baa42dcb7a0d46ec311f628e38e24e17.ppt
- Количество слайдов: 31

Expanding TAVR Beyond High Risk and Inoperable Patients Jeffrey J. Popma, MD Director, Interventional Cardiology Clinical Services Beth Israel Deaconess Medical Center Associate Professor of Medicine Harvard Medical School Boston, MA 1

Jeffrey J. Popma, MD Consulting: Abbott Vascular and Boston Scientific Corporation Grant Support: Abbott Vascular, Abiomed Inc. , Atrium Medical Corporation, Boston Scientific Corporation, Cordis Corporation, IDEV Technologies, Inc. and Medtronic, Inc.
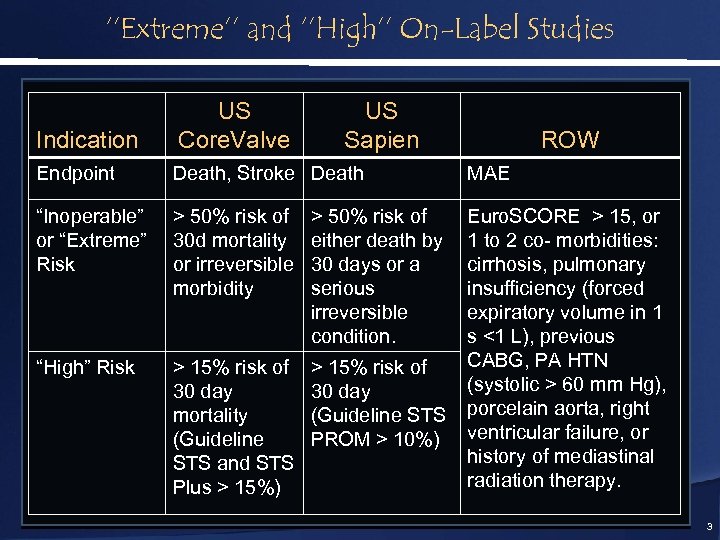
‘’Extreme’’ and ‘’High’’ On-Label Studies Indication US Core. Valve US Sapien Endpoint Death, Stroke Death MAE “Inoperable” or “Extreme” Risk > 50% risk of 30 d mortality or irreversible morbidity > 50% risk of either death by 30 days or a serious irreversible condition. “High” Risk > 15% risk of 30 day mortality (Guideline STS and STS Plus > 15%) > 15% risk of 30 day (Guideline STS PROM > 10%) Euro. SCORE > 15, or 1 to 2 co- morbidities: cirrhosis, pulmonary insufficiency (forced expiratory volume in 1 s <1 L), previous CABG, PA HTN (systolic > 60 mm Hg), porcelain aorta, right ventricular failure, or history of mediastinal radiation therapy. ROW 3
