aafc44d0dfc10dc9d0be84eff23a26e6.ppt
- Количество слайдов: 65

F-Tag 428 Medication Regimen Review Drug Use Problems in Long Term Care Residents and Key Elements to Performing a Drug Regimen Review Robert L. Maher Jr. , Pharm. D, BCPS, CGP Assistant Professor of Clinical Pharmacy Duquesne University School of Pharmacy Vice-President of Clinical Services Mission Pharmacy Services October 26 th , 2007

Timeline for Pharmacy Tags • Reminder: Appendix N Deleted - Effective June 2004 • Effective date/implementation scheduled for DECEMBER 18, 2006

Tags Combined • Pharmaceutical Services – New Tag F 428 = Old Tags F 428, F 429, F 430 • DRR/MRR

F 428 - MRR Regulations • The drug regimen of each resident must be reviewed at least once a month by a licensed pharmacist • The pharmacist must report any irregularities to the attending physician and the director of nursing • And, these reports must be acted upon

MRR What does it say currently? • More Frequent Reviews – Weekly Reviews depending on the resident’s condition and the drugs they are taking • High Risk Residents – Drug Therapy With High Potential for Less Severe Adverse Outcomes In Persons Over 65 (AKA: Beers list) • Note – Review by the surveyor is not necessary for drug therapy given the first seven consecutive days upon admission/readmission, unless there is an immediate threat to health and safety

MRR What does it say currently? • The director of nursing and the attending physician are not required to agree with the pharmacist’s report, • Nor are they required to provide a rationale for their acceptance or rejection of the report • They must, however, act upon the report • This may be accomplished by indicating acceptance or rejection of the report and signing their names • The facility is encouraged to provide the medical director with a copy of drug regimen review reports and to involve the medical director in reports that have not been acted upon

Prior to F-Tag 428 • The director of nursing and the attending physician are not required to agree with the pharmacist’s report, • Nor are they required to provide a rationale for their acceptance or rejection of the report • They must, however, act upon the report • This may be accomplished by indicating acceptance or rejection of the report and signing their names • The facility is encouraged to provide the medical director with a copy of drug regimen review reports and to involve the medical director in reports that have not been acted upon

F 428 - MRR • Definition in glossary: – Goal of promoting positive outcomes and minimizing adverse consequences associated with medications; • The review includes the following with medicationrelated problems and med errors 1. Identifying 2. Reporting 3. Resolving • Done by collaborating with others members of the interdisciplinary team.

F 428 - MRR – What are these So “things” we’re preventing, identifying, reporting, and resolving…how are MRPs, med errors, and irregularities defined?

F 428 - MRR MRPs • A Medication-Related Problem (MRP) is: (NOTE HOW SIMILAR THESE ARE TO THE UNNECESSARY MED ‘CATEGORIES’ IN F-TAG 329) – Use of a medication without adequate indication for use – Use of a medication without identifiable evidence that safer alternatives or more clinically appropriate medications have been considered

F 428 - MRR MRPs – Use of an appropriate medication that is not reaching treatment goals for reasons such as timing or techniques of administration, dosing intervals, etc. – Use of a medication in an excessive dose (including duplicate therapy) or for excessive duration – Presence of an adverse consequence associated with medication(s)
F 428 - MRR MRPs – Use of a medication without adequate monitoring • Inadequate monitoring of response to med, or • Inadequate response to findings/results – Presence of or risk for medication errors – Presence of a clinical condition that might warrant initiation of medication – Medication interaction - “TOP 10 DIs in LTC”

F 428 - MRR Med Errors • A medication error isn’t actually defined in document, but NCCMERP definition is: “A medication error is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing; order communication; product labeling, packaging, and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; and use. ”

F 428 - MRR Irregularities • An irregularity is: “Any event that is inconsistent with usual, proper, accepted, or right approaches to providing pharmaceutical services (as defined by F 425), or that impedes or interferes with achieving the intended outcomes of those services. ”

F 428 - MRR • Given those definitions, important to note that document also states: “This guidance is not intended to imply that all adverse consequences related to medications are preventable, but rather to specify that a SYSTEM exists to assure that medication usage is evaluated on an ongoing basis…”

