a24ec52661c6a18526130f41b05d205b.ppt
- Количество слайдов: 13
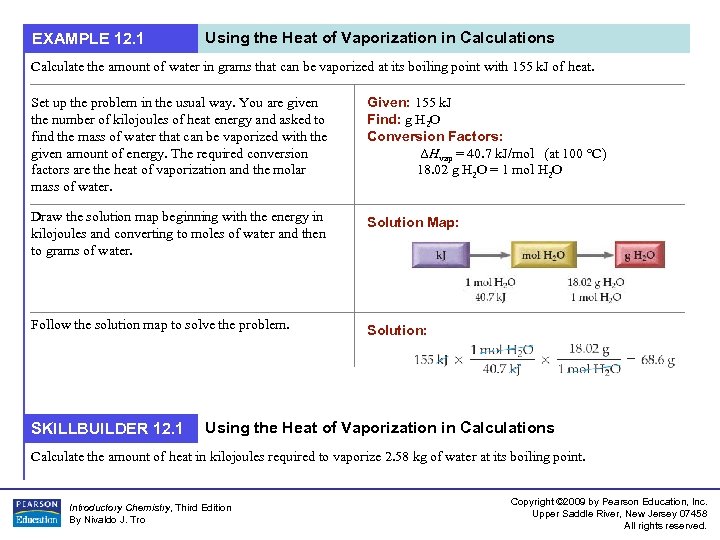 EXAMPLE 12. 1 Using the Heat of Vaporization in Calculations Calculate the amount of water in grams that can be vaporized at its boiling point with 155 k. J of heat. Set up the problem in the usual way. You are given the number of kilojoules of heat energy and asked to find the mass of water that can be vaporized with the given amount of energy. The required conversion factors are the heat of vaporization and the molar mass of water. Given: 155 k. J Find: g H 2 O Conversion Factors: ΔHvap = 40. 7 k. J/mol (at 100 °C) 18. 02 g H 2 O = 1 mol H 2 O Draw the solution map beginning with the energy in kilojoules and converting to moles of water and then to grams of water. Solution Map: Follow the solution map to solve the problem. Solution: SKILLBUILDER 12. 1 Using the Heat of Vaporization in Calculations Calculate the amount of heat in kilojoules required to vaporize 2. 58 kg of water at its boiling point. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 1 Using the Heat of Vaporization in Calculations Calculate the amount of water in grams that can be vaporized at its boiling point with 155 k. J of heat. Set up the problem in the usual way. You are given the number of kilojoules of heat energy and asked to find the mass of water that can be vaporized with the given amount of energy. The required conversion factors are the heat of vaporization and the molar mass of water. Given: 155 k. J Find: g H 2 O Conversion Factors: ΔHvap = 40. 7 k. J/mol (at 100 °C) 18. 02 g H 2 O = 1 mol H 2 O Draw the solution map beginning with the energy in kilojoules and converting to moles of water and then to grams of water. Solution Map: Follow the solution map to solve the problem. Solution: SKILLBUILDER 12. 1 Using the Heat of Vaporization in Calculations Calculate the amount of heat in kilojoules required to vaporize 2. 58 kg of water at its boiling point. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 1 Using the Heat of Vaporization in Calculations Continued SKILLBUILDER PLUS A drop of water weighing 0. 48 g condenses on the surface of a 55 -g block of aluminum that is initially at 25 °C. If the heat released during condensation goes only toward heating the metal, what is the final temperature in Celsius of the metal block? (The specific heat capacity of aluminum is 0. 903 J/g °C. ) FOR MORE PRACTICE Example 12. 7; Problems 51, 52, 53, 54, 55, 56. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 1 Using the Heat of Vaporization in Calculations Continued SKILLBUILDER PLUS A drop of water weighing 0. 48 g condenses on the surface of a 55 -g block of aluminum that is initially at 25 °C. If the heat released during condensation goes only toward heating the metal, what is the final temperature in Celsius of the metal block? (The specific heat capacity of aluminum is 0. 903 J/g °C. ) FOR MORE PRACTICE Example 12. 7; Problems 51, 52, 53, 54, 55, 56. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
