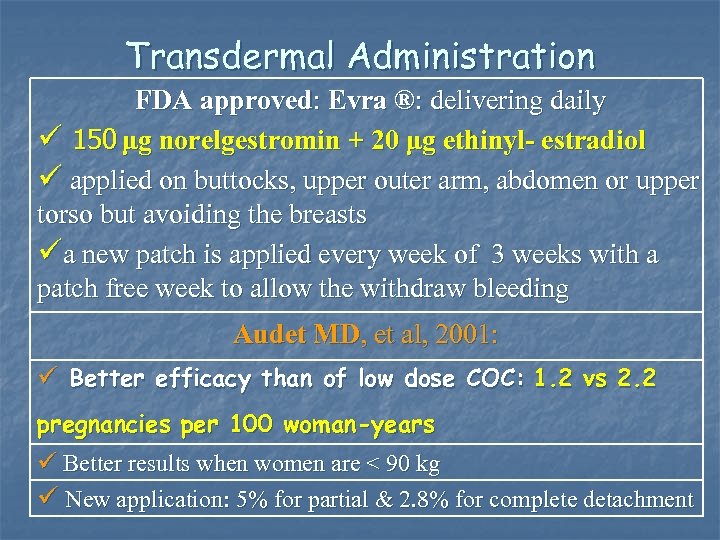 Transdermal Administration FDA approved: Evra ®: delivering daily ü 150 μg norelgestromin + 20 μg ethinyl- estradiol ü applied on buttocks, upper outer arm, abdomen or upper torso but avoiding the breasts üa new patch is applied every week of 3 weeks with a patch free week to allow the withdraw bleeding Audet MD, et al, 2001: ü Better efficacy than of low dose COC: 1. 2 vs 2. 2 pregnancies per 100 woman-years ü Better results when women are < 90 kg ü New application: 5% for partial & 2. 8% for complete detachment
Transdermal Administration FDA approved: Evra ®: delivering daily ü 150 μg norelgestromin + 20 μg ethinyl- estradiol ü applied on buttocks, upper outer arm, abdomen or upper torso but avoiding the breasts üa new patch is applied every week of 3 weeks with a patch free week to allow the withdraw bleeding Audet MD, et al, 2001: ü Better efficacy than of low dose COC: 1. 2 vs 2. 2 pregnancies per 100 woman-years ü Better results when women are < 90 kg ü New application: 5% for partial & 2. 8% for complete detachment
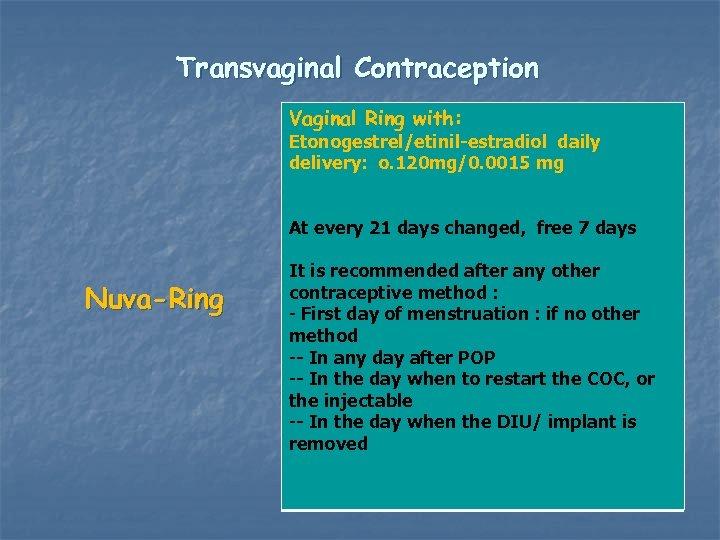 Transvaginal Contraception Vaginal Ring with: Etonogestrel/etinil-estradiol daily delivery: o. 120 mg/0. 0015 mg At every 21 days changed, free 7 days Nuva-Ring It is recommended after any other contraceptive method : - First day of menstruation : if no other method -- In any day after POP -- In the day when to restart the COC, or the injectable -- In the day when the DIU/ implant is removed
Transvaginal Contraception Vaginal Ring with: Etonogestrel/etinil-estradiol daily delivery: o. 120 mg/0. 0015 mg At every 21 days changed, free 7 days Nuva-Ring It is recommended after any other contraceptive method : - First day of menstruation : if no other method -- In any day after POP -- In the day when to restart the COC, or the injectable -- In the day when the DIU/ implant is removed
 Progestogen only Pills (minipills) (1) ü ü ü § § The lowest dose that prevents pregnancy Alternative for women with estrogens intolerance Mechanism of action: Influence cervical mucus and ovarian follicles maturation & ovulation blockade- in a less degree Little effects on carbohydrate metabolism & cardiovascular diseases (thrombosis, migraines, elder women, smokers) ü § § § Disadvantages: become contraindications Ectopic pregnancy Abnormal uterine bleeding: irregular, spotting, amenorrhea Functional ovarian cysts
Progestogen only Pills (minipills) (1) ü ü ü § § The lowest dose that prevents pregnancy Alternative for women with estrogens intolerance Mechanism of action: Influence cervical mucus and ovarian follicles maturation & ovulation blockade- in a less degree Little effects on carbohydrate metabolism & cardiovascular diseases (thrombosis, migraines, elder women, smokers) ü § § § Disadvantages: become contraindications Ectopic pregnancy Abnormal uterine bleeding: irregular, spotting, amenorrhea Functional ovarian cysts
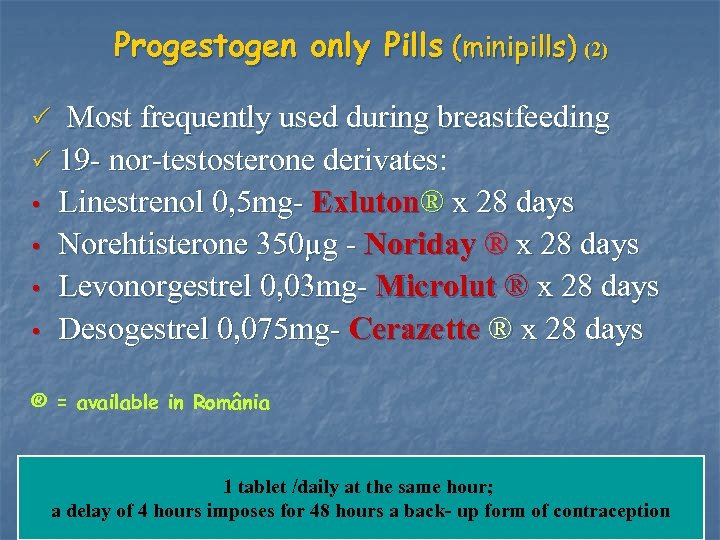 Progestogen only Pills (minipills) (2) Most frequently used during breastfeeding P 19 - nor-testosterone derivates: derivates • Linestrenol 0, 5 mg- Exluton® x 28 days • Norehtisterone 350μg - Noriday ® x 28 days • Levonorgestrel 0, 03 mg- Microlut ® x 28 days • Desogestrel 0, 075 mg- Cerazette ® x 28 days P ® = available in România 1 tablet /daily at the same hour; a delay of 4 hours imposes for 48 hours a back- up form of contraception
Progestogen only Pills (minipills) (2) Most frequently used during breastfeeding P 19 - nor-testosterone derivates: derivates • Linestrenol 0, 5 mg- Exluton® x 28 days • Norehtisterone 350μg - Noriday ® x 28 days • Levonorgestrel 0, 03 mg- Microlut ® x 28 days • Desogestrel 0, 075 mg- Cerazette ® x 28 days P ® = available in România 1 tablet /daily at the same hour; a delay of 4 hours imposes for 48 hours a back- up form of contraception
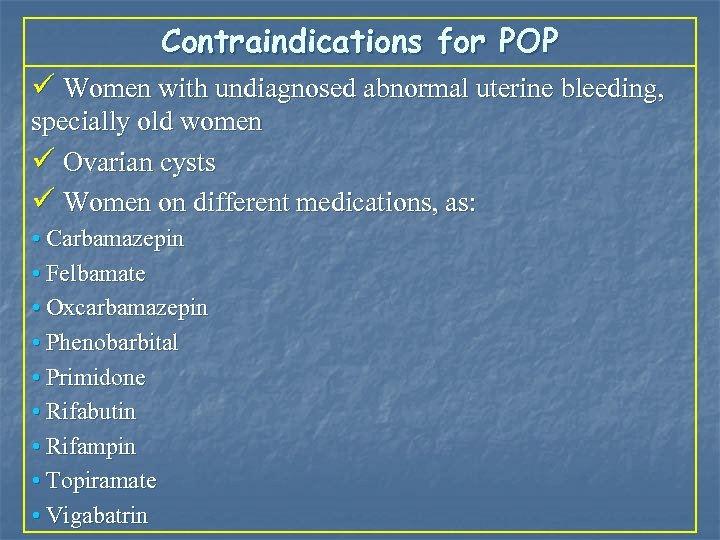 Contraindications for POP ü Women with undiagnosed abnormal uterine bleeding, specially old women ü Ovarian cysts ü Women on different medications, as: • Carbamazepin • Felbamate • Oxcarbamazepin • Phenobarbital • Primidone • Rifabutin • Rifampin • Topiramate • Vigabatrin
Contraindications for POP ü Women with undiagnosed abnormal uterine bleeding, specially old women ü Ovarian cysts ü Women on different medications, as: • Carbamazepin • Felbamate • Oxcarbamazepin • Phenobarbital • Primidone • Rifabutin • Rifampin • Topiramate • Vigabatrin