F 428 - MRR Frequency of Review • Monthly or more frequently, depending on: – the resident’s condition, and – the risks for adverse consequences related to current medications • This sounds alarming, but it is virtually the same as current survey guidelines

F 428 - MRR Where to Conduct the Review • Generally within facility because important info may be attainable only by talking to staff, reviewing “paper” chart, observing/speaking with resident • BUT new technology (electronic health records) may permit the PHARMACIST to conduct some components of the review outside of the facility

F 428 - MRR Sources of Information • May include, but are not limited to: – – – MARs Prescribers’ orders Progress, nursing, consultants’ notes RAI/MDS Lab reports Forms/reports reflecting behavioral monitoring and/or changes in condition – QM/QI reports – Attending physician, facility staff – Interviewing, assessing, and/or observing the resident • Ask yourself, how many of these do I use and should I be using more sources or different types of sources than I am now?

F 428 - MRR Considerations • MRR considers factors, such as: – Has MD/staff documented objective findings, diagnoses, symptoms to support indication? – Has MD/staff identified and acted upon, or should they be notified about, resident’s allergies, potential interactions/averse consequences? – Is dose, frequency, route, duration consistent with resident’s condition, manufacturer’s recommendations, and applicable standards of practice?

F 428 - MRR Considerations – Has MD/staff documented progress towards or maintenance of the goal(s) for medications therapy? – Has MD/staff obtained and acted upon lab results, diagnostic studies, or other measurements? – Do med errors exist or do circumstances exist that make errors likely to occur?

F 428 - MRR Considerations – Has MD/staff noted and acted upon possible medication-related causes of recent or persistent changes in the resident’s condition? …think “Geriatric Syndromes” • • Anorexia and/or unplanned weight loss, or weight gain Behavioral changes, unusual behavior patterns Bowel function changes Confusion, cognitive decline, worsening of dementia Dehydration, fluid/electrolyte imbalance Depression, mood disturbance Dysphagia, swallowing difficulty Excessive sedation, insomnia, or sleep disturbance

F 428 - MRR Considerations • • Falls, dizziness, impaired coordination GI bleeding Headaches, muscle pain, generalized aching/pain Rash, pruritis Seizure activity Spontaneous or unexplained bleeding, bruising Unexplained decline in functional status Urinary retention or incontinence

F 428 - MRR Notification of Findings • Pharmacist is expected to document either that no irregularity was identified or the nature of the irregularity(ies), if any were identified – If none, pharmacist would include a signed and dated statement to that effect • Different iterations of this requirement throughout the various drafts, but final focus is on the use of the word “report” as a verb rather than a noun

F 428 - MRR Notification of Findings • Timeliness of notification depends on potential for or presence of serious adverse consequences – Examples include: • Bleeding resident on anticoagulants • Possible allergic reactions to antibiotic • Collaborate with facility to identify the most effective means of notification/documentation • Notification/documentation may be done electronically

F 428 - MRR Notification of Findings • Pharmacist’s findings are part of clinical record – If not maintained within active clinical record, it must still be maintained within facility and readily available • Find balance between: – Encouraging/facilitating other HC professionals to utilize – Allowing facilities flexibility in determining a consistent location that suits their needs

F 428 - MRR Response to Findings • Physician either: – Accepts recommendation and acts, OR – Rejects the recommendation and provides a brief explanation, such as in a dated progress note • “It is not acceptable for a physician to document only that he/she disagrees with the report without providing some basis for disagreeing. ” • For those direct care issues that do not require physician intervention, DON or designated nurse can address and document action taken

F 428 - MRR Lack of Action or Rejection • What about when MD does not act upon or rejects MRR report/recommendations and there is the potential for serious harm? – Facility and CP should contact Medical Director, OR – When attending and MD are same, follow established facility procedure to resolve the situation • No specific timeframe provided for when a report that is not acted upon officially becomes delinquent or “not acted upon”

F 428 - MRR Lack of Action or Rejection • What about continuing to document an issue that the physician has disregarded or rejected? – “Pharmacist does not need to document a continuing irregularity each month if it’s deemed to be clinically insignificant or there is evidence of valid clinical reason for rejection” – “In these situations, pharmacist need only reconsider annually whether to report again or make new recommendation. ”

How to sort through all the MRPs in Long Term care

Types of Suboptimal Drug Use 1. Overutilization (polypharmacy) 2. Underutilization 3. Inappropriate utilization Hanlon JT, et al. J Am Geriatr Soc 2001; 49: 200 -9.

