fd2d8f0e33f5947fa07a87ffea92d7f4.ppt
- Количество слайдов: 11
 EXCi. PACTTM International Pharmaceutical Excipients Certification Minimize risks – maximize benefits
EXCi. PACTTM International Pharmaceutical Excipients Certification Minimize risks – maximize benefits
 Why EXCi. PACTTM? • • • Risks in the pharmaceutical supply chain are not just API related, excipients may be impacted Regulators expect Market Authorization Holders to secure their supply chain The appropriate way to achieve this is by a substantial increase of periodical, physical audits • The economical burden associated to these requirements are impacting both manufacturers and users è Excipact Certification scheme is the industry response to help mitigate the risks and address the auditing challenge EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
Why EXCi. PACTTM? • • • Risks in the pharmaceutical supply chain are not just API related, excipients may be impacted Regulators expect Market Authorization Holders to secure their supply chain The appropriate way to achieve this is by a substantial increase of periodical, physical audits • The economical burden associated to these requirements are impacting both manufacturers and users è Excipact Certification scheme is the industry response to help mitigate the risks and address the auditing challenge EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
 What is EXCi. PACTTM? Vision • A voluntary self regulated initiative of the global pharmaceutical excipient manufacturing, distributing and processing industry supported by excipient users • EXCi. PACTTM ensures current GMP and GDP requirements are applied to pharmaceutical excipients through a recognized auditing and certification process thereby increasing safety and reliability as well as transparency of the pharmaceutical supply chain · EXCi. PACTTM is accepted from all major stakeholders including relevant authorities globally · As an independent organization EXCi. PACTTM will be able to objectively set c. GMP and c. GDP standards today and in the future · EXCi. PACTTM certification provides a cost efficient method of ensuring c. GMP and c. GDP are applied throughout the pharmaceutical supply chain by reducing the audit burden EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 3
What is EXCi. PACTTM? Vision • A voluntary self regulated initiative of the global pharmaceutical excipient manufacturing, distributing and processing industry supported by excipient users • EXCi. PACTTM ensures current GMP and GDP requirements are applied to pharmaceutical excipients through a recognized auditing and certification process thereby increasing safety and reliability as well as transparency of the pharmaceutical supply chain · EXCi. PACTTM is accepted from all major stakeholders including relevant authorities globally · As an independent organization EXCi. PACTTM will be able to objectively set c. GMP and c. GDP standards today and in the future · EXCi. PACTTM certification provides a cost efficient method of ensuring c. GMP and c. GDP are applied throughout the pharmaceutical supply chain by reducing the audit burden EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 3