 Long lasting Contraceptive Methods Injectables (1) Represent a method of contraception with synthetic steroid hormones; intramuscularly administrated high doses with long lasting effects, which allow long intervals between doses P Time of action: 3 months injectable : DMPA: Depo-Provera®; Megestron®; Depo-Ralovera®: 150 mg • 2 months injectable : NET- EN: Noristerat®; Norgest®: 200 mg • monthly injectable : (estrogen + progestogen): Cyclofem ®; Mesigyna ®; Lunelle®: 5 mg estradiol cypionate • + 25 mg MPA (USA, 1999) ® available in Romania
Long lasting Contraceptive Methods Injectables (1) Represent a method of contraception with synthetic steroid hormones; intramuscularly administrated high doses with long lasting effects, which allow long intervals between doses P Time of action: 3 months injectable : DMPA: Depo-Provera®; Megestron®; Depo-Ralovera®: 150 mg • 2 months injectable : NET- EN: Noristerat®; Norgest®: 200 mg • monthly injectable : (estrogen + progestogen): Cyclofem ®; Mesigyna ®; Lunelle®: 5 mg estradiol cypionate • + 25 mg MPA (USA, 1999) ® available in Romania
 Long lasting Contraceptive Methods Injectables (2) Mechanism of action ü Ovulation inhibition: by antigonadotrophic & antiestrogenic effect; directly ovarian blockade ü Increased cervical mucus viscosity, which becomes a barrier for spermatozoa ü Changes in tubal rate transport ü Endometrial changes : improper for implantation
Long lasting Contraceptive Methods Injectables (2) Mechanism of action ü Ovulation inhibition: by antigonadotrophic & antiestrogenic effect; directly ovarian blockade ü Increased cervical mucus viscosity, which becomes a barrier for spermatozoa ü Changes in tubal rate transport ü Endometrial changes : improper for implantation
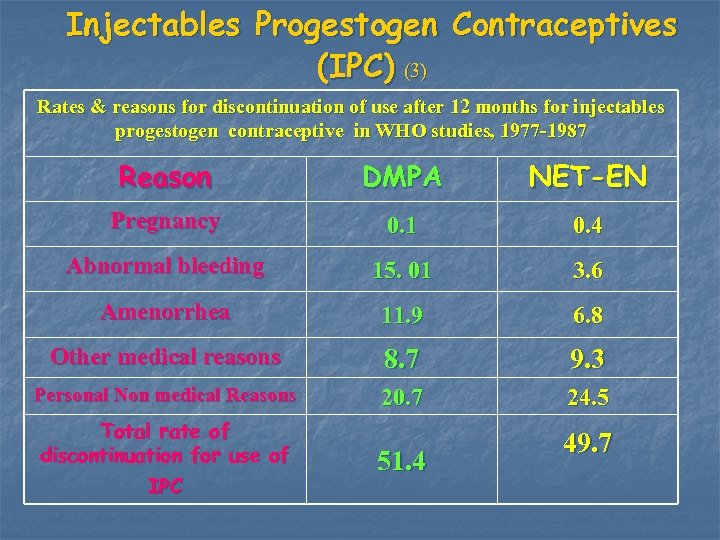 Injectables Progestogen Contraceptives (IPC) (3) Rates & reasons for discontinuation of use after 12 months for injectables progestogen contraceptive in WHO studies, 1977 -1987 Reason DMPA NET-EN Pregnancy 0. 1 0. 4 Abnormal bleeding 15. 01 3. 6 Amenorrhea 11. 9 6. 8 Other medical reasons 8. 7 9. 3 Personal Non medical Reasons 20. 7 24. 5 Total rate of discontinuation for use of IPC 51. 4 49. 7
Injectables Progestogen Contraceptives (IPC) (3) Rates & reasons for discontinuation of use after 12 months for injectables progestogen contraceptive in WHO studies, 1977 -1987 Reason DMPA NET-EN Pregnancy 0. 1 0. 4 Abnormal bleeding 15. 01 3. 6 Amenorrhea 11. 9 6. 8 Other medical reasons 8. 7 9. 3 Personal Non medical Reasons 20. 7 24. 5 Total rate of discontinuation for use of IPC 51. 4 49. 7
 Long lasting Contraceptive Methods Injectables(4) Reversibility delay, but not a loss: fertility recovers at injectable discontinuation ü ü ü Mean time from discontinuation to conception is ~ 9 months, inclusively those 3 months during which the drug is active; > 90% of women conceive within first 2 years after DMPA discontinuation The delay for fertility recover is shorter for NET-EN than for DMPA Women with amenorrhea during NET-EN use have a longer delay for fertility recover vs those with regular menses
Long lasting Contraceptive Methods Injectables(4) Reversibility delay, but not a loss: fertility recovers at injectable discontinuation ü ü ü Mean time from discontinuation to conception is ~ 9 months, inclusively those 3 months during which the drug is active; > 90% of women conceive within first 2 years after DMPA discontinuation The delay for fertility recover is shorter for NET-EN than for DMPA Women with amenorrhea during NET-EN use have a longer delay for fertility recover vs those with regular menses
 Long lasting Contraceptive Methods Injectables (5) Interferences & interactions (a) Lipids and lipoproteins: Ø No effect, or reduction of triglycerides and total cholesterol (in connection to antiestrogenic effect of Pgs) P DMPA decrease with 10 -l 5% of HDL- cholesterol, HDL 2, HDL 3 , Apo Al and Apo Bl 00. P NET-EN decrease with 25% of HDL- cholesterol Carbohydrates: Ø No report of overt DM after use of DMPA, even if progestogens induce the reducing of oral glucose tolerence, hyperinsulinemia and insuline resistence. Proteins: Ø No interference with synthesis of globulins, angiotensinogen (some exceptions for NET-EN), fibrinogen, clotting factors: II, VII, X, XIII, antithrombine III and anti-factor Xa
