bedeefdb5eeb5c2f92c6dce70544864a.ppt
- Количество слайдов: 16

False Biomarker Discovery due to Reactivity of a Commercial ELISA for CUZD 1 with Cancer Antigen CA 125 I. Prassas, D. Brinc, S. Farkona, F. Leung, A. Dimitromanolakis, C. C. Chrystoja, R. Brand, V. Kulasingam, I. M. Blasutig, and E. P. Diamandis February 2014 www. clinchem. org/content/60/2/381. full © Copyright 2014 by the American Association for Clinical Chemistry
Introduction: The Biomarker Problem Ø Despite the recent advances in throughput of genomic and proteomic technologies, very few (<15) novel biomarkers have been introduced in the clinic the last 2 decades Ø Bringing a biomarker to the clinic is a multi-step process: discovery, verification, preclinical validation, large blinded clinical validation Ø Every step of this process is prone to errors that can put the whole pipeline at risk © Copyright 2009 by the American Association for Clinical Chemistry
Introduction: The problem with commercial ELISAs Ø The most common method for biomarker validation is the development of antibody-based assays (ELISAs) Ø In house ELISA development is costly, labor-intensive and expensive Ø Commercially available kits represent the easiest option Ø As our case illustrates, these reagents are sometimes sold without having been rigorously validated and this can result in substantial amounts of wasted time and investments. © Copyright 2009 by the American Association for Clinical Chemistry
Question Ø What are some common errors (analytical, statistical, methodological) that can happen during the different steps of a biomarker discovery and validation pipeline? © Copyright 2009 by the American Association for Clinical Chemistry