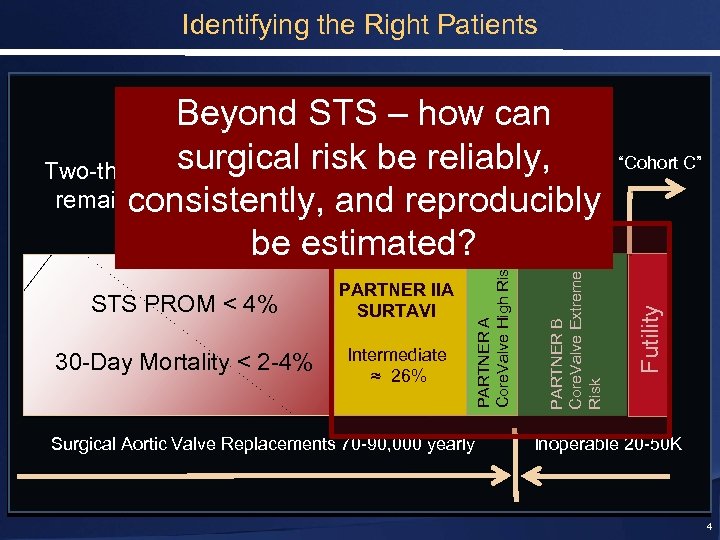
Identifying the Right Patients Top 33% Surgical Risk STS ≥ 4 Intermediate ≈ 26% Surgical Aortic Valve Replacements 70 -90, 000 yearly Futility 30 -Day Mortality < 2 -4% PARTNER B Core. Valve Extreme Risk STS PROM < 4% PARTNER IIA SURTAVI PARTNER A Core. Valve High Risk Beyond STS – how can Surgical surgical risk be. Top 7%STS > 8 Risk “Cohort C” reliably, Two-thirds of patients will Extreme remainconsistently, and reproducibly optimal surgical Risk candidates be estimated? Inoperable 20 -50 K 4
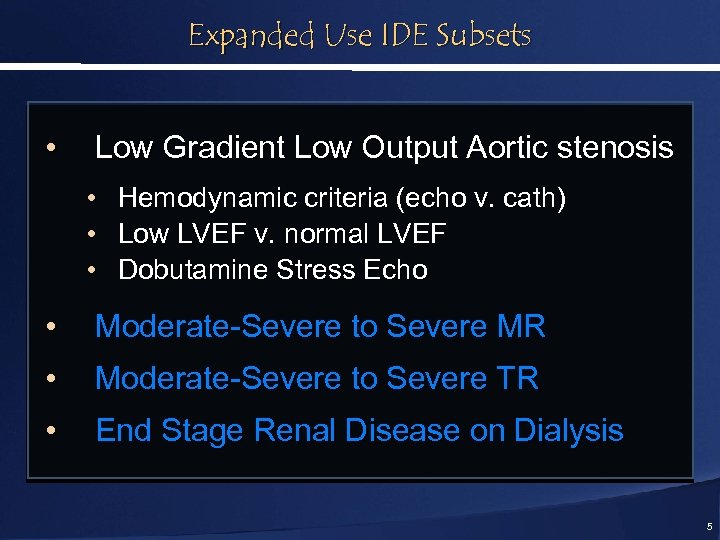
Expanded Use IDE Subsets • Low Gradient Low Output Aortic stenosis • • • Hemodynamic criteria (echo v. cath) Low LVEF v. normal LVEF Dobutamine Stress Echo • Moderate-Severe to Severe MR • Moderate-Severe to Severe TR • End Stage Renal Disease on Dialysis 5
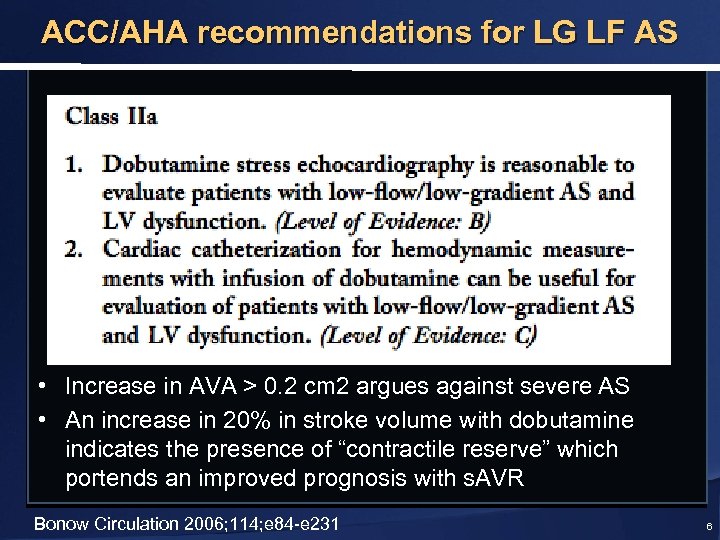
ACC/AHA recommendations for LG LF AS • Increase in AVA > 0. 2 cm 2 argues against severe AS • An increase in 20% in stroke volume with dobutamine indicates the presence of “contractile reserve” which portends an improved prognosis with s. AVR Bonow Circulation 2006; 114; e 84 -e 231 6