Long lasting Contraceptive Methods Injectables (5) Interferences & interactions (a) Lipids and lipoproteins: Ø No effect, or reduction of triglycerides and total cholesterol (in connection to antiestrogenic effect of Pgs) P DMPA decrease with 10 -l 5% of HDL- cholesterol, HDL 2, HDL 3 , Apo Al and Apo Bl 00. P NET-EN decrease with 25% of HDL- cholesterol Carbohydrates: Ø No report of overt DM after use of DMPA, even if progestogens induce the reducing of oral glucose tolerence, hyperinsulinemia and insuline resistence. Proteins: Ø No interference with synthesis of globulins, angiotensinogen (some exceptions for NET-EN), fibrinogen, clotting factors: II, VII, X, XIII, antithrombine III and anti-factor Xa
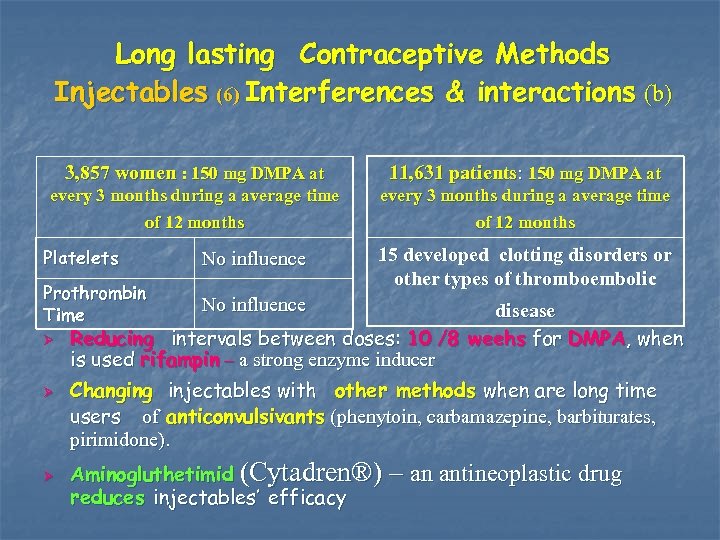 Long lasting Contraceptive Methods Injectables (6) Interferences & interactions (b) 3, 857 women : 150 mg DMPA at 11, 631 patients: 150 mg DMPA at every 3 months during a average time of 12 months Platelets No influence Prothrombin Time 15 developed clotting disorders or other types of thromboembolic No influence Ø Ø Ø disease Reducing intervals between doses: 10 /8 weehs for DMPA, when is used rifampin – a strong enzyme inducer Changing injectables with other methods when are long time users of anticonvulsivants (phenytoin, carbamazepine, barbiturates, pirimidone). Aminogluthetimid (Cytadren®) – an antineoplastic drug reduces injectables’ efficacy
Long lasting Contraceptive Methods Injectables (6) Interferences & interactions (b) 3, 857 women : 150 mg DMPA at 11, 631 patients: 150 mg DMPA at every 3 months during a average time of 12 months Platelets No influence Prothrombin Time 15 developed clotting disorders or other types of thromboembolic No influence Ø Ø Ø disease Reducing intervals between doses: 10 /8 weehs for DMPA, when is used rifampin – a strong enzyme inducer Changing injectables with other methods when are long time users of anticonvulsivants (phenytoin, carbamazepine, barbiturates, pirimidone). Aminogluthetimid (Cytadren®) – an antineoplastic drug reduces injectables’ efficacy
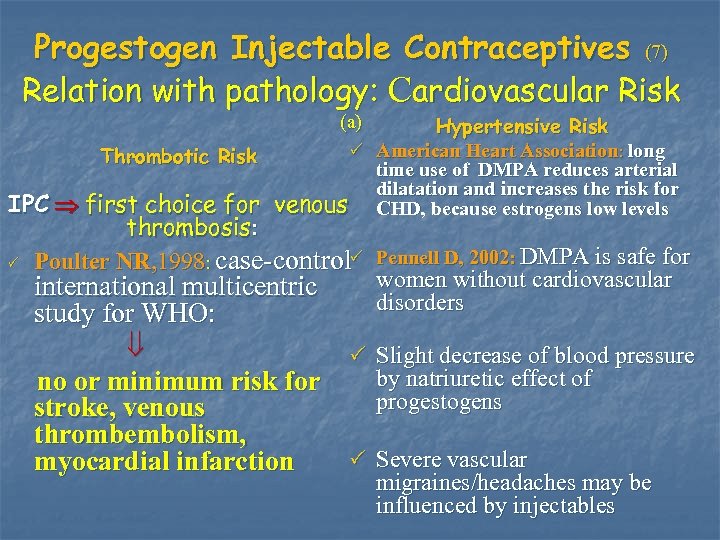 Progestogen Injectable Contraceptives (7) Relation with pathology: Cardiovascular Risk (a) Hypertensive Risk P American Heart Association: long Thrombotic Risk time use of DMPA reduces arterial dilatation and increases the risk for IPC first choice for venous CHD, because estrogens low levels P thrombosis: Poulter NR, 1998: case-control. P Pennell D, 2002: DMPA is safe for women without cardiovascular international multicentric disorders study for WHO: no or minimum risk for stroke, venous thrombembolism, myocardial infarction P Slight decrease of blood pressure by natriuretic effect of progestogens P Severe vascular migraines/headaches may be influenced by injectables
Progestogen Injectable Contraceptives (7) Relation with pathology: Cardiovascular Risk (a) Hypertensive Risk P American Heart Association: long Thrombotic Risk time use of DMPA reduces arterial dilatation and increases the risk for IPC first choice for venous CHD, because estrogens low levels P thrombosis: Poulter NR, 1998: case-control. P Pennell D, 2002: DMPA is safe for women without cardiovascular international multicentric disorders study for WHO: no or minimum risk for stroke, venous thrombembolism, myocardial infarction P Slight decrease of blood pressure by natriuretic effect of progestogens P Severe vascular migraines/headaches may be influenced by injectables
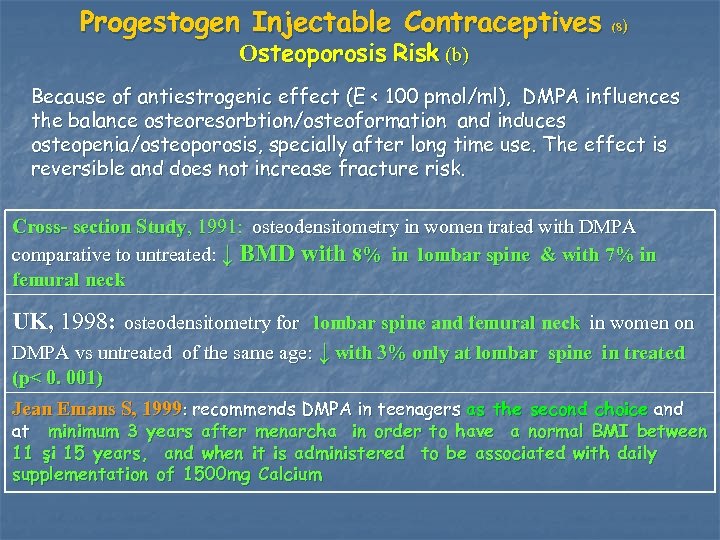 Progestogen Injectable Contraceptives Osteoporosis Risk (b) (8) Because of antiestrogenic effect (E < 100 pmol/ml), DMPA influences the balance osteoresorbtion/osteoformation and induces osteopenia/osteoporosis, specially after long time use. The effect is reversible and does not increase fracture risk. Cross- section Study, 1991: osteodensitometry in women trated with DMPA comparative to untreated: ↓ BMD with 8% in lombar spine & with 7% in femural neck UK, 1998: osteodensitometry for lombar spine and femural neck in women on DMPA vs untreated of the same age: ↓ with 3% only at lombar spine in treated (p< 0. 001) Jean Emans S, 1999: recommends DMPA in teenagers as the second choice and at minimum 3 years after menarcha in order to have a normal BMI between 11 şi 15 years, and when it is administered to be associated with daily supplementation of 1500 mg Calcium