Total Drug Therapy Cost Control • Total Drug Cost $= (Product Cost + Distribution Cost) x Utilization + Medication Related Problems (Therapeutic Failures + ADRS)


Performing MRR • Familiarize with Medicare and Medicaid requirements • Familiarize with recent facility surveys • Familiarize with documentation procedures • Familiarize with lines of communication • Familiarize with Medical and Nursing Staff • Set dates and times for doing MRR

Performing MRR • Get to know the following people – ADON, Medical Director, Medical Records • What reports – – – Infection control Restraints Behavioral QI Meeting to attend Committee to involve

Performing MRR • The Chart – Admission Records – History and Physical Examination – Physician or Prescriber Orders – MARS • Omissions (reasons) • Prn use – frequency – documented effect – Nursing Progress Notes – Hospital Discharge Note - ? ? Fax to the pharmacy

Performing MRR • The Chart – Nursing Progress Notes • • • Nursing Staff Communication Resident Condition Daily Progress Treatment Plans Vital Signs Monthly Summaries Monitoring of Outcomes of Therapy Documentation of Adverse Effects Functional Ability of Resident Complaints

Performing DRR • The Chart – Physician or Prescriber Progress Notes • Diagnosis, Rationale, Therapeutic Outcomes – Consultant Notes • Psych, Dietary, Social Services, etc. . • DRR Documentation, Justification of Med use – Clinical Lab Data • Urinalysis, Serum Drug Concentration, CBC, Renal Function test, Thyroid Test • Timing of labs
Performing MRR • Timing of MRR – Prospective DRR • Upon Admission – Target high risk medications • Concurrent MRR • Retrospective MRR – Discontinued medications – question of why? ?

Performing MRR • DRR Time Requirements – No more than 100 reviews in one day – Industry standard according to open surveys 9 minutes/chart – Factors to consider • • • The complexity of MRR Number of Chronic Conditions Medical Acuity Level of the Resident Duration of residency in the facility Chronic Care or postacute care The pharmacist familiarity with a particular resident

Targeting the High Risk Elderly Patient • Specific Medications NTD Renally Cleared Medications Phase I metabolized medications • Class of Medications anticonvulants antipsychotics anticholinergics narcotic analgesics sedative/hypnotics

Targeting the High Risk Elderly Patient • Patient’s on Beer’s Criteria Drugs • Cr. Cl <50 ml/min • Low BMI <22 kg/m 2 • >6 chronic active medical conditions • Polypharmacy > 9 or more chronic meds

Targeting the High Risk Elderly Patient • Prior history of an adverse drug reaction • Advanced age (>85) • Those with a history of non-compliance • Those recently discharged from the hospital • Those with certain illness (e. g. dementia)

Preventing ADRs in the Elderly • • • 28% - 56% or ADEs are preventable Most ADEs result from errors in order writing 78% are due to “systems failure” Improve information systems when ordering meds Increase patient education Systematic review of medications – DUE and DUR

Principles for Optimizing Drug Use in the Elderly • Consider whether drug therapy is necessary • Promote the use of a small number of drugs to treat common problems • Adjust doses and or/dosage intervals for medications • Establish reasonable therapeutic endpoints and monitor for desired outcome • Monitor for adverse drug reactions • Regularly review the need for chronic medications

Chronic Medication Review Steps • Assess whether ADRs are the cause of any symptoms • Match problem list with drug list • If on drug but no match with problem list consider whether drug is necessary • If has a chronic condition and not on a medication consider whethere is an evidence based drug to tx the condition • Assess the monitoring for efficacy/safety/appropriateness of the remaining medications