Echocardiographic Criteria • Dobutamine stress echocardiography in setting of LV dysfunction starting at 2. 5 or 5 mg/kg/min with an incremental increase in the infusion every 3– 5 min to a maximum dose of 10– 20 mg/kg/min. • The infusion should be stopped as soon as a positive result is obtained or when the heart rate begins to rise more than 10– 20 bpm over baseline or exceeds 100 bpm, • Severe stenosis is suggested by an AS jet > 4. 0 or a mean gradient > 40 mm. Hg provided that valve area does not exceed 1. 0 cm 2 at any flow rate Baumgartner JASE 2009; 22: 1 7
Criteria Used for US Core. Valve IDE Protocols • Left Ventricular Ejection Fraction < 50% ü DS gradient > 40 mm. Hg or > 4 M/sec ü DS gradient < 40 mm. Hg AND < 4 M/sec • Current protocol indicates either cardiac catheterization or tranthoracic echocardiography can be used to determine mean or peak gradients, at rest or with dobutamine stress (DS) • Liberal acceptance of either measurement method 8
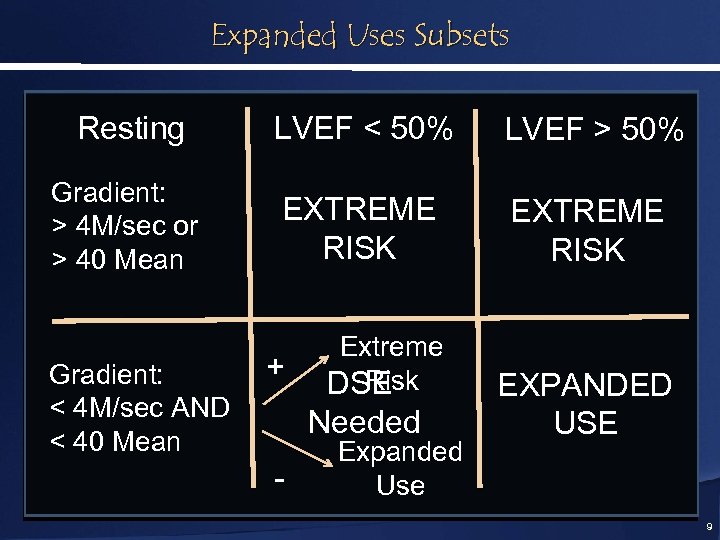
Expanded Uses Subsets Resting LVEF < 50% LVEF > 50% Gradient: > 4 M/sec or > 40 Mean EXTREME RISK Gradient: < 4 M/sec AND < 40 Mean + Extreme Risk DSE Needed - Expanded Use EXPANDED USE 9