Progestogen Injectable Contraceptives Osteoporosis Risk (b) (8) Because of antiestrogenic effect (E < 100 pmol/ml), DMPA influences the balance osteoresorbtion/osteoformation and induces osteopenia/osteoporosis, specially after long time use. The effect is reversible and does not increase fracture risk. Cross- section Study, 1991: osteodensitometry in women trated with DMPA comparative to untreated: ↓ BMD with 8% in lombar spine & with 7% in femural neck UK, 1998: osteodensitometry for lombar spine and femural neck in women on DMPA vs untreated of the same age: ↓ with 3% only at lombar spine in treated (p< 0. 001) Jean Emans S, 1999: recommends DMPA in teenagers as the second choice and at minimum 3 years after menarcha in order to have a normal BMI between 11 şi 15 years, and when it is administered to be associated with daily supplementation of 1500 mg Calcium
 Progestogen Injectable Contraceptives (9) Relation with pathology Neoplasias (c) P Endometrial cancer : ↓ 5 times the risk, protection of minimum 8 yrs after discontinuation P Epithelial Ovarian cancer : RR=1. 1 vs non-users P Cervical cancer: RR= 1. 2; DMPA ↑ risk for in situ CC, reversible, & induced lesions do not progress to invasive disease. DOES NOT increase the incidence of invasive cervical cancer: WHO Collaborative Study, 1995 P Primary Liver Cancer : RR= 1. 1 (Kenya) & 0. 3 (Thailand)
Progestogen Injectable Contraceptives (9) Relation with pathology Neoplasias (c) P Endometrial cancer : ↓ 5 times the risk, protection of minimum 8 yrs after discontinuation P Epithelial Ovarian cancer : RR=1. 1 vs non-users P Cervical cancer: RR= 1. 2; DMPA ↑ risk for in situ CC, reversible, & induced lesions do not progress to invasive disease. DOES NOT increase the incidence of invasive cervical cancer: WHO Collaborative Study, 1995 P Primary Liver Cancer : RR= 1. 1 (Kenya) & 0. 3 (Thailand)
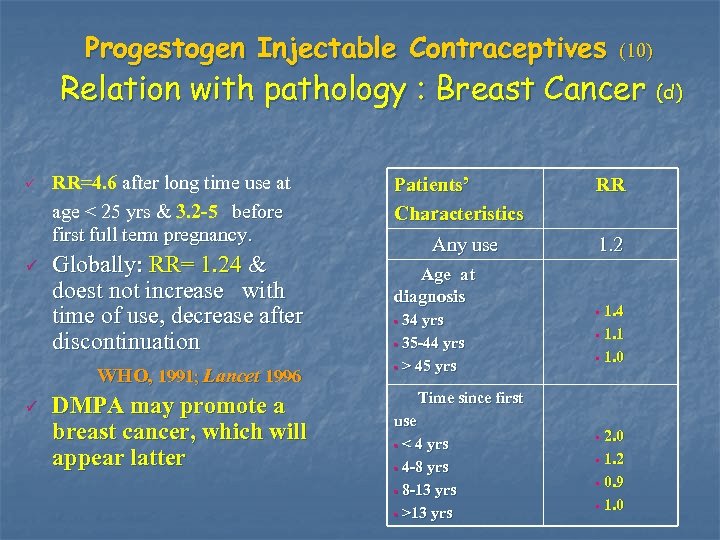 Progestogen Injectable Contraceptives (10) Relation with pathology : Breast Cancer (d) P P RR=4. 6 after long time use at age < 25 yrs & 3. 2 -5 before first full term pregnancy. Globally: RR= 1. 24 & doest not increase with time of use, decrease after discontinuation WHO, 1991; Lancet 1996 P DMPA may promote a breast cancer, which will appear latter Patients’ Characteristics Any use Age at diagnosis • 34 yrs • 35 -44 yrs • > 45 yrs Time since first use • < 4 yrs • 4 -8 yrs • 8 -13 yrs • >13 yrs RR 1. 2 • 1. 4 • 1. 1 • 1. 0 • 2. 0 • 1. 2 • 0. 9 • 1. 0
Progestogen Injectable Contraceptives (10) Relation with pathology : Breast Cancer (d) P P RR=4. 6 after long time use at age < 25 yrs & 3. 2 -5 before first full term pregnancy. Globally: RR= 1. 24 & doest not increase with time of use, decrease after discontinuation WHO, 1991; Lancet 1996 P DMPA may promote a breast cancer, which will appear latter Patients’ Characteristics Any use Age at diagnosis • 34 yrs • 35 -44 yrs • > 45 yrs Time since first use • < 4 yrs • 4 -8 yrs • 8 -13 yrs • >13 yrs RR 1. 2 • 1. 4 • 1. 1 • 1. 0 • 2. 0 • 1. 2 • 0. 9 • 1. 0
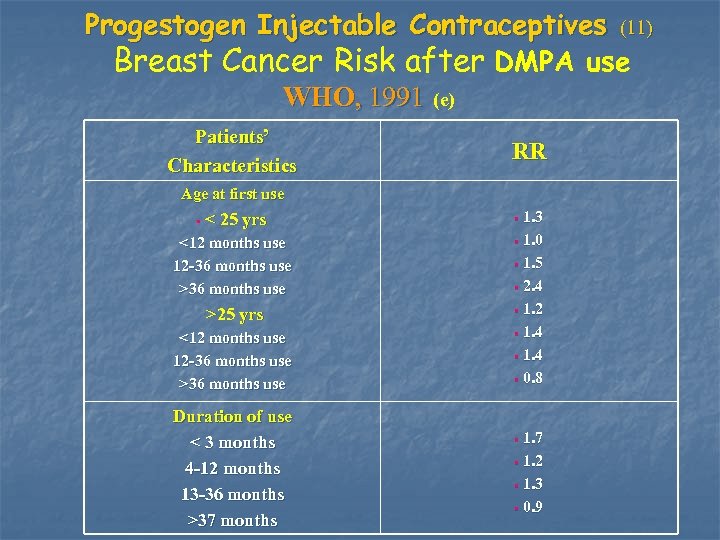 Progestogen Injectable Contraceptives (11) Breast Cancer Risk after DMPA use WHO, 1991 (e) Patients’ Characteristics RR Age at first use < 25 yrs • 1. 3 <12 months use 12 -36 months use >36 months use • 1. 0 >25 yrs • 1. 2 • <12 months use 12 -36 months use >36 months use Duration of use < 3 months 4 -12 months 13 -36 months >37 months • 1. 5 • 2. 4 • 1. 4 • 0. 8 • 1. 7 • 1. 2 • 1. 3 • 0. 9
Progestogen Injectable Contraceptives (11) Breast Cancer Risk after DMPA use WHO, 1991 (e) Patients’ Characteristics RR Age at first use < 25 yrs • 1. 3 <12 months use 12 -36 months use >36 months use • 1. 0 >25 yrs • 1. 2 • <12 months use 12 -36 months use >36 months use Duration of use < 3 months 4 -12 months 13 -36 months >37 months • 1. 5 • 2. 4 • 1. 4 • 0. 8 • 1. 7 • 1. 2 • 1. 3 • 0. 9
 Progestogen Injectable Contraceptives Advantages Ø Ø Ø (12) High efficiency: > COC’s Appropriate – no connection to intercourse Reversible (with same delay) Do not have estrogens Do noy implay genitalia manipulation ↓ menstrual cycle disorders Protection for PID and candidiosis Prevention for ectopic pregnancy (ovulation blockade) ↓ functional ovarian cysts Protection of endometrial cancer Benefits for women with menstrual scissures