Assessing Prescribing Appropriateness Using the Medication Appropriateness Index • • • Is there an indication for the drug? Is the medication effective for this condition? Is the dosage correct? Are the directions practical? Are there clinically significant drug-drug interactions? Are there clinically significant drug-disease interactions? Is there unnecessary duplications of drugs? Is the duration of therapy acceptable? Is this drug on the formulary or the least expensive alternative compared to others of equal utility? – (Hanlon, et al)
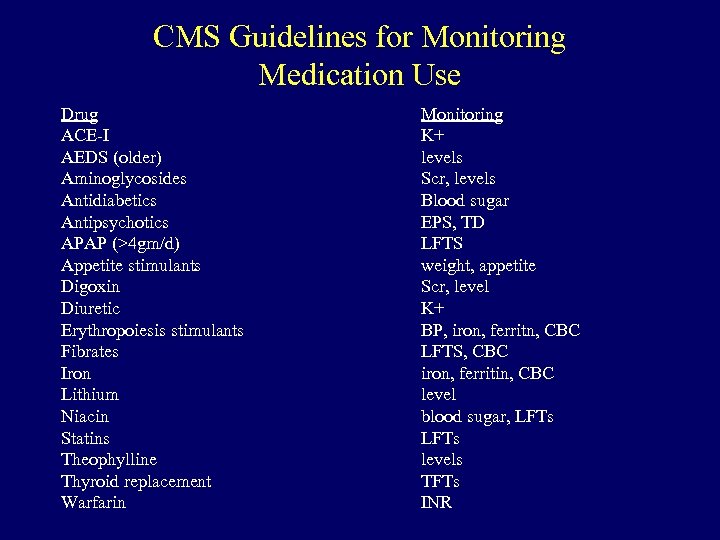
CMS Guidelines for Monitoring Medication Use Drug ACE-I AEDS (older) Aminoglycosides Antidiabetics Antipsychotics APAP (>4 gm/d) Appetite stimulants Digoxin Diuretic Erythropoiesis stimulants Fibrates Iron Lithium Niacin Statins Theophylline Thyroid replacement Warfarin Monitoring K+ levels Scr, levels Blood sugar EPS, TD LFTS weight, appetite Scr, level K+ BP, iron, ferritn, CBC LFTS, CBC iron, ferritin, CBC level blood sugar, LFTs levels TFTs INR
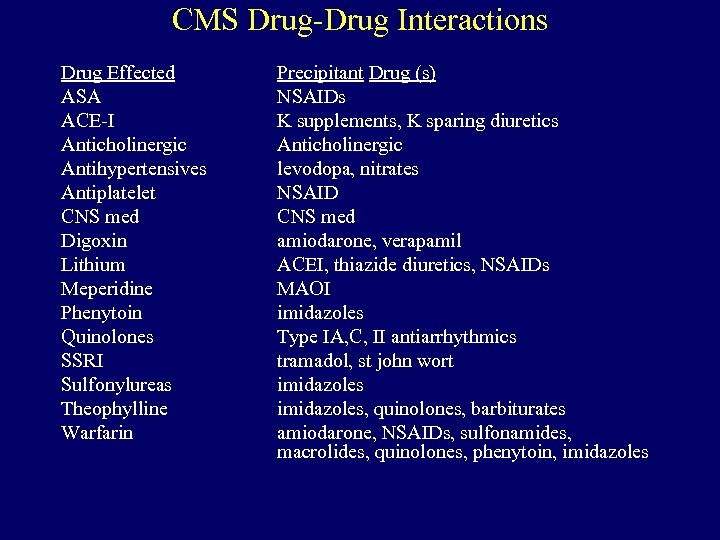
CMS Drug-Drug Interactions Drug Effected ASA ACE-I Anticholinergic Antihypertensives Antiplatelet CNS med Digoxin Lithium Meperidine Phenytoin Quinolones SSRI Sulfonylureas Theophylline Warfarin Precipitant Drug (s) NSAIDs K supplements, K sparing diuretics Anticholinergic levodopa, nitrates NSAID CNS med amiodarone, verapamil ACEI, thiazide diuretics, NSAIDs MAOI imidazoles Type IA, C, II antiarrhythmics tramadol, st john wort imidazoles, quinolones, barbiturates amiodarone, NSAIDs, sulfonamides, macrolides, quinolones, phenytoin, imidazoles

