 Factor V Leiden Detection and Genotyping August 6, 2008 Marylin Bicak
Factor V Leiden Detection and Genotyping August 6, 2008 Marylin Bicak
 Objective l l l 1. To provide an overview of the Factor V Leiden assay 2. To explain the pathophysiology and epidemiology of the Factor V Leiden mutation. 3. To evaluate the efficacy of the assay.
Objective l l l 1. To provide an overview of the Factor V Leiden assay 2. To explain the pathophysiology and epidemiology of the Factor V Leiden mutation. 3. To evaluate the efficacy of the assay.
 Pathophysiology l l l l inherited condition autosomal dominant chromosome 1, gene F 5 Single point mutation, G 1691 A (arginine to glutamine at 506 th amino acid mutation prevents inactivation of factor V in clotting process (resistant to inactivation by Protein C) overproduction of thrombin= excess fibrin formation excess clotting (DVT and PE)
Pathophysiology l l l l inherited condition autosomal dominant chromosome 1, gene F 5 Single point mutation, G 1691 A (arginine to glutamine at 506 th amino acid mutation prevents inactivation of factor V in clotting process (resistant to inactivation by Protein C) overproduction of thrombin= excess fibrin formation excess clotting (DVT and PE)
 Epidemiology l l l l l Most common inherited coagulation disorder in U. S. 5% Caucasians 2% Hispanics 1. 2% African Americans <0. 5% Asian Americans Heterozygous= 3 -8 fold increase risk of thrombosis Homozygous= 30 -140 increase risk of thrombosis risk factors treatment
Epidemiology l l l l l Most common inherited coagulation disorder in U. S. 5% Caucasians 2% Hispanics 1. 2% African Americans <0. 5% Asian Americans Heterozygous= 3 -8 fold increase risk of thrombosis Homozygous= 30 -140 increase risk of thrombosis risk factors treatment
 Factor V Leiden Assay l 1. Extraction- Mag. NA Pure Compact Nucleic Acid Isolation Kit and instrument (Roche Diagnostics) l 2. Amplification/Detection/Genotyping- Factor V Leiden Kit and Light. Cycler 2. 0 instrument (Roche Diagnostics)
Factor V Leiden Assay l 1. Extraction- Mag. NA Pure Compact Nucleic Acid Isolation Kit and instrument (Roche Diagnostics) l 2. Amplification/Detection/Genotyping- Factor V Leiden Kit and Light. Cycler 2. 0 instrument (Roche Diagnostics)
