e3e976aa10456bf4bfc1a3e065ede709.ppt
- Количество слайдов: 22
 “Ex Vivo Retroviral Gene Transfer for Treatment of X-linked Severe Combined Immunodeficiency (XSCID)” Treatment of patients with persistent immune defects despite allogeneic bone marrow transplantation Harry L. Malech, MD and Jennifer M. Puck, MD National Institute of Allergy and Infectious Diseases and National Human Genome Research Institute National Institutes of Health Bethesda, Maryland
“Ex Vivo Retroviral Gene Transfer for Treatment of X-linked Severe Combined Immunodeficiency (XSCID)” Treatment of patients with persistent immune defects despite allogeneic bone marrow transplantation Harry L. Malech, MD and Jennifer M. Puck, MD National Institute of Allergy and Infectious Diseases and National Human Genome Research Institute National Institutes of Health Bethesda, Maryland
 Current Standard Therapy for SCID Bone marrow transplantation 60 -90% survival Better outcome with HLA-matched sibling donor (available to only 25% of patients) Less success with haploidentical (from parent) or matched unrelated donor Better outcome with diagnosis and transplant by 3 months of age
Current Standard Therapy for SCID Bone marrow transplantation 60 -90% survival Better outcome with HLA-matched sibling donor (available to only 25% of patients) Less success with haploidentical (from parent) or matched unrelated donor Better outcome with diagnosis and transplant by 3 months of age
 Limitations of Haploidential BMT • Graft vs. host disease • Incomplete immune reconstitution or graft loss: Poor B cell function (IVIG dependence) Immune dysregulation, autoimmunity Recurrent infections Growth retardation, nutritional problems, and chronic lung disease
Limitations of Haploidential BMT • Graft vs. host disease • Incomplete immune reconstitution or graft loss: Poor B cell function (IVIG dependence) Immune dysregulation, autoimmunity Recurrent infections Growth retardation, nutritional problems, and chronic lung disease
 NIH XSCID Gene Therapy Protocol Design: Up to 6 XSCID patients, 2 -20 years old with persistent immune defects despite BMT Ex vivo retrovirus gene transfer to cytokine mobilized autologous CD 34+ peripheral blood hematopoietic stem cells Single infusion of gene corrected CD 34+ cells with no marrow conditioning to enhance engraftment (no radiation, no chemotherapy) Long term follow up of immune reconstitution, vector marking, and changes in clinical status
NIH XSCID Gene Therapy Protocol Design: Up to 6 XSCID patients, 2 -20 years old with persistent immune defects despite BMT Ex vivo retrovirus gene transfer to cytokine mobilized autologous CD 34+ peripheral blood hematopoietic stem cells Single infusion of gene corrected CD 34+ cells with no marrow conditioning to enhance engraftment (no radiation, no chemotherapy) Long term follow up of immune reconstitution, vector marking, and changes in clinical status
 Protocol Design Subjects: An important feature of our study is that all subjects have already received one or more allogeneic bone marrow transplants, but demonstrate persistent immune defects which result in IVIG dependence, recurrent infections, growth failure, chronic gastrointestinal problems, chronic inflammatory skin conditions and chronic lung disease.
Protocol Design Subjects: An important feature of our study is that all subjects have already received one or more allogeneic bone marrow transplants, but demonstrate persistent immune defects which result in IVIG dependence, recurrent infections, growth failure, chronic gastrointestinal problems, chronic inflammatory skin conditions and chronic lung disease.
 Patient Selection: Pre-enrollment Evaluation No patients have yet enrolled in this gene therapy protocol. We have studied 8 post-BMT XSCID patients dependent on IVIG who have a variety of persistent clinical problems.
Patient Selection: Pre-enrollment Evaluation No patients have yet enrolled in this gene therapy protocol. We have studied 8 post-BMT XSCID patients dependent on IVIG who have a variety of persistent clinical problems.