Clinically Important Drug-Disease Interactions Determined by Expert Panel Consensus Drug Disease – Alpha blockers – Anticholinergics – – – – Aspirin Barbiturates Benzodiazepines Bupropion CCB 1 st generation Corticosteroids Digoxin Syncope BPH, constipation, dementia, glaucoma (narrow angle) PUD Dementia, falls Seizures CHF (systolic dysfunction) DM Heart block Lindblad C, Hanlon J et al. Clin Ther 2006 (in press)

Clinically Important Drug-Disease Interactions Determined by Expert Panel Consensus Drug – – – Disease Metoclopramide Non-aspirin NSAIDs Opioid analgesics Sedative/hypnotics Thioridazine Tricyclic antidepressants – Typical antipsychotics Parkinson’s disease CRF, PUD Constipation Falls Postural hypotension BPH, constipation dementia, falls, heart block postural hypotension Falls Lindblad C, Hanlon J et al. Clin Ther 2006 (in press)

Overutilization (Polypharmacy) in the Elderly • Polypharmacy defined as : 1. Concomitant use of multiple drugs 2. Use of more medications than are clinically indicated

Risks Associated with Polypharmacy • • • Functional status decline ADRs Inappropriate drug use Increased medication administration errors Increased risk of geriatric syndromes
Underutilization of Medication • • Undiagnosed and untreated condition (e. g. , depression) Diagnosed condition but omitted treatment (e. g. , post-MI) Underuse of preventive treatment (e. g. , vaccinations) One study found that 50% of 372 vulnerable adults not prescribed an indicated medication; Biggest problems with no gastroprotective agent for high risk NSAID users, no ACE-I in diabetics with proteinuria, no calciumVit. D for those with osteoporosis (Higashi T et al. Ann Intern Med 2004; 140: 714 -20) • Another study found that between 38 -76% of assisted living residents had medication undertreatment; Biggest problems with no ASA or beta blocker post MI; non ACE-I in CHF patients; and no calciumVit. D for those with osteoporosis (Sloane PD et al. Arch Int Med 2004; 164: 2031 -37)

Inappropriate Prescribing • Prescribing of medications that does not agree with accepted medical standards
The I’s of Geriatrics and MRPs Immobility Isolation Incontinence Infection Inanition Impaction Impaired senses Instability Intellectual Impairment Impotence Immunodeficiency Insomnia Iatrogenesis