Progestogen Injectable Contraceptives Advantages Ø Ø Ø (12) High efficiency: > COC’s Appropriate – no connection to intercourse Reversible (with same delay) Do not have estrogens Do noy implay genitalia manipulation ↓ menstrual cycle disorders Protection for PID and candidiosis Prevention for ectopic pregnancy (ovulation blockade) ↓ functional ovarian cysts Protection of endometrial cancer Benefits for women with menstrual scissures
 Progestogen Injectable Contraceptives Disadvantages P P P P P (13) The method can not be stoped once it is administered Injectable form may be not suitable for some women Menstrual disorders Weight gain Delayed of fertility return Osteoporosis Chloasma – after exposure to ultraviolet radiation Depression Subjective side effects
Progestogen Injectable Contraceptives Disadvantages P P P P P (13) The method can not be stoped once it is administered Injectable form may be not suitable for some women Menstrual disorders Weight gain Delayed of fertility return Osteoporosis Chloasma – after exposure to ultraviolet radiation Depression Subjective side effects
 Progestogen Injectable Contraceptives Indications P Venous Thrombembolism or known blood clotting abnormalities: SEL P Preparation for surgery P P P Forgetful pills’ women P Patients in need for maximum protection after immunisation against rubela, women partners of men going to vasectomy, postpartum patients who avoid sterilization or still waiting for tubal sterilization (14) During the treatment with Accutane (induces offspring’s congenital defects) or during oral anticoagulant therapy or valproic acid Patients with long term progestogens use, as: premenstrual syndrome, endometriosis P Alternative to IUD, in cases with high risk for IUD (young women whose male partners have many other female partners) P Sickle cell anemia Women with scissures At woman’s choice P P
Progestogen Injectable Contraceptives Indications P Venous Thrombembolism or known blood clotting abnormalities: SEL P Preparation for surgery P P P Forgetful pills’ women P Patients in need for maximum protection after immunisation against rubela, women partners of men going to vasectomy, postpartum patients who avoid sterilization or still waiting for tubal sterilization (14) During the treatment with Accutane (induces offspring’s congenital defects) or during oral anticoagulant therapy or valproic acid Patients with long term progestogens use, as: premenstrual syndrome, endometriosis P Alternative to IUD, in cases with high risk for IUD (young women whose male partners have many other female partners) P Sickle cell anemia Women with scissures At woman’s choice P P
 Contraindications for Injectables Obstetrical/Gynecologic Conditions Breast feeding < 6 weeks from delivery Pregnancy Unexplained abnormal uterine bleedong Breast Cancer (current) Chronic diseases /Other conditions Cardiovascular Conditions mild ( BP <160/100) to moderate HBP (BP <180/110) severe HBP (BP >180/110) and/or HBP with vascular disease CHD and stroke Severe. reccurent headache, inclusive migrene with neurological signs of focal (at any age) Diabetes mellitus with rethinopathy, nefropathy, neuropathy Diabetes mellitus of >20 years Breast diseases (history of cancer without signs of evolution in the last 5 years) Hepatites (active); Cirrhosis (severe); Jaundice Liver tumors (benign/ malignant) Othosclerozsis
Contraindications for Injectables Obstetrical/Gynecologic Conditions Breast feeding < 6 weeks from delivery Pregnancy Unexplained abnormal uterine bleedong Breast Cancer (current) Chronic diseases /Other conditions Cardiovascular Conditions mild ( BP <160/100) to moderate HBP (BP <180/110) severe HBP (BP >180/110) and/or HBP with vascular disease CHD and stroke Severe. reccurent headache, inclusive migrene with neurological signs of focal (at any age) Diabetes mellitus with rethinopathy, nefropathy, neuropathy Diabetes mellitus of >20 years Breast diseases (history of cancer without signs of evolution in the last 5 years) Hepatites (active); Cirrhosis (severe); Jaundice Liver tumors (benign/ malignant) Othosclerozsis