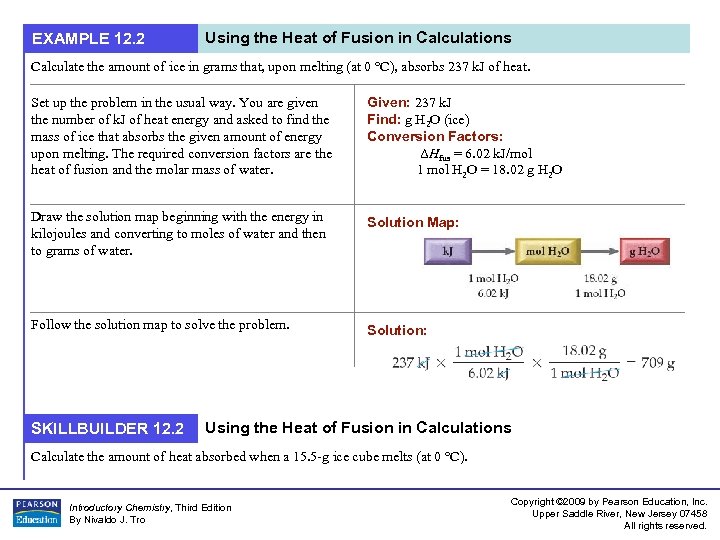 EXAMPLE 12. 2 Using the Heat of Fusion in Calculations Calculate the amount of ice in grams that, upon melting (at 0 °C), absorbs 237 k. J of heat. Set up the problem in the usual way. You are given the number of k. J of heat energy and asked to find the mass of ice that absorbs the given amount of energy upon melting. The required conversion factors are the heat of fusion and the molar mass of water. Given: 237 k. J Find: g H 2 O (ice) Conversion Factors: ΔHfus = 6. 02 k. J/mol 1 mol H 2 O = 18. 02 g H 2 O Draw the solution map beginning with the energy in kilojoules and converting to moles of water and then to grams of water. Solution Map: Follow the solution map to solve the problem. Solution: SKILLBUILDER 12. 2 Using the Heat of Fusion in Calculations Calculate the amount of heat absorbed when a 15. 5 -g ice cube melts (at 0 °C). Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 2 Using the Heat of Fusion in Calculations Calculate the amount of ice in grams that, upon melting (at 0 °C), absorbs 237 k. J of heat. Set up the problem in the usual way. You are given the number of k. J of heat energy and asked to find the mass of ice that absorbs the given amount of energy upon melting. The required conversion factors are the heat of fusion and the molar mass of water. Given: 237 k. J Find: g H 2 O (ice) Conversion Factors: ΔHfus = 6. 02 k. J/mol 1 mol H 2 O = 18. 02 g H 2 O Draw the solution map beginning with the energy in kilojoules and converting to moles of water and then to grams of water. Solution Map: Follow the solution map to solve the problem. Solution: SKILLBUILDER 12. 2 Using the Heat of Fusion in Calculations Calculate the amount of heat absorbed when a 15. 5 -g ice cube melts (at 0 °C). Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 2 Using the Heat of Fusion in Calculations Continued SKILLBUILDER PLUS A 5. 6 -g ice cube (at 0 °C) is placed into 195 g of water initially at 25 °C. If the heat absorbed for melting the ice comes only from the 195 g of water, what is the temperature change of the 195 g of water? FOR MORE PRACTICE Example 12. 8; Problems 59, 60, 61, 62. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 2 Using the Heat of Fusion in Calculations Continued SKILLBUILDER PLUS A 5. 6 -g ice cube (at 0 °C) is placed into 195 g of water initially at 25 °C. If the heat absorbed for melting the ice comes only from the 195 g of water, what is the temperature change of the 195 g of water? FOR MORE PRACTICE Example 12. 8; Problems 59, 60, 61, 62. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 3 Dispersion Forces Which halogen, Cl 2 or I 2 has the higher boiling point? Solution: The molar mass of Cl 2 is 70. 90 g/mol and the molar mass of I 2 is 253. 80 g/mol. Since I 2 has the higher molar mass, it has stronger dispersion forces and therefore the higher boiling point. SKILLBUILDER 12. 5 Dispersion Forces Which hydrocarbon, CH 4 or C 2 H 6, has the higher boiling point? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 67, 68. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 3 Dispersion Forces Which halogen, Cl 2 or I 2 has the higher boiling point? Solution: The molar mass of Cl 2 is 70. 90 g/mol and the molar mass of I 2 is 253. 80 g/mol. Since I 2 has the higher molar mass, it has stronger dispersion forces and therefore the higher boiling point. SKILLBUILDER 12. 5 Dispersion Forces Which hydrocarbon, CH 4 or C 2 H 6, has the higher boiling point? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 67, 68. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
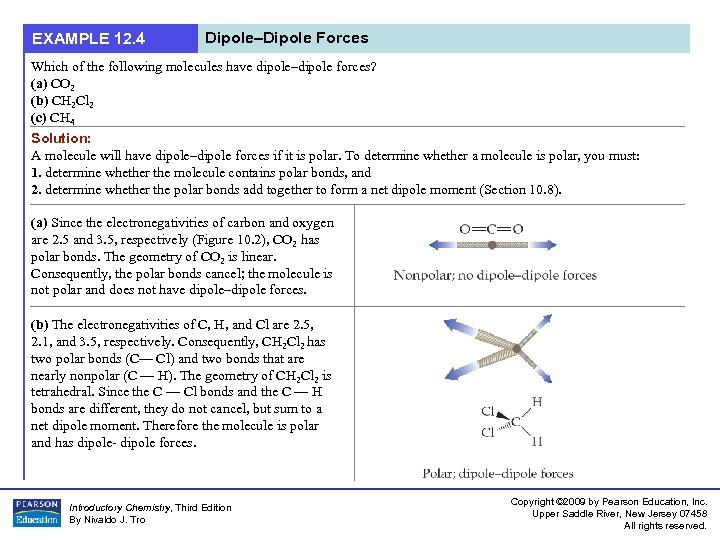 EXAMPLE 12. 4 Dipole–Dipole Forces Which of the following molecules have dipole–dipole forces? (a) CO 2 (b) CH 2 Cl 2 (c) CH 4 Solution: A molecule will have dipole–dipole forces if it is polar. To determine whether a molecule is polar, you must: 1. determine whether the molecule contains polar bonds, and 2. determine whether the polar bonds add together to form a net dipole moment (Section 10. 8). (a) Since the electronegativities of carbon and oxygen are 2. 5 and 3. 5, respectively (Figure 10. 2), CO 2 has polar bonds. The geometry of CO 2 is linear. Consequently, the polar bonds cancel; the molecule is not polar and does not have dipole–dipole forces. (b) The electronegativities of C, H, and Cl are 2. 5, 2. 1, and 3. 5, respectively. Consequently, CH 2 Cl 2 has two polar bonds (C— Cl) and two bonds that are nearly nonpolar (C — H). The geometry of CH 2 Cl 2 is tetrahedral. Since the C — Cl bonds and the C — H bonds are different, they do not cancel, but sum to a net dipole moment. Therefore the molecule is polar and has dipole- dipole forces. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 4 Dipole–Dipole Forces Which of the following molecules have dipole–dipole forces? (a) CO 2 (b) CH 2 Cl 2 (c) CH 4 Solution: A molecule will have dipole–dipole forces if it is polar. To determine whether a molecule is polar, you must: 1. determine whether the molecule contains polar bonds, and 2. determine whether the polar bonds add together to form a net dipole moment (Section 10. 8). (a) Since the electronegativities of carbon and oxygen are 2. 5 and 3. 5, respectively (Figure 10. 2), CO 2 has polar bonds. The geometry of CO 2 is linear. Consequently, the polar bonds cancel; the molecule is not polar and does not have dipole–dipole forces. (b) The electronegativities of C, H, and Cl are 2. 5, 2. 1, and 3. 5, respectively. Consequently, CH 2 Cl 2 has two polar bonds (C— Cl) and two bonds that are nearly nonpolar (C — H). The geometry of CH 2 Cl 2 is tetrahedral. Since the C — Cl bonds and the C — H bonds are different, they do not cancel, but sum to a net dipole moment. Therefore the molecule is polar and has dipole- dipole forces. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