Expanded Uses Subsets • Low Gradient Low Output Aortic stenosis • • • Hemodynamic criteria (echo v. cath) Low LVEF v. normal LVEF Dobutamine Stress Echo • Moderate-Severe to Severe MR • Moderate-Severe to Severe TR • End Stage Renal Disease on Dialysis 10
Valve in Valve 11
Valve in Valve 12
Valve in Valve 13
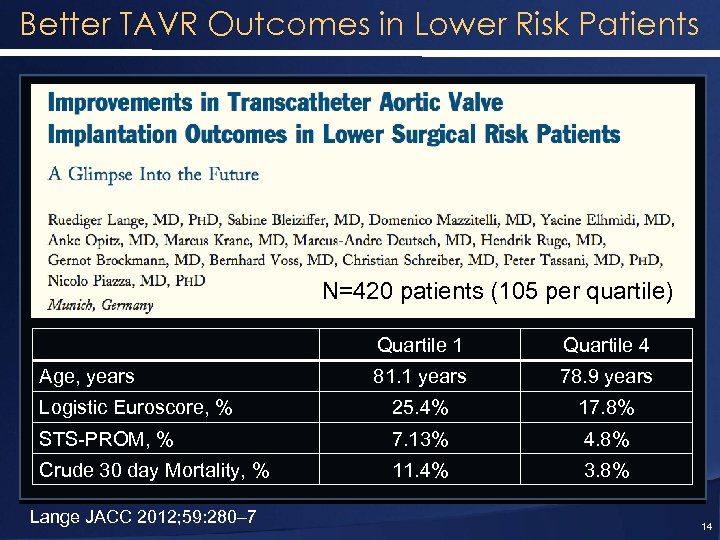
Better TAVR Outcomes in Lower Risk Patients N=420 patients (105 per quartile) Quartile 1 Quartile 4 81. 1 years 78. 9 years Logistic Euroscore, % 25. 4% 17. 8% STS-PROM, % 7. 13% 4. 8% Crude 30 day Mortality, % 11. 4% 3. 8% Age, years Lange JACC 2012; 59: 280– 7 14
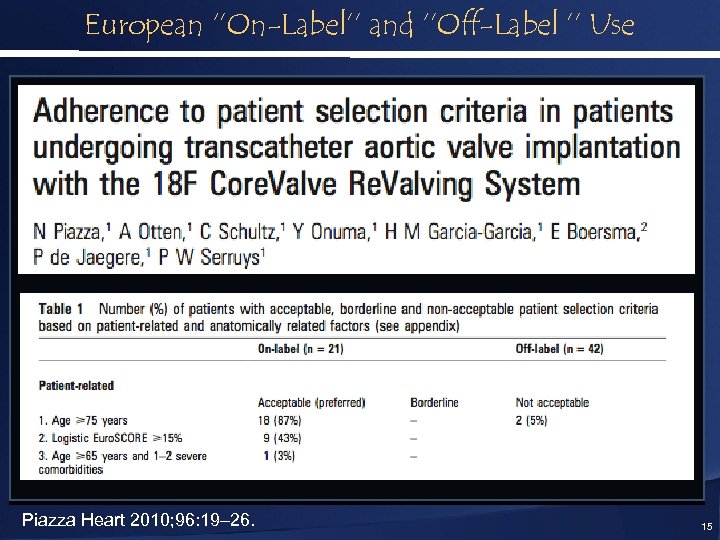
European ‘’On-Label’’ and ‘’Off-Label ’’ Use Piazza Heart 2010; 96: 19– 26. 15
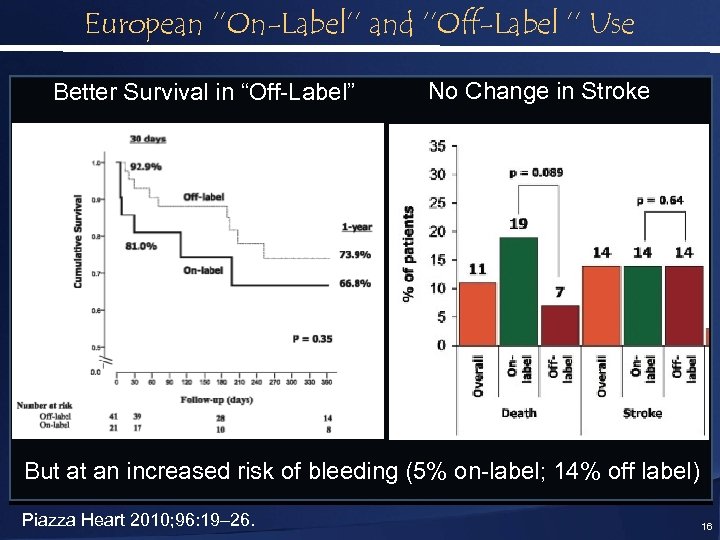
European ‘’On-Label’’ and ‘’Off-Label ’’ Use Better Survival in “Off-Label” No Change in Stroke But at an increased risk of bleeding (5% on-label; 14% off label) Piazza Heart 2010; 96: 19– 26. 16

Intermediate Risk - Patient Selection Presented By Prof. Patrick Serruys, MD on behalf of 17

SURTAVI Trial Leadership Chairmen: • Prof. P. W. Serruys (Chair) • Dr. N. van Mieghem (Deputy Chair) Principal Investigators: • Prof. S. Windecker • Prof. A. P. Kappetein • Prof. R. Lange • Prof. T. Walther • Dr. J. Popma • Dr. D. Adams • Dr. M. Reardon Serruys SURTAVI TCT 2011 18