 EXCi. PACTTM Certification Benefits · Ease of Access: Certification from many 3 rd party audit organizations · Evolutionary: builds on existing ISO 9001 certification and uses well known IPEC-PQG GMP guides · Simple: easy to understand apply for all stakeholders · Inclusive: applicable to all pharmaceutical excipients manufacturers and distributors · Permits the supplier to proactively demonstrate commitment to c. GMP and c. GDP in the manufacture and supply of their pharmaceutical excipient EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 4
EXCi. PACTTM Certification Benefits · Ease of Access: Certification from many 3 rd party audit organizations · Evolutionary: builds on existing ISO 9001 certification and uses well known IPEC-PQG GMP guides · Simple: easy to understand apply for all stakeholders · Inclusive: applicable to all pharmaceutical excipients manufacturers and distributors · Permits the supplier to proactively demonstrate commitment to c. GMP and c. GDP in the manufacture and supply of their pharmaceutical excipient EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 4
 EXCi. PACTTM Certification Key Milestones for Implementation ü ü • • • Development of concept / business plan by EXCi. PACTTM GSC April 2011 Cross referenced acceptance of Rx 360 and EXi. PACTTM by 3. Q. 2011 Trademark protection by 2 Q. 2011 Acceptance and acknowledgement by IPEC, CEFIC. . . by 3 Q. 2011 Base funding completed by 90% by 4 Q. 2011 Hiring of staff, establishement of the advisory board by 1. Q 2012 Start of operations by 1 Q. 2012 EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 5
EXCi. PACTTM Certification Key Milestones for Implementation ü ü • • • Development of concept / business plan by EXCi. PACTTM GSC April 2011 Cross referenced acceptance of Rx 360 and EXi. PACTTM by 3. Q. 2011 Trademark protection by 2 Q. 2011 Acceptance and acknowledgement by IPEC, CEFIC. . . by 3 Q. 2011 Base funding completed by 90% by 4 Q. 2011 Hiring of staff, establishement of the advisory board by 1. Q 2012 Start of operations by 1 Q. 2012 EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 5
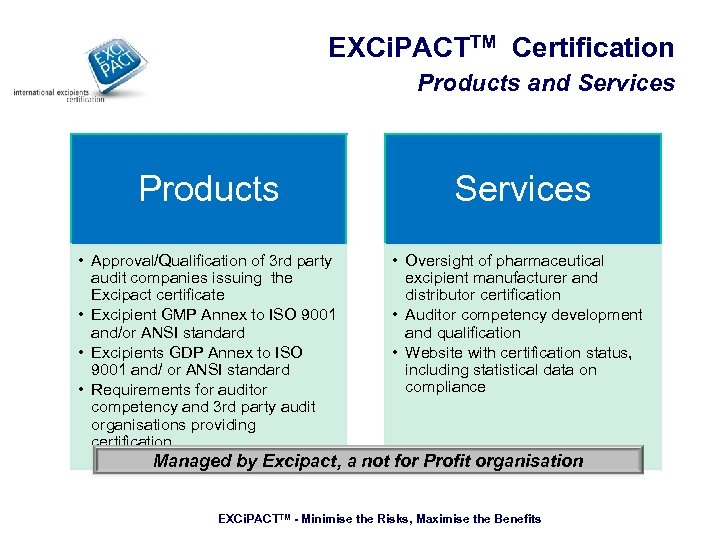 EXCi. PACTTM Certification Products and Services Products • Approval/Qualification of 3 rd party audit companies issuing the Excipact certificate • Excipient GMP Annex to ISO 9001 and/or ANSI standard • Excipients GDP Annex to ISO 9001 and/ or ANSI standard • Requirements for auditor competency and 3 rd party audit organisations providing certification Services • Oversight of pharmaceutical excipient manufacturer and distributor certification • Auditor competency development and qualification • Website with certification status, including statistical data on compliance Managed by Excipact, a not for Profit organisation EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