 How to use Progestogen Injectable Contraceptives P Deep i. m. (buttock, thigh, deltoid muscle); with needle of 2. 5 - 4 cm lengh & of 21 -23 gauge; the area of injection must not be massed, for not reducing the effectiveness P In menstruated women: first injection is administered in the first 5 days of menstrual cycle; when on COC or POP the injectable can be started whenever is necessary P Postpartum (breastfeeding or not) the first injection is administered in the 6 th week post delivery P After miscarriage /post abortion : • in first trimester: in first 7 days after abortion and is not imposed an extra method • in the 2 nd trimester: delay of administration to reduce the risk of prolonged and abundant bleeding ü Time during injections: 90 7 days for DMPA, 60 5 days for NET-EN P Accepted delay: maximum 2 weeks
How to use Progestogen Injectable Contraceptives P Deep i. m. (buttock, thigh, deltoid muscle); with needle of 2. 5 - 4 cm lengh & of 21 -23 gauge; the area of injection must not be massed, for not reducing the effectiveness P In menstruated women: first injection is administered in the first 5 days of menstrual cycle; when on COC or POP the injectable can be started whenever is necessary P Postpartum (breastfeeding or not) the first injection is administered in the 6 th week post delivery P After miscarriage /post abortion : • in first trimester: in first 7 days after abortion and is not imposed an extra method • in the 2 nd trimester: delay of administration to reduce the risk of prolonged and abundant bleeding ü Time during injections: 90 7 days for DMPA, 60 5 days for NET-EN P Accepted delay: maximum 2 weeks
 Emergency Contraception (postcoital, “after morning”) (1) Conditions: First 72 hs after unprotect intercourse, ü Only for emergency (NOT RUTINE) ü After method application, condom use!!! ü Pregnancy suspicion, if period does not appear in the next three weeks ü
Emergency Contraception (postcoital, “after morning”) (1) Conditions: First 72 hs after unprotect intercourse, ü Only for emergency (NOT RUTINE) ü After method application, condom use!!! ü Pregnancy suspicion, if period does not appear in the next three weeks ü
 Emergency Contraception : Available types (2) A. Worldwide: - Danazol® (400; 600 mg/d x 5 days) - RU 486 (Mifepristone )( 600 mg- single dose) - Ellaone ® (ulipristal acetate 30 mg/tb): in the first 120 h after unprotected sex - Epostane (3 -β hidroxy steroid dehydrogenase inhibitor) B. România: • Progestogen only pills: Levonorgestrel: 0. 75 mg x 2 (Postinor 2®) (1 tb /12 hs) Levonorgestrel: 1. 50 mg x 1 tb (Escapelle ): single dose; 1 tb • Estrogen only pills: Ethinyl-estradiol®: 2 -5 mg/day x 5 days Premarin® 10 mg/day x 5 days • Medicated IUD: levonorgestrel/ progesterone Mirena
Emergency Contraception : Available types (2) A. Worldwide: - Danazol® (400; 600 mg/d x 5 days) - RU 486 (Mifepristone )( 600 mg- single dose) - Ellaone ® (ulipristal acetate 30 mg/tb): in the first 120 h after unprotected sex - Epostane (3 -β hidroxy steroid dehydrogenase inhibitor) B. România: • Progestogen only pills: Levonorgestrel: 0. 75 mg x 2 (Postinor 2®) (1 tb /12 hs) Levonorgestrel: 1. 50 mg x 1 tb (Escapelle ): single dose; 1 tb • Estrogen only pills: Ethinyl-estradiol®: 2 -5 mg/day x 5 days Premarin® 10 mg/day x 5 days • Medicated IUD: levonorgestrel/ progesterone Mirena
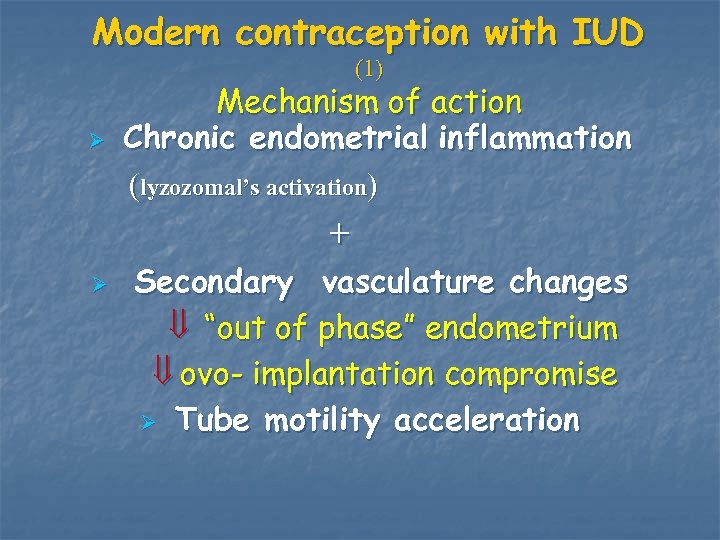 Modern contraception with IUD (1) Ø Mechanism of action Chronic endometrial inflammation (lyzozomal’s activation) + Ø Secondary vasculature changes “out of phase” endometrium ovo- implantation compromise Ø Tube motility acceleration
Modern contraception with IUD (1) Ø Mechanism of action Chronic endometrial inflammation (lyzozomal’s activation) + Ø Secondary vasculature changes “out of phase” endometrium ovo- implantation compromise Ø Tube motility acceleration
 Modern contraception with IUD Adverse effects (2) P Low abdominal pains (at insertion moment) P Uterine bleeding P Infections: PID P Pregnancy: intra and ectopic P Secondary infertility P Uterine perforation and miscarriage
Modern contraception with IUD Adverse effects (2) P Low abdominal pains (at insertion moment) P Uterine bleeding P Infections: PID P Pregnancy: intra and ectopic P Secondary infertility P Uterine perforation and miscarriage