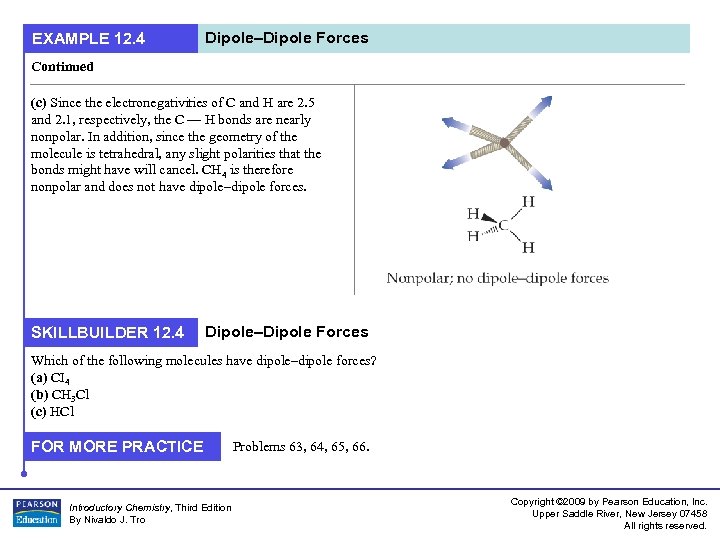 EXAMPLE 12. 4 Dipole–Dipole Forces Continued (c) Since the electronegativities of C and H are 2. 5 and 2. 1, respectively, the C — H bonds are nearly nonpolar. In addition, since the geometry of the molecule is tetrahedral, any slight polarities that the bonds might have will cancel. CH 4 is therefore nonpolar and does not have dipole–dipole forces. SKILLBUILDER 12. 4 Dipole–Dipole Forces Which of the following molecules have dipole–dipole forces? (a) CI 4 (b) CH 3 Cl (c) HCl FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 63, 64, 65, 66. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 4 Dipole–Dipole Forces Continued (c) Since the electronegativities of C and H are 2. 5 and 2. 1, respectively, the C — H bonds are nearly nonpolar. In addition, since the geometry of the molecule is tetrahedral, any slight polarities that the bonds might have will cancel. CH 4 is therefore nonpolar and does not have dipole–dipole forces. SKILLBUILDER 12. 4 Dipole–Dipole Forces Which of the following molecules have dipole–dipole forces? (a) CI 4 (b) CH 3 Cl (c) HCl FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 63, 64, 65, 66. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 5 Hydrogen Bonding One of the following compounds is a liquid at room temperature. Which one and why? Solution: The three compounds have similar molar masses. formaldehyde 30. 03 g/mol fluoromethane 34. 04 g/mol hydrogen peroxide 34. 02 g/mol Therefore, the strengths of their dispersion forces are similar. All three compounds are also polar, so they have dipole –dipole forces. Hydrogen peroxide, however, is the only compound that also contains H bonded directly to F, O, or N. Therefore it also has hydrogen bonding and is most likely to have the highest boiling point of the three. Since the problem stated that only one of the compounds was a liquid, we can safely assume that hydrogen peroxide is the liquid. Note that although fluoromethane contains both H and F, H is not directly bonded to F, so fluoromethane does not have hydrogen bonding as an intermolecular force. Similarly, although formaldehyde contains both H and O, H is not directly bonded to O, so formaldehyde does not have hydrogen bonding either. SKILLBUILDER 12. 5 Hydrogen Bonding Which has the higher boiling point, HF or HCl? Why? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 12. 10; Problems 69, 70, 71, 72, 73, 74. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 5 Hydrogen Bonding One of the following compounds is a liquid at room temperature. Which one and why? Solution: The three compounds have similar molar masses. formaldehyde 30. 03 g/mol fluoromethane 34. 04 g/mol hydrogen peroxide 34. 