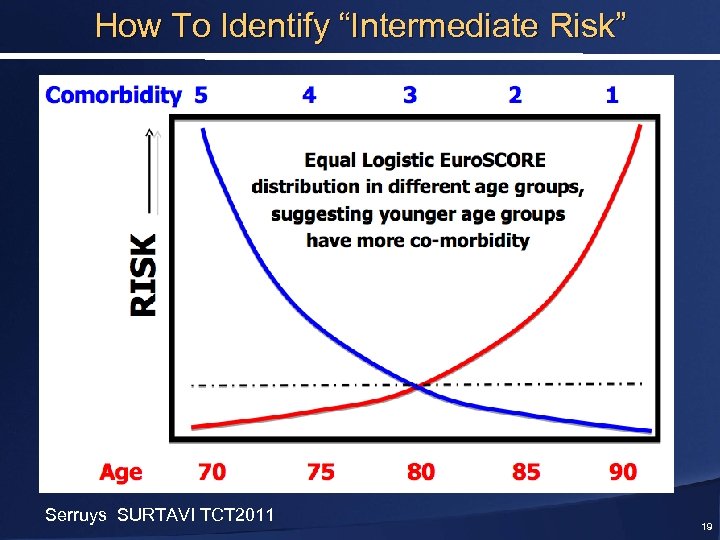
How To Identify “Intermediate Risk” Serruys SURTAVI TCT 2011 19
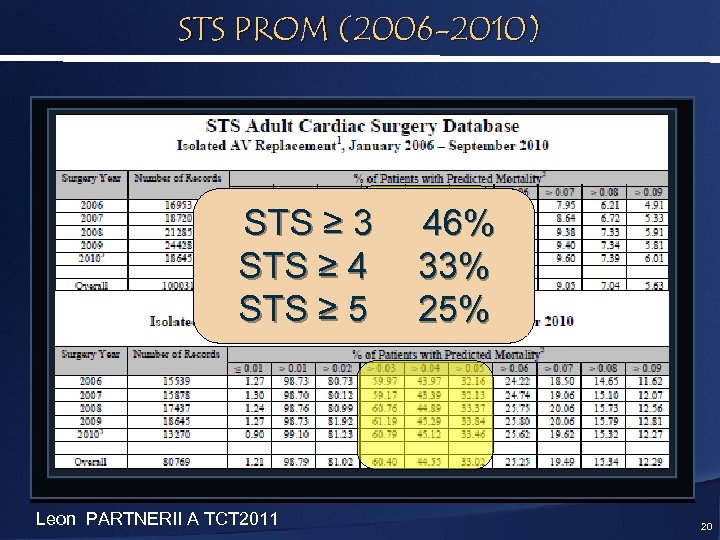
STS PROM (2006 -2010) STS ≥ 3 STS ≥ 4 STS ≥ 5 Leon PARTNERII A TCT 2011 46% 33% 25% 20
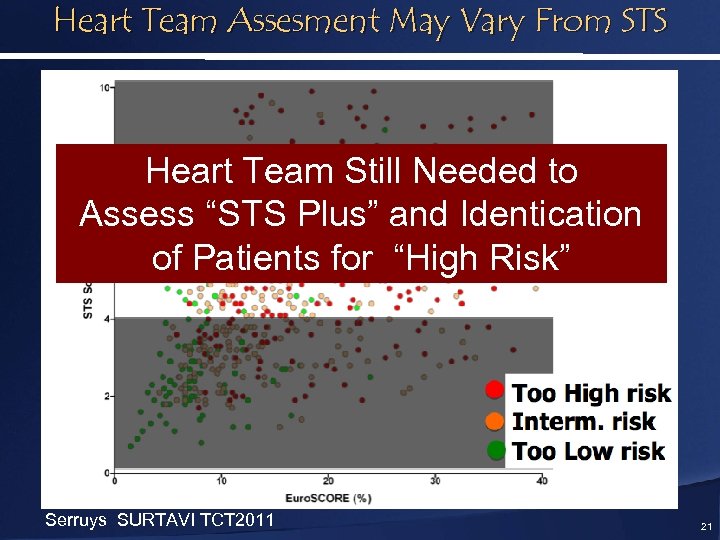
Heart Team Assesment May Vary From STS Heart Team Still Needed to Assess “STS Plus” and Identication of Patients for “High Risk” Serruys SURTAVI TCT 2011 21

Core. Valve® SURTAVI Trial Population • Symptomatic severe aortic stenosis • Intermediate surgical risk, defined by Society of Thoracic Surgeons (STS) mortality risk of ≥ 4% and ≤ 10% 1. Medtronic Core. Valve® SURTAVI Trial. Version 4. 0. Mounds View, MN: Medtronic, Inc. Clinical Research; Approval pending. 22
Core. Valve® SURTAVI Trial Coronary Artery Disease • Subjects with CAD and a Syntax score <22 will be eligible – Concomitant PCI and TAVI can be performed; staging is left at discretion of operator – CABG should be conducted during the index SAVR procedure • Randomization stratified by need for coronary revascularization 2. Medtronic Core. Valve® SURTAVI Trial. Version 3. 0. Mounds View, MN: Medtronic, Inc. Clinical Research; 2011. 23