 Extraction l l l Specimen- EDTA whole blood (200 ul whole blood= 100 ul purified product) Mag. NA Pure Kit- pre-sealed reagent cartridge; disposable pipette tip tray assembly; sample tube; elution tube Mag. NA Pure Compact Instrument-automated method based on magnetic glass particle technology
Extraction l l l Specimen- EDTA whole blood (200 ul whole blood= 100 ul purified product) Mag. NA Pure Kit- pre-sealed reagent cartridge; disposable pipette tip tray assembly; sample tube; elution tube Mag. NA Pure Compact Instrument-automated method based on magnetic glass particle technology
 Principle of Magnetic Glass Particle Technology l Four step process: 1 -lysis; 2 -bind to magnetic particles; 3 -wash; 4 -elution l
Principle of Magnetic Glass Particle Technology l Four step process: 1 -lysis; 2 -bind to magnetic particles; 3 -wash; 4 -elution l
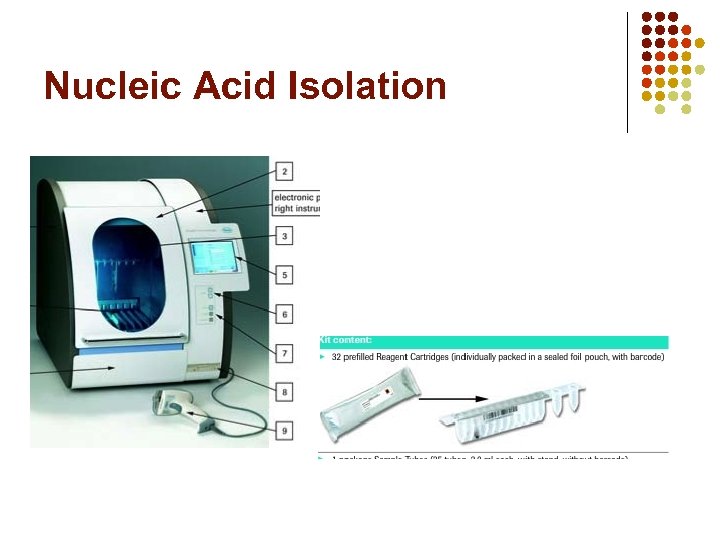 Nucleic Acid Isolation
Nucleic Acid Isolation
 Amplification-real-time PCR l l l l target- 222 bp fragment of Factor V gene Factor V Leiden kit- master mix reagents (primers and probes) Light. Cycler 2. 0 instrument- rapid- 45 cycles (30 min) Cycle temperatures- denaturation 95 C; annealing 60 C; elongation 72 C thermal cycler- heat/ambient air cycle reaction vessel- 20 ul glass capillary
Amplification-real-time PCR l l l l target- 222 bp fragment of Factor V gene Factor V Leiden kit- master mix reagents (primers and probes) Light. Cycler 2. 0 instrument- rapid- 45 cycles (30 min) Cycle temperatures- denaturation 95 C; annealing 60 C; elongation 72 C thermal cycler- heat/ambient air cycle reaction vessel- 20 ul glass capillary
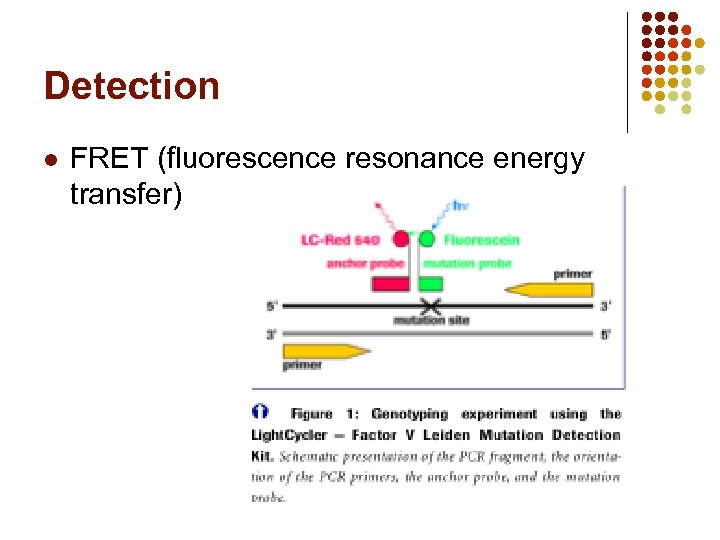 Detection l FRET (fluorescence resonance energy transfer)
Detection l FRET (fluorescence resonance energy transfer)
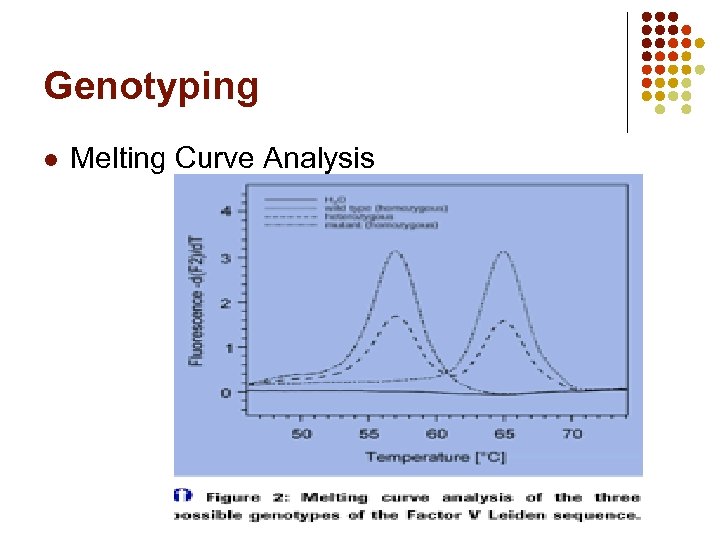 Genotyping l Melting Curve Analysis
Genotyping l Melting Curve Analysis
 Summary l l l Limitations- technical process/ physical space/ instrumentation Erroneous results- a. false positive (3 rare mutations span same mutation probe) and b. patient sample with elevated WBC Overall, innovative system and important diagnostic tool for clinical lab
Summary l l l Limitations- technical process/ physical space/ instrumentation Erroneous results- a. false positive (3 rare mutations span same mutation probe) and b. patient sample with elevated WBC Overall, innovative system and important diagnostic tool for clinical lab