 Contraindications for IUD (1) General: Pregnancy: known or suspected Abnormalities of the uterus resulting in distorsion of the uterine cavity Acute PID or history Postpartum endometritis or infected abortion in the past 3 months Known or suspected uterine or cervical malignancy, including unresolved abnormal Pap smear Genital bleeding of unknown etiology Untreated acute cervicitis or vaginitis, bacterial vaginosis, untill infection is controlled Patient or her partner has multiple partners Conditions associated with increased susceptibility to infections with microorganisms, including: leukemia, AIDS, intravenous drug abuse Genital actinomycosis A previously inserted IUD that has not been removed Modified from Physicians Desk Reference, 2004
Contraindications for IUD (1) General: Pregnancy: known or suspected Abnormalities of the uterus resulting in distorsion of the uterine cavity Acute PID or history Postpartum endometritis or infected abortion in the past 3 months Known or suspected uterine or cervical malignancy, including unresolved abnormal Pap smear Genital bleeding of unknown etiology Untreated acute cervicitis or vaginitis, bacterial vaginosis, untill infection is controlled Patient or her partner has multiple partners Conditions associated with increased susceptibility to infections with microorganisms, including: leukemia, AIDS, intravenous drug abuse Genital actinomycosis A previously inserted IUD that has not been removed Modified from Physicians Desk Reference, 2004
 Contraindications for IUD (2) Specific: ü IUD with Cooper: • Wilson disease • Cooper alergy ü Mirena*; levosert 20 * • Hypersensitivity to levonorgestrel • Known or suspected carcinoma of the breast • History of ectopic pregnancy or condition that predispose to ectopic pregnancy Modified from Physicians Desk Reference, 2004
Contraindications for IUD (2) Specific: ü IUD with Cooper: • Wilson disease • Cooper alergy ü Mirena*; levosert 20 * • Hypersensitivity to levonorgestrel • Known or suspected carcinoma of the breast • History of ectopic pregnancy or condition that predispose to ectopic pregnancy Modified from Physicians Desk Reference, 2004
 Natural Contraceptive Methods (periodic/rhythmic abstinence, self observation) (1) Calendar Rhythm Method (Ogino- Knaus method) (a) 8 days of menstrual cycle = fertile days Ovulation= days: 12 -16 of cycle Oocyte : life of 12 - 24 h, is fecundable Spermatozoa: survival 3 days (maximum 7 dys) in female genital tract
Natural Contraceptive Methods (periodic/rhythmic abstinence, self observation) (1) Calendar Rhythm Method (Ogino- Knaus method) (a) 8 days of menstrual cycle = fertile days Ovulation= days: 12 -16 of cycle Oocyte : life of 12 - 24 h, is fecundable Spermatozoa: survival 3 days (maximum 7 dys) in female genital tract
 Natural Contraceptive Methods (2) Calendar Rhythm Method (Ogino- Knaus) (b) P § § regular periods with variation Index of variability (i. v. ) (27, 28, 29…. ) FMD = LP + duration of the longest cycle Last day fertile interval = FMD - 12 First day of fertile interval = FMD - (19 + i. v. ) P regular periods without variation § FMD = LP + cycle duration § § Last day fertile interval = FMD - 12 First day of fertile interval = FMD - 19 irregular periods § FMD = LP + duration of the longest cycle Intercourse: only 11 days before FMD ü
Natural Contraceptive Methods (2) Calendar Rhythm Method (Ogino- Knaus) (b) P § § regular periods with variation Index of variability (i. v. ) (27, 28, 29…. ) FMD = LP + duration of the longest cycle Last day fertile interval = FMD - 12 First day of fertile interval = FMD - (19 + i. v. ) P regular periods without variation § FMD = LP + cycle duration § § Last day fertile interval = FMD - 12 First day of fertile interval = FMD - 19 irregular periods § FMD = LP + duration of the longest cycle Intercourse: only 11 days before FMD ü
 Natural Contraceptive Methods (3) Temperature Rhythmic Method ü Progesterone effect: the increase with 0. 4º F in the morning basal body temperature, just before ovulation last day of basal plateau Ovulation 2 nd or 3 rd ↑ body basal temperature ü Cycle of 28 days: intercourse permited: 17 - 28 days of menstrual cycle
Natural Contraceptive Methods (3) Temperature Rhythmic Method ü Progesterone effect: the increase with 0. 4º F in the morning basal body temperature, just before ovulation last day of basal plateau Ovulation 2 nd or 3 rd ↑ body basal temperature ü Cycle of 28 days: intercourse permited: 17 - 28 days of menstrual cycle