EXCi. PACTTM Certification Products and Services Products • Approval/Qualification of 3 rd party audit companies issuing the Excipact certificate • Excipient GMP Annex to ISO 9001 and/or ANSI standard • Excipients GDP Annex to ISO 9001 and/ or ANSI standard • Requirements for auditor competency and 3 rd party audit organisations providing certification Services • Oversight of pharmaceutical excipient manufacturer and distributor certification • Auditor competency development and qualification • Website with certification status, including statistical data on compliance Managed by Excipact, a not for Profit organisation EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
 EXCi. PACTTM International Pharmaceutical Excipients Certification Backups Minimize risks – maximize benefits
EXCi. PACTTM International Pharmaceutical Excipients Certification Backups Minimize risks – maximize benefits
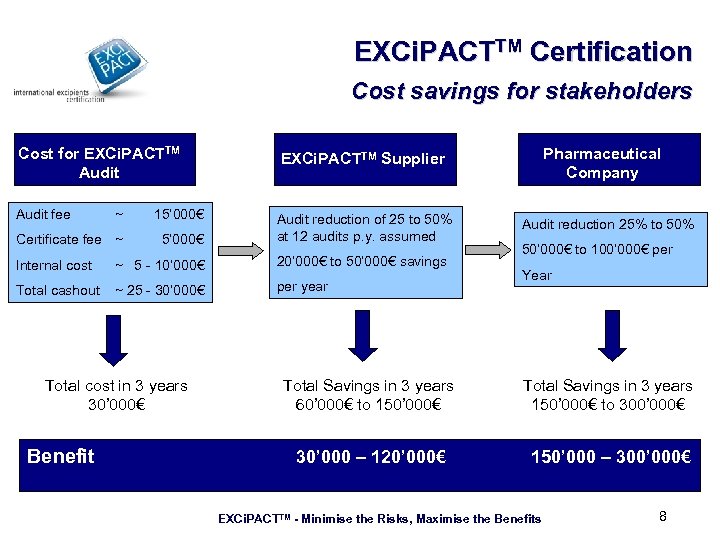 EXCi. PACTTM Certification Cost savings for stakeholders Cost for EXCi. PACTTM Audit EXCi. PACTTM Supplier Audit fee Audit reduction of 25 to 50% at 12 audits p. y. assumed ~ 15’ 000€ Certificate fee ~ 5’ 000€ Internal cost Total cashout ~ 5 - 10’ 000€ 20’ 000€ to 50’ 000€ savings ~ 25 - 30’ 000€ per year Total cost in 3 years 30’ 000€ Benefit Pharmaceutical Company Audit reduction 25% to 50% 50’ 000€ to 100’ 000€ per Year Total Savings in 3 years 60’ 000€ to 150’ 000€ Total Savings in 3 years 150’ 000€ to 300’ 000€ 30’ 000 – 120’ 000€ 150’ 000 – 300’ 000€ EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 8
EXCi. PACTTM Certification Cost savings for stakeholders Cost for EXCi. PACTTM Audit EXCi. PACTTM Supplier Audit fee Audit reduction of 25 to 50% at 12 audits p. y. assumed ~ 15’ 000€ Certificate fee ~ 5’ 000€ Internal cost Total cashout ~ 5 - 10’ 000€ 20’ 000€ to 50’ 000€ savings ~ 25 - 30’ 000€ per year Total cost in 3 years 30’ 000€ Benefit Pharmaceutical Company Audit reduction 25% to 50% 50’ 000€ to 100’ 000€ per Year Total Savings in 3 years 60’ 000€ to 150’ 000€ Total Savings in 3 years 150’ 000€ to 300’ 000€ 30’ 000 – 120’ 000€ 150’ 000 – 300’ 000€ EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 8
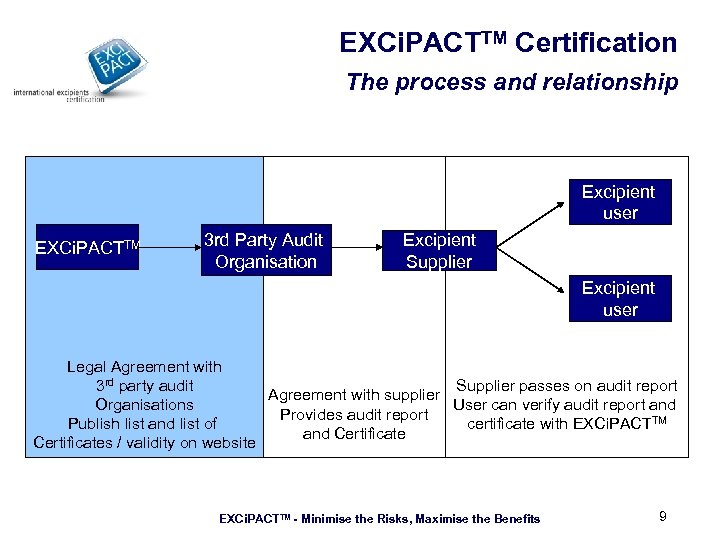 EXCi. PACTTM Certification The process and relationship Excipient user EXCi. PACTTM 3 rd Party Audit Organisation Excipient Supplier Excipient user Legal Agreement with 3 rd party audit Supplier passes on audit report Agreement with supplier Organisations User can verify audit report and Provides audit report Publish list and list of certificate with EXCi. PACTTM and Certificates / validity on website EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 9