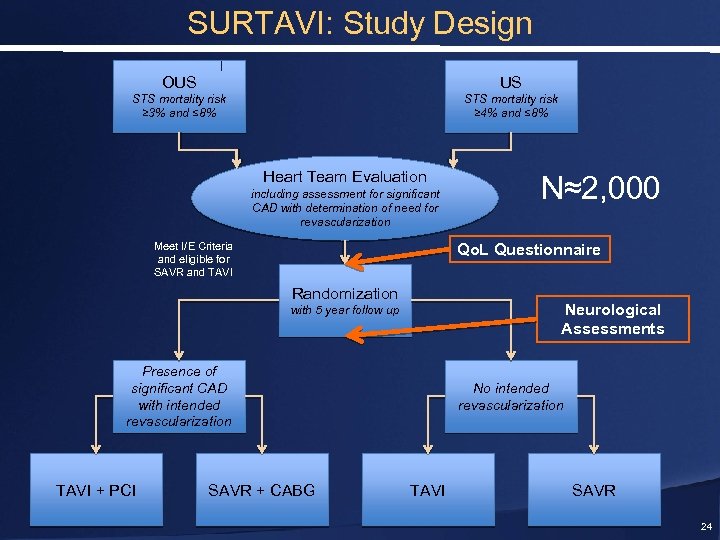
SURTAVI: Study Design OUS US STS mortality risk ≥ 3% and ≤ 8% STS mortality risk ≥ 4% and ≤ 8% Heart Team Evaluation including assessment for significant CAD with determination of need for revascularization Meet I/E Criteria and eligible for SAVR and TAVI Qo. L Questionnaire Randomization Neurological Assessments with 5 year follow up Presence of significant CAD with intended revascularization TAVI + PCI N≈2, 000 SAVR + CABG No intended revascularization TAVI SAVR 24
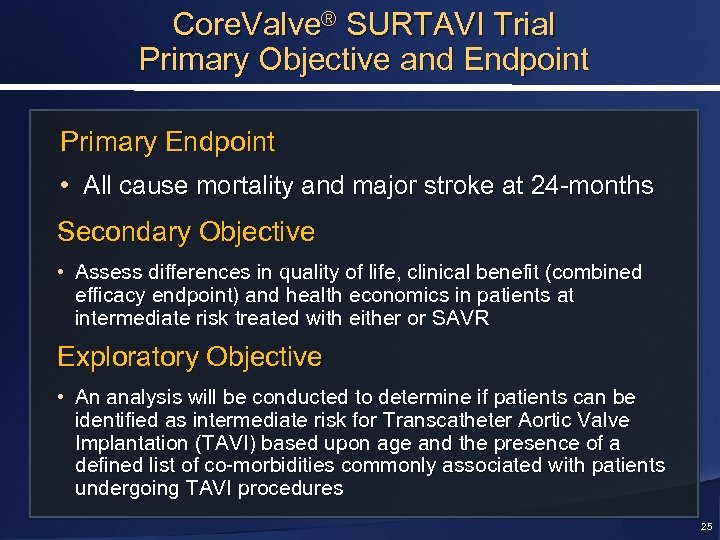
Core. Valve® SURTAVI Trial Primary Objective and Endpoint Primary Endpoint • All cause mortality and major stroke at 24 -months Secondary Objective • Assess differences in quality of life, clinical benefit (combined efficacy endpoint) and health economics in patients at intermediate risk treated with either or SAVR Exploratory Objective • An analysis will be conducted to determine if patients can be identified as intermediate risk for Transcatheter Aortic Valve Implantation (TAVI) based upon age and the presence of a defined list of co-morbidities commonly associated with patients undergoing TAVI procedures 25