Introduction: Our case Ø Commercial ELISA kits were used to assess the performance of our top-ranked (recently discovered) candidate pancreatic cancer (PDAC) biomarkers Ø Of these, CUZD 1 appeared as the most promising candidate biomarker for the diagnosis of pancreatic cancer Ø In our early pre-clinical verification studies of CUZD 1 (measured by a commercial CUZD 1 ELISA kit), this biomarker showed strong diagnostic performance Ø Before we moved to further clinical validation studies, we rigorously characterized the specificity of this commercial kit © Copyright 2009 by the American Association for Clinical Chemistry
Materials and Methods Ø We performed detailed experiments to investigate the specificity of the commercial CUZD 1 ELISA assay: 1. CUZD 1 protein was cloned and expressed in house in both bacteria and yeast expression vector systems 2. Recombinant pure CUZD 1 antigen and several biological samples containing CUZD 1, as well as commercial CUZD 1 ELISA standards were analyzed by: § Western Blot § Size exclusion HPLC § Mass-Spectrometry (LC-MS Orbitrap) © Copyright 2009 by the American Association for Clinical Chemistry
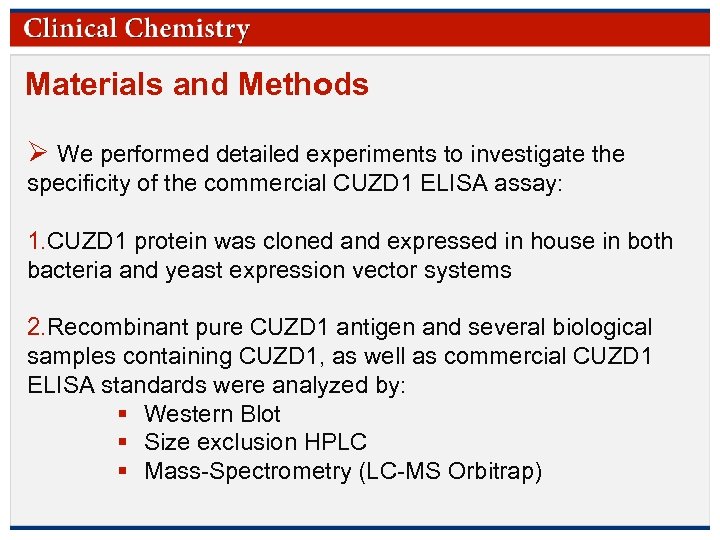
Results Figure 1. (A), Assessment of CUZD 1 immunoreactivity (USCN ELISA) in eluted fractions of an SEC column revealed that peak signal was always observed in the void volume (molecular weight, 500 k. D). The same elution profile was obtained irrespective of the sample type used, including CUZD 1 calibrators from the ELISA kit , different pancreatic ascites samples, pooled ovarian cancer serum. (B), Western blots of several CUZD 1 immunoreactive sources, as shown, confirm the high molecular weight (MW) (250 k. Da) of the target antigen (no bands were detected in the expected MW of CUZD 1 at approximately 70 k. Da). © Copyright 2009 by the American Association for Clinical Chemistry
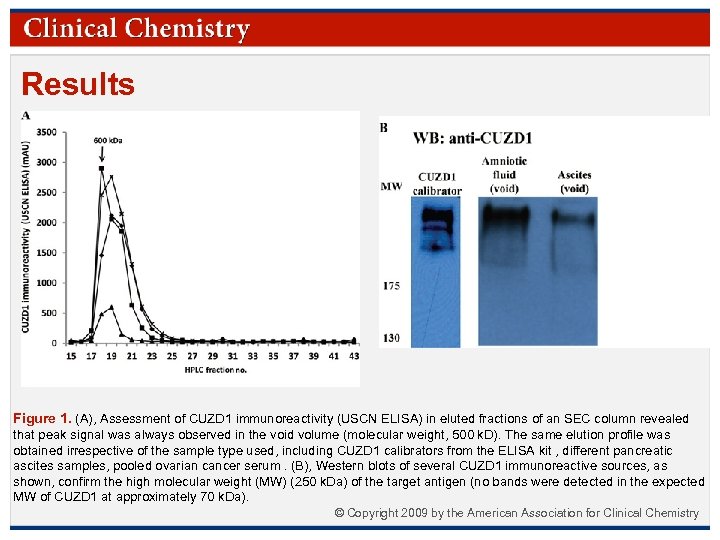