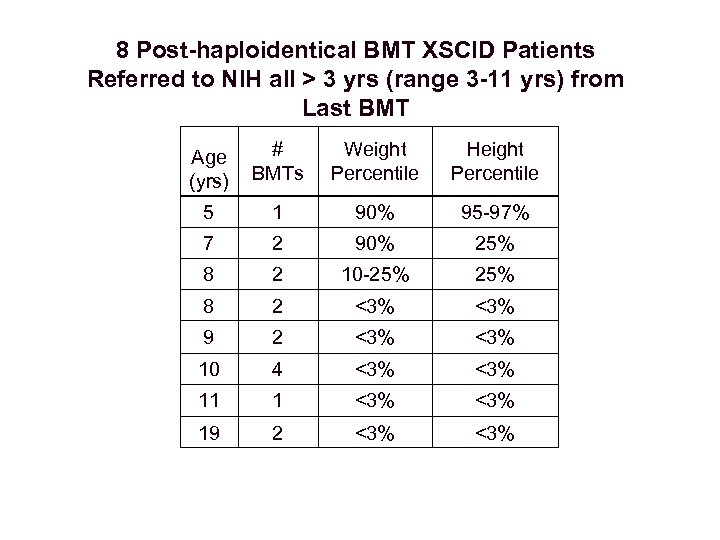 8 Post-haploidentical BMT XSCID Patients Referred to NIH all > 3 yrs (range 3 -11 yrs) from Last BMT Age (yrs) # BMTs Weight Percentile Height Percentile 5 1 90% 95 -97% 7 2 90% 25% 8 2 10 -25% 8 2 <3% 9 2 <3% 10 4 <3% 11 1 <3% 19 2 <3%
8 Post-haploidentical BMT XSCID Patients Referred to NIH all > 3 yrs (range 3 -11 yrs) from Last BMT Age (yrs) # BMTs Weight Percentile Height Percentile 5 1 90% 95 -97% 7 2 90% 25% 8 2 10 -25% 8 2 <3% 9 2 <3% 10 4 <3% 11 1 <3% 19 2 <3%
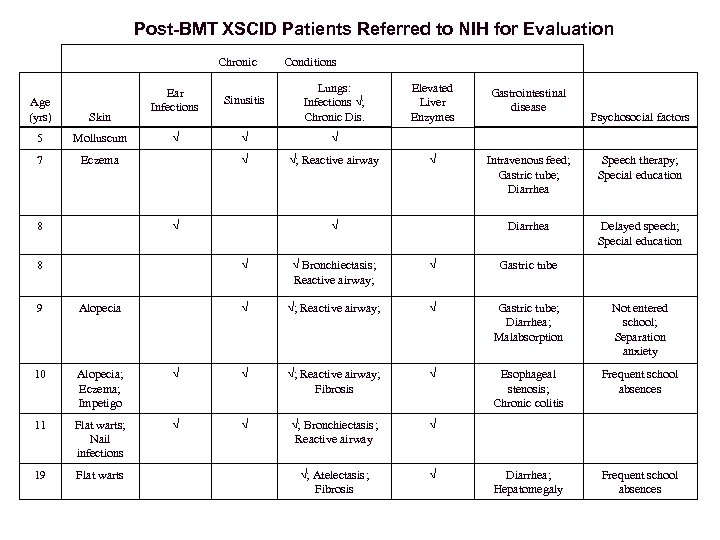 Post-BMT XSCID Patients Referred to NIH for Evaluation Chronic Conditions Lungs: Infections √; Chronic Dis. Elevated Liver Enzymes Gastrointestinal disease √ √ √ √; Reactive airway √ Intravenous feed; Gastric tube; Diarrhea Speech therapy; Special education √ √ Diarrhea Delayed speech; Special education 8 √ √ Bronchiectasis; Reactive airway; √ Gastric tube 9 Alopecia √ √; Reactive airway; √ Gastric tube; Diarrhea; Malabsorption Not entered school; Separation anxiety 10 Alopecia; Eczema; Impetigo √ √ √; Reactive airway; Fibrosis √ Esophageal stenosis; Chronic colitis Frequent school absences 11 Flat warts; Nail infections √ √ √; Bronchiectasis; Reactive airway √ 19 Flat warts √; Atelectasis; Fibrosis √ Diarrhea; Hepatomegaly Frequent school absences Ear Infections Sinusitis Molluscum √ 7 Eczema 8 Age (yrs) Skin 5 Psychosocial factors
Post-BMT XSCID Patients Referred to NIH for Evaluation Chronic Conditions Lungs: Infections √; Chronic Dis. Elevated Liver Enzymes Gastrointestinal disease √ √ √ √; Reactive airway √ Intravenous feed; Gastric tube; Diarrhea Speech therapy; Special education √ √ Diarrhea Delayed speech; Special education 8 √ √ Bronchiectasis; Reactive airway; √ Gastric tube 9 Alopecia √ √; Reactive airway; √ Gastric