02 g/mol Therefore, the strengths of their dispersion forces are similar. All three compounds are also polar, so they have dipole –dipole forces. Hydrogen peroxide, however, is the only compound that also contains H bonded directly to F, O, or N. Therefore it also has hydrogen bonding and is most likely to have the highest boiling point of the three. Since the problem stated that only one of the compounds was a liquid, we can safely assume that hydrogen peroxide is the liquid. Note that although fluoromethane contains both H and F, H is not directly bonded to F, so fluoromethane does not have hydrogen bonding as an intermolecular force. Similarly, although formaldehyde contains both H and O, H is not directly bonded to O, so formaldehyde does not have hydrogen bonding either. SKILLBUILDER 12. 5 Hydrogen Bonding Which has the higher boiling point, HF or HCl? Why? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 12. 10; Problems 69, 70, 71, 72, 73, 74. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 6 Identifying Types of Crystalline Solids Identify each of the following solids as molecular, ionic, or atomic. (a) Ca. Cl 2(s) (b) Co(s) (c) CS 2(s) Solution: (a) Ca. Cl 2 is an ionic compound (metal and nonmetal) and therefore forms an ionic solid (Ca. Cl 2 melts at 772 °C). (b) Co is a metal and therefore forms a metallic atomic solid (Co melts at 1768 °C). (c) CS 2 is a molecular compound (nonmetal bonded to a nonmetal) and therefore forms a molecular solid (CS 2 melts at – 110 °C). SKILLBUILDER 12. 6 Identifying Types of Crystalline Solids Identify each of the following solids as molecular, ionic, or atomic. (a) NH 3(s) (b) Ca. O(s) (c) Kr(s) FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 77, 78, 79, 80. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 6 Identifying Types of Crystalline Solids Identify each of the following solids as molecular, ionic, or atomic. (a) Ca. Cl 2(s) (b) Co(s) (c) CS 2(s) Solution: (a) Ca. Cl 2 is an ionic compound (metal and nonmetal) and therefore forms an ionic solid (Ca. Cl 2 melts at 772 °C). (b) Co is a metal and therefore forms a metallic atomic solid (Co melts at 1768 °C). (c) CS 2 is a molecular compound (nonmetal bonded to a nonmetal) and therefore forms a molecular solid (CS 2 melts at – 110 °C). SKILLBUILDER 12. 6 Identifying Types of Crystalline Solids Identify each of the following solids as molecular, ionic, or atomic. (a) NH 3(s) (b) Ca. O(s) (c) Kr(s) FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 77, 78, 79, 80. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
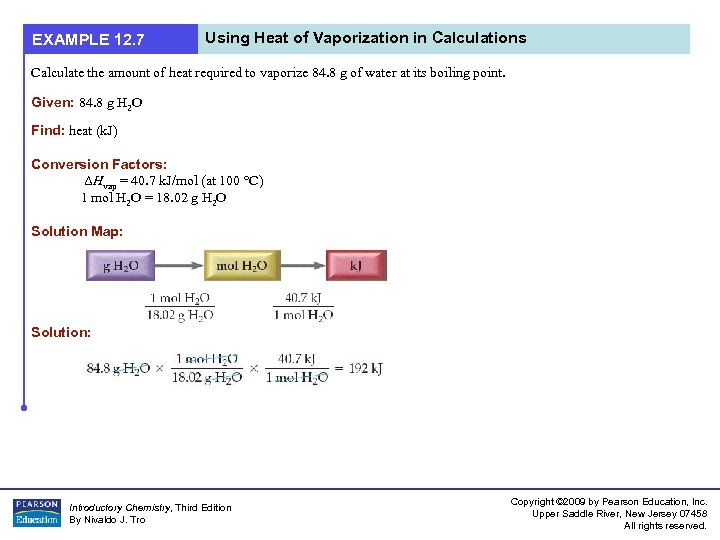 EXAMPLE 12. 7 Using Heat of Vaporization in Calculations Calculate the amount of heat required to vaporize 84. 8 g of water at its boiling point. Given: 84. 8 g H 2 O Find: heat (k. J) Conversion Factors: ΔHvap = 40. 7 k. J/mol (at 100 °C) 1 mol H 2 O = 18. 02 g H 2 O Solution Map: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 7 Using Heat of Vaporization in Calculations Calculate the amount of heat required to vaporize 84. 8 g of water at its boiling point. Given: 84. 8 g H 2 O Find: heat (k. J) Conversion Factors: ΔHvap = 40. 7 k. J/mol (at 100 °C) 1 mol H 2 O = 18. 02 g H 2 O Solution Map: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