Results Figure 2. Comparison of MS-verified CUZD 1 signal with commercial “CUZD 1” immunoreactivity. We used mass spectrometry (LC-MS Orbitrap) to compare CUZD 1 expression (MS-verified) with the immuno-reactivity of the “CUZD 1” kit against several biological fluids. As shown in the figure, all CUZD 1 containing samples were negative for the CUZD 1 kit and all CUZD 1 non-containing samples were immunoreacting with the commercial “CUZD 1” ELISA kit. © Copyright 2009 by the American Association for Clinical Chemistry
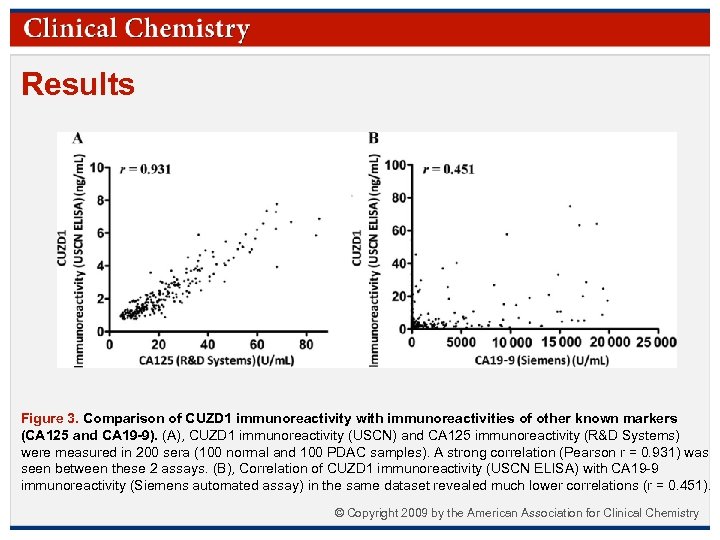
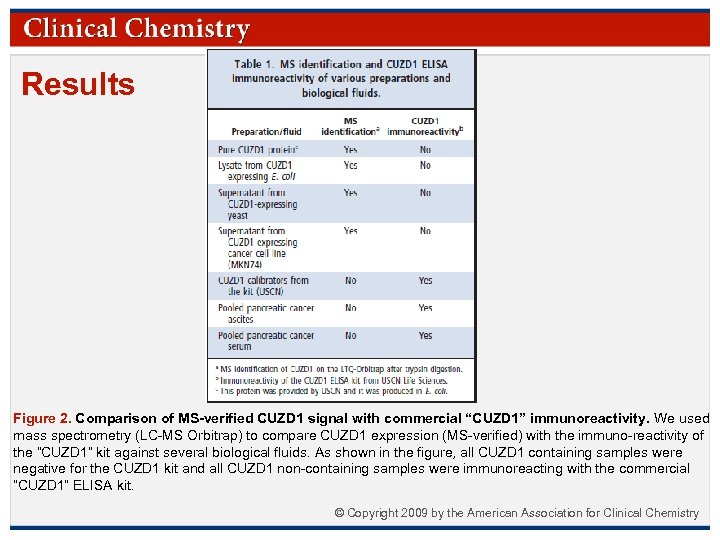
Results Figure 3. Comparison of CUZD 1 immunoreactivity with immunoreactivities of other known markers (CA 125 and CA 19 -9). (A), CUZD 1 immunoreactivity (USCN) and CA 125 immunoreactivity (R&D Systems) were measured in 200 sera (100 normal and 100 PDAC samples). A strong correlation (Pearson r = 0. 931) was seen between these 2 assays. (B), Correlation of CUZD 1 immunoreactivity (USCN ELISA) with CA 19 -9 immunoreactivity (Siemens automated assay) in the same dataset revealed much lower correlations (r = 0. 451). © Copyright 2009 by the American Association for Clinical Chemistry
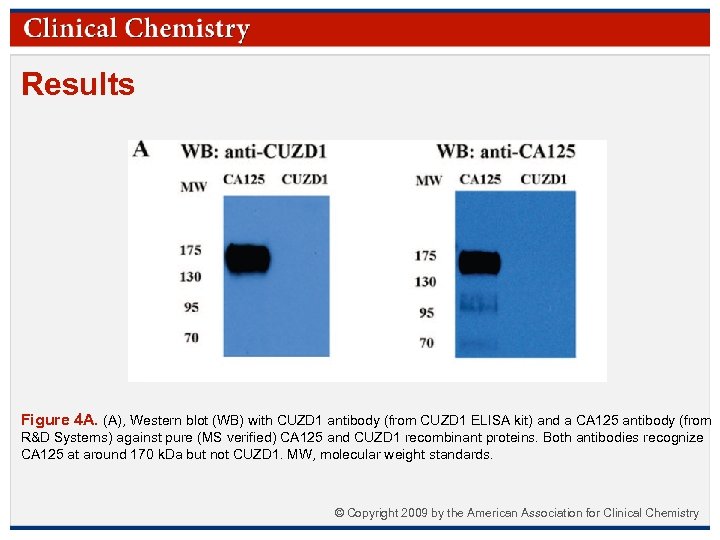
Results Figure 4 A. (A), Western blot (WB) with CUZD 1 antibody (from CUZD 1 ELISA kit) and a CA 125 antibody (from R&D Systems) against pure (MS verified) CA 125 and CUZD 1 recombinant proteins. Both antibodies recognize CA 125 at around 170 k. Da but not CUZD 1. MW, molecular weight standards. © Copyright 2009 by the American Association for Clinical Chemistry
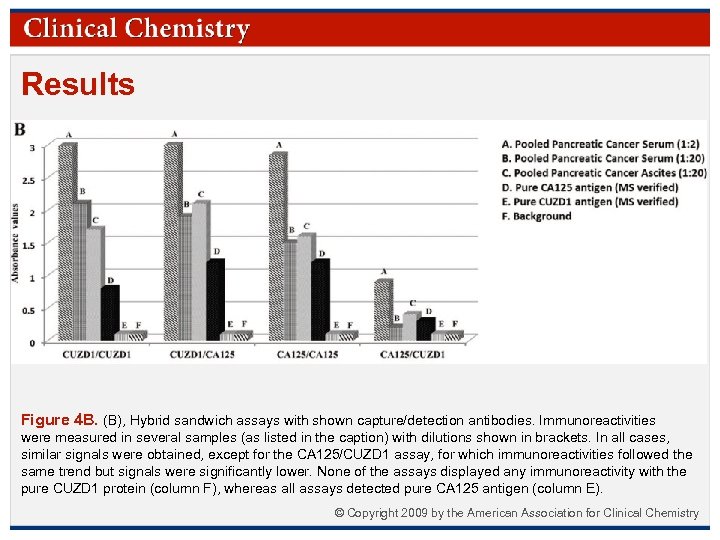
Results Figure 4 B. (B), Hybrid sandwich assays with shown capture/detection antibodies. Immunoreactivities were measured in several samples (as listed in the caption) with dilutions shown in brackets. In all cases, similar signals were obtained, except for the CA 125/CUZD 1 assay, for which immunoreactivities followed the same trend but signals were significantly lower. None of the assays displayed any immunoreactivity with the pure CUZD 1 protein (column F), whereas all assays detected pure CA 125 antigen (column E). © Copyright 2009 by the American Association for Clinical Chemistry