 Natural Contraceptive Methods Cervical Mucus Rhythm Method (Billings: Eve & John) P P (4) First days of m. c. : cervical mucus – missing Estrogen’s influence: more fluid, but viscous, opaque Ovulation ”peak days”: very fluid, clear, elastic: wet P Progesterone’s influence: less fluid and slow vagina P Ø vagina disappear: dry Intercourse: days 2 -4 after period “dry” days after ovulation (3 -4 days) till to period Although this method has not achieved popularity, when it is used accurately, the first- year failure rate is approximately 3%
Natural Contraceptive Methods Cervical Mucus Rhythm Method (Billings: Eve & John) P P (4) First days of m. c. : cervical mucus – missing Estrogen’s influence: more fluid, but viscous, opaque Ovulation ”peak days”: very fluid, clear, elastic: wet P Progesterone’s influence: less fluid and slow vagina P Ø vagina disappear: dry Intercourse: days 2 -4 after period “dry” days after ovulation (3 -4 days) till to period Although this method has not achieved popularity, when it is used accurately, the first- year failure rate is approximately 3%
 Natural Contraceptive Methods (5) Symptothermal Method Preovulatory: Cervical mucus method = onset of fertile period 2 -4 days after period + Postovulatory: cervical mucus method + temperature method at 4 days after “peak” at 3 days of increase from basal level The use of home kits to detect LH increase in woman’s urine on the day prior to ovulation may improve the accuracy of periodic abstinence methods Hatcher RA, 1998
Natural Contraceptive Methods (5) Symptothermal Method Preovulatory: Cervical mucus method = onset of fertile period 2 -4 days after period + Postovulatory: cervical mucus method + temperature method at 4 days after “peak” at 3 days of increase from basal level The use of home kits to detect LH increase in woman’s urine on the day prior to ovulation may improve the accuracy of periodic abstinence methods Hatcher RA, 1998
 Patients’ Selection & Assessment Recommended Procedures (1) Assessment for appreciation of adecquation and choose of the most proper method To assess: P P P Indications & advantages of the method The presence of situations which limit method’s choose The presence of special issues which impose supplementary medical examinations
Patients’ Selection & Assessment Recommended Procedures (1) Assessment for appreciation of adecquation and choose of the most proper method To assess: P P P Indications & advantages of the method The presence of situations which limit method’s choose The presence of special issues which impose supplementary medical examinations
 Patients’ Selection & Assessment Recommended Procedures (2) History: family, individual ’s physiologic & pathologic, focused on pe: Ø Date of birth Ø period’s history (menarcha, regularity, menstrual blood flow, abnormal uterine bleeding) Ø First day of LMP Ø Obstetrical history (parity, miscarriages & abortions, date of last delivery, breast feeding, gestational diabetes mellitus, gestational hypertension & cholestasis) Physic examination : Ø weight Ø blood pressure – Ø breast examination and women teaching for self breast examination, without transforming this fact in an excessive ritual Ø Bimanual genital examination and cervico vaginal smears
Patients’ Selection & Assessment Recommended Procedures (2) History: family, individual ’s physiologic & pathologic, focused on pe: Ø Date of birth Ø period’s history (menarcha, regularity, menstrual blood flow, abnormal uterine bleeding) Ø First day of LMP Ø Obstetrical history (parity, miscarriages & abortions, date of last delivery, breast feeding, gestational diabetes mellitus, gestational hypertension & cholestasis) Physic examination : Ø weight Ø blood pressure – Ø breast examination and women teaching for self breast examination, without transforming this fact in an excessive ritual Ø Bimanual genital examination and cervico vaginal smears
 Sterilization voluntary Female: 28% (USA) o Puerperal Tubal Sterilization o Non -puerperal Tubal Sterilization o Reversal Tubal Sterilization o Hysterectomy Surgical/ Laparoscopy § Transcervical Sterilization § Intratubal Chemical Methods § Intratubal Devices Male: Bilateral vasectomy
Sterilization voluntary Female: 28% (USA) o Puerperal Tubal Sterilization o Non -puerperal Tubal Sterilization o Reversal Tubal Sterilization o Hysterectomy Surgical/ Laparoscopy § Transcervical Sterilization § Intratubal Chemical Methods § Intratubal Devices Male: Bilateral vasectomy