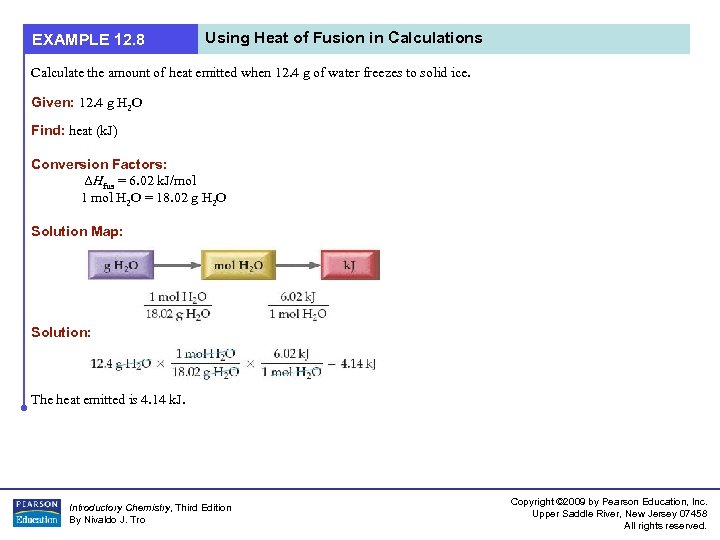 EXAMPLE 12. 8 Using Heat of Fusion in Calculations Calculate the amount of heat emitted when 12. 4 g of water freezes to solid ice. Given: 12. 4 g H 2 O Find: heat (k. J) Conversion Factors: ΔHfus = 6. 02 k. J/mol 1 mol H 2 O = 18. 02 g H 2 O Solution Map: Solution: The heat emitted is 4. 14 k. J. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 8 Using Heat of Fusion in Calculations Calculate the amount of heat emitted when 12. 4 g of water freezes to solid ice. Given: 12. 4 g H 2 O Find: heat (k. J) Conversion Factors: ΔHfus = 6. 02 k. J/mol 1 mol H 2 O = 18. 02 g H 2 O Solution Map: Solution: The heat emitted is 4. 14 k. J. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 9 Determining the Types of Intermolecular Forces in a Compound Determine the types of intermolecular forces present in each of the following substances. (a) N 2 (b) CO (c) NH 3 Solution: (a) N 2 is nonpolar and therefore has only dispersion forces. (b) CO is polar and therefore has dipole–dipole forces (in addition to dispersion forces). (c) NH 3 has hydrogen bonding (in addition to dispersion forces and dipole–dipole forces). Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 9 Determining the Types of Intermolecular Forces in a Compound Determine the types of intermolecular forces present in each of the following substances. (a) N 2 (b) CO (c) NH 3 Solution: (a) N 2 is nonpolar and therefore has only dispersion forces. (b) CO is polar and therefore has dipole–dipole forces (in addition to dispersion forces). (c) NH 3 has hydrogen bonding (in addition to dispersion forces and dipole–dipole forces). Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
 EXAMPLE 12. 10 Using Intermolecular Forces to Determine Melting and/or Boiling Points Arrange each of the following in order of increasing boiling point. (a) F 2 , Cl 2 , Br 2 (b) HF, HCl, HBr Solution: (a) Since these all have only dispersion forces, and since they are similar substances (all halogens), the strength of the dispersion force will increase with increasing molar mass. Therefore, the correct order is F 2 < Cl 2 < Br 2. (b) Since HF has hydrogen bonding, it has the highest boiling point. Between HCl and HBr, HBr (because of its higher molar mass) has a higher boiling point. Therefore the correct order is HCl < HBr < HF. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
EXAMPLE 12. 10 Using Intermolecular Forces to Determine Melting and/or Boiling Points Arrange each of the following in order of increasing boiling point. (a) F 2 , Cl 2 , Br 2 (b) HF, HCl, HBr Solution: (a) Since these all have only dispersion forces, and since they are similar substances (all halogens), the strength of the dispersion force will increase with increasing molar mass. Therefore, the correct order is F 2 < Cl 2 < Br 2. (b) Since HF has hydrogen bonding, it has the highest boiling point. Between HCl and HBr, HBr (because of its higher molar mass) has a higher boiling point. Therefore the correct order is HCl < HBr < HF. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.