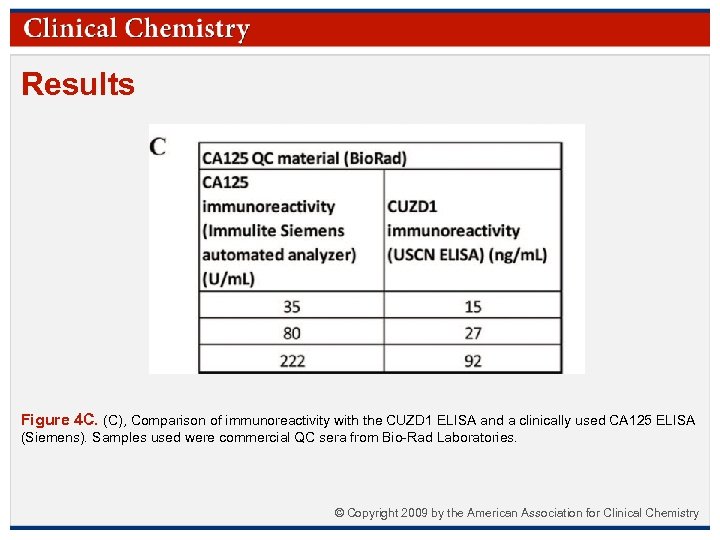
Results Figure 4 C. (C), Comparison of immunoreactivity with the CUZD 1 ELISA and a clinically used CA 125 ELISA (Siemens). Samples used were commercial QC sera from Bio-Rad Laboratories. © Copyright 2009 by the American Association for Clinical Chemistry

Question #2: Ø Mass Spectrometry can provide very definitive answers regarding the presence (and the quantity) of a protein in a sample. If this is the case, why do ELISAs remain the most common method in serum biomarker validation studies? © Copyright 2009 by the American Association for Clinical Chemistry
Conclusions Ø Commercial CUZD 1 assay recognizes a completely different antigen (CA 125) than CUZD 1 Ø This phenomenon is not a cross-reactivity issue, since the “CUZD 1” kit cannot recognize any form of CUZD 1 protein Ø All observed “CUZD 1 immunoreactivity” corresponds to CA 125 immunoreactivity Ø Rigorous validation of commercial assays can prevent substantial amounts of wasted time and investments © Copyright 2009 by the American Association for Clinical Chemistry

Question #3: Ø FDA poses very strict regulations when a commercial assay is intended for clinical use. However, rules are not as strict in the case of reagents designated as “for research use only. ” Do you think that stricter regulations also should be imposed for the latter category of reagents? © Copyright 2009 by the American Association for Clinical Chemistry

Thank you for participating in this month’s Clinical Chemistry Journal Club. Additional Journal Clubs are available at www. clinchem. org Download the free Clinical Chemistry app on i. Tunes for additional content! Follow us © Copyright 2009 by the American Association for Clinical Chemistry
bedeefdb5eeb5c2f92c6dce70544864a.ppt