Medications with Anticholinergic Activity • • • Anti-emetics/anti-vertigo and - (e. g. meclizine) Antiparkinsonians - (e. g. trihexyphenidyl) Antispasmodics- (e. g. belladonna, oxybutynin) Cold and allergy drugs- (e. g hydroxyzine) Sleep aids- (e. g. diphenhydramine) Skeletal muscle relaxants - (e. g. cyclobenzaprine)
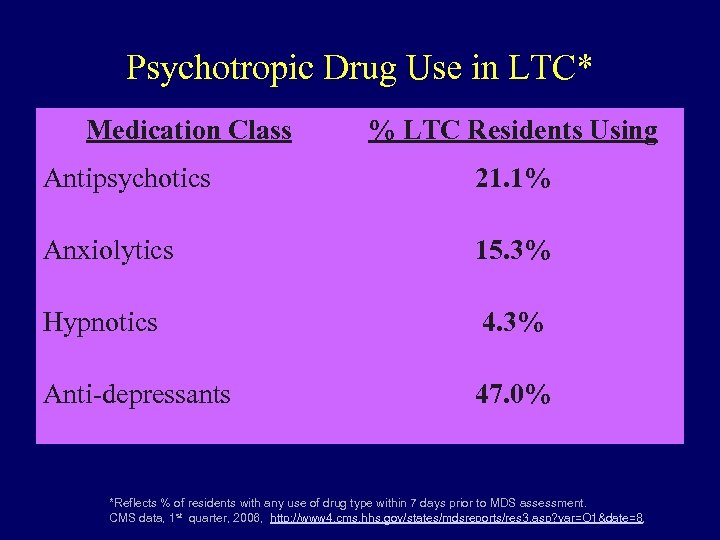
Psychotropic Drug Use in LTC* Medication Class % LTC Residents Using Antipsychotics 21. 1% Anxiolytics 15. 3% Hypnotics 4. 3% Anti-depressants 47. 0% *Reflects % of residents with any use of drug type within 7 days prior to MDS assessment. CMS data, 1 st quarter, 2006, http: //www 4. cms. hhs. gov/states/mdsreports/res 3. asp? var=O 1&date=8,
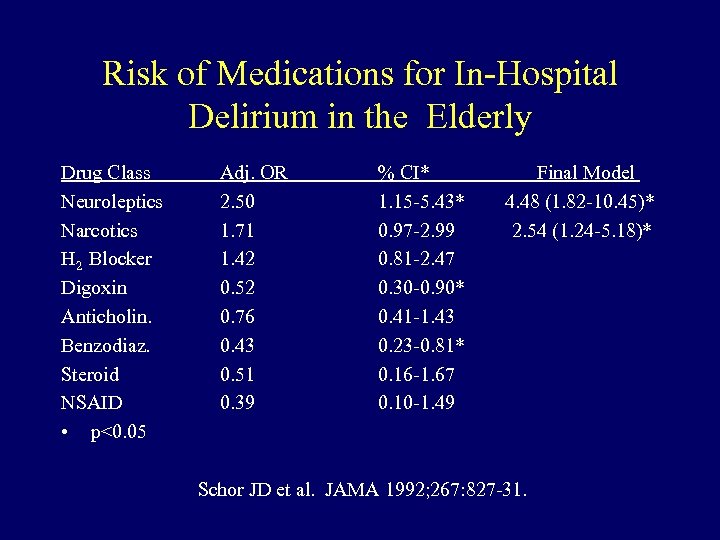
Risk of Medications for In-Hospital Delirium in the Elderly Drug Class Neuroleptics Narcotics H 2 Blocker Digoxin Anticholin. Benzodiaz. Steroid NSAID • p<0. 05 Adj. OR 2. 50 1. 71 1. 42 0. 52 0. 76 0. 43 0. 51 0. 39 % CI* 1. 15 -5. 43* 0. 97 -2. 99 0. 81 -2. 47 0. 30 -0. 90* 0. 41 -1. 43 0. 23 -0. 81* 0. 16 -1. 67 0. 10 -1. 49 Final Model 4. 48 (1. 82 -10. 45)* 2. 54 (1. 24 -5. 18)* Schor JD et al. JAMA 1992; 267: 827 -31.

Communication • Consultant Pharmacist Communication Techniques – Meet your physicians • • • What is the best type of communication? When do the physicians make rounds? Type written vs hand written recommendations

Communication • What physicians say they want from pharmacists • • • Recommendations designed to achieve improved efficacy and decreased risk of adverse drug reactions Help in reducing unnecessary drug use Information about drug side effects and interactions

Communication • What physicians say they want from pharmacists • • Medication-related information and in-services for facility staff Monitoring and dosing of Narrow therapeutic drugs Help in developing processes for detecting and reporting adverse drug reactions Performance of drug regimen review as close as possible to point of prescribing

Communication • • Many physicians feel it is the content that is lacking in recommendations from pharmacists Physician Pet Peeves • • Recommending changes from computer generated pharmacy profiles Closing the sale • • Communicate the solving of the problem not the perception of the problem. Communicating the regulatory issues and addressing the true patient concerns.

Communication • To Cite or Not to Cite • • What if the physician does not respond? • • • Refer to guidelines and the medical literature, make sure it is relative to the elderly resident. Follow the paper trail Are they being sent back in a timely manner Meet with the medical director and create a good professional relationship Maintain a presence in the facility. Choice of words is always a plus

Communication • Consultant Software – – Communication examples In-house pharmacy reporting examples

Questions ?
aafc44d0dfc10dc9d0be84eff23a26e6.ppt