AOA® Anti-Mineralization Treatment • Alpha-amino oleic acid (AOA) – Naturally occurring long-chain fatty acid – Binds to aldehyde groups blocking calcification of prosthetic valve leaflets • Demonstrated Long-Term Results – AOA has 20 years of proven clinical success on surgical valves 1 – A randomized study of the AOA treated Freestyle® aortic root replacement reported 8 -year freedom from reoperation of 100% 2 1. 2. Medtronic Freestyle Aortic Root Bioprosthesis was first implanted clinically in August 1992. El-Hamamsy I, et. al. Late Outcomes Following Freestyle Versus Homograft Aortic Root Replacement. JACC. 2010; 4; 368 -76. 26
Access Options Direct Aortic Subclavian Transfemoral 27
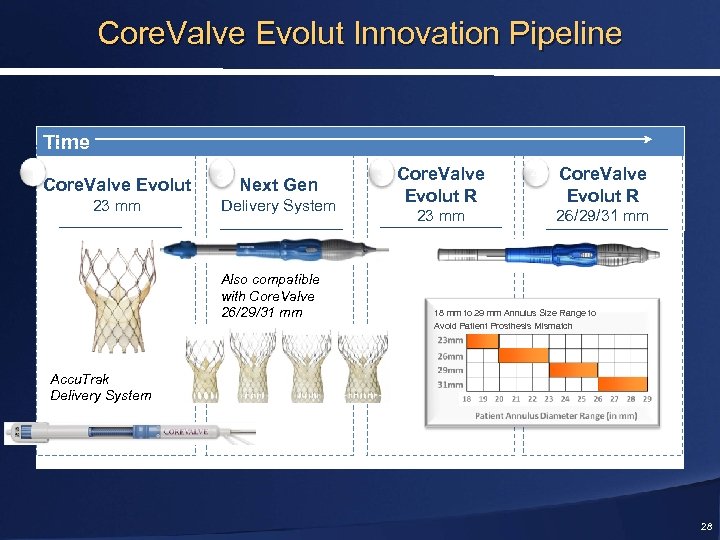
Core. Valve Evolut Innovation Pipeline Time 1 Core. Valve Evolut 23 mm 2 Next Gen Delivery System Also compatible with Core. Valve 26/29/31 mm 3 Core. Valve Evolut R 23 mm 4 Core. Valve Evolut R 26/29/31 mm 18 mm to 29 mm Annulus Size Range to Avoid Patient Prosthesis Mismatch Accu. Trak Delivery System 28
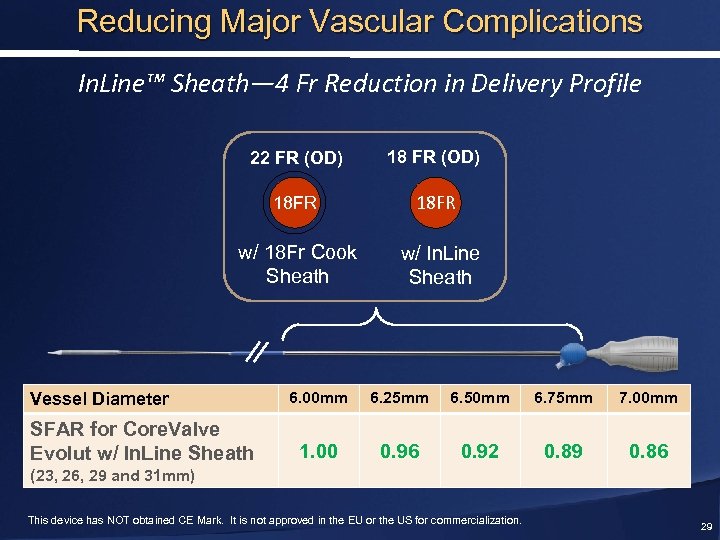
Reducing Major Vascular Complications In. Line™ Sheath— 4 Fr Reduction in Delivery Profile 22 FR (OD) 18 FR w/ 18 Fr Cook Sheath w/ In. Line Sheath Vessel Diameter SFAR for Core. Valve Evolut w/ In. Line Sheath 6. 