EXCi. PACTTM Certification The process and relationship Excipient user EXCi. PACTTM 3 rd Party Audit Organisation Excipient Supplier Excipient user Legal Agreement with 3 rd party audit Supplier passes on audit report Agreement with supplier Organisations User can verify audit report and Provides audit report Publish list and list of certificate with EXCi. PACTTM and Certificates / validity on website EXCi. PACTTM - Minimise the Risks, Maximise the Benefits 9
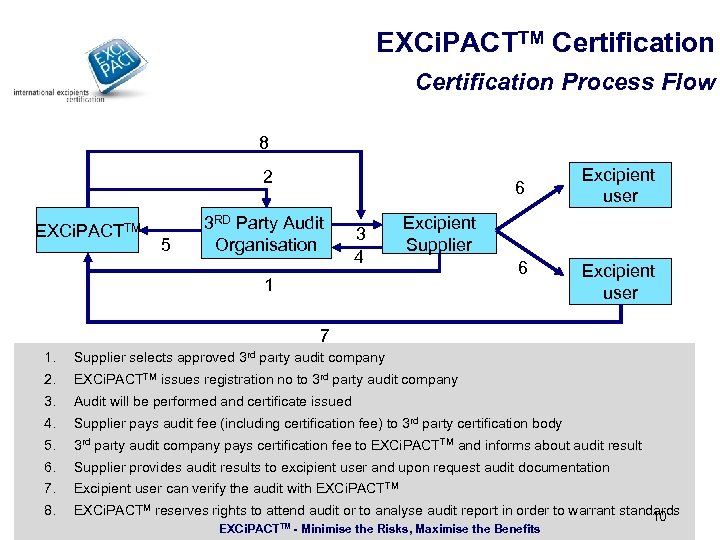 EXCi. PACTTM Certification Process Flow 8 2 EXCi. PACTTM 5 6 3 RD Party Audit Organisation 3 4 Excipient user Excipient Supplier 1 6 Excipient user 7 1. Supplier selects approved 3 rd party audit company 2. EXCi. PACTTM issues registration no to 3 rd party audit company 3. Audit will be performed and certificate issued 4. Supplier pays audit fee (including certification fee) to 3 rd party certification body 5. 3 rd party audit company pays certification fee to EXCi. PACTTM and informs about audit result 6. Supplier provides audit results to excipient user and upon request audit documentation 7. Excipient user can verify the audit with EXCi. PACTTM 8. EXCi. PACTM reserves rights to attend audit or to analyse audit report in order to warrant standards 10 EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
EXCi. PACTTM Certification Process Flow 8 2 EXCi. PACTTM 5 6 3 RD Party Audit Organisation 3 4 Excipient user Excipient Supplier 1 6 Excipient user 7 1. Supplier selects approved 3 rd party audit company 2. EXCi. PACTTM issues registration no to 3 rd party audit company 3. Audit will be performed and certificate issued 4. Supplier pays audit fee (including certification fee) to 3 rd party certification body 5. 3 rd party audit company pays certification fee to EXCi. PACTTM and informs about audit result 6. Supplier provides audit results to excipient user and upon request audit documentation 7. Excipient user can verify the audit with EXCi. PACTTM 8. EXCi. PACTM reserves rights to attend audit or to analyse audit report in order to warrant standards 10 EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
 EXCi. PACTTM Certification Quality Assurance of Certification • • Certificate on file and accessible on the web site Audit of third party certification body for Excipact standard compliance Access to third party auditing process for Quality assurance purpose Periodic review and renewal of 3 rd party authorisation to certify against Excipact standards EXCi. PACTTM - Minimise the Risks, Maximise the Benefits
EXCi. PACTTM Certification Quality Assurance of Certification • • Certificate on file and accessible on the web site Audit of third party certification body for Excipact standard compliance Access to third party auditing process for Quality assurance purpose Periodic review and renewal of 3 rd party authorisation to certify against Excipact standards EXCi. PACTTM - Minimise the Risks, Maximise the Benefits