00 mm 6. 25 mm 6. 50 mm 6. 75 mm 7. 00 mm 1. 00 0. 96 0. 92 0. 89 0. 86 (23, 26, 29 and 31 mm) This device has NOT obtained CE Mark. It is not approved in the EU or the US for commercialization. 29
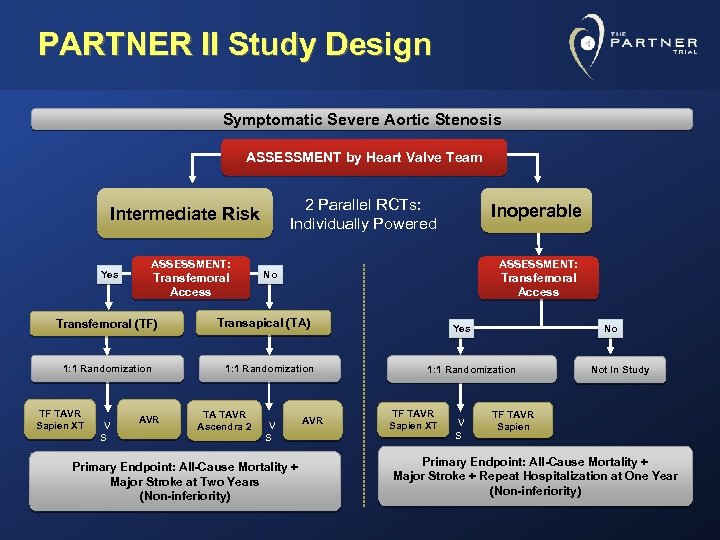
PARTNER II Study Design Symptomatic Severe Aortic Stenosis ASSESSMENT by Heart Valve Team 2 Parallel RCTs: Individually Powered Intermediate Risk Yes ASSESSMENT: Transfemoral Access Transfemoral (TF) 1: 1 Randomization TF TAVR Sapien XT V S AVR ASSESSMENT: No Transfemoral Access Transapical (TA) 1: 1 Randomization TA TAVR Ascendra 2 Inoperable V S Primary Endpoint: All-Cause Mortality + Major Stroke at Two Years (Non-inferiority) AVR Yes No 1: 1 Randomization TF TAVR Sapien XT V S Not In Study TF TAVR Sapien Primary Endpoint: All-Cause Mortality + Major Stroke + Repeat Hospitalization at One Year (Non-inferiority)

Intermediate Risk Populations: Summary • Outcomes will definitely be better with TAVI in lower risk populations, but so will the outcomes in patients undergoing s. AVR • Two big concerns: Perivalvular AR and Stroke • Aim to lower PPM rate with good implantation technique • Randomized trials are essential 31
baa42dcb7a0d46ec311f628e38e24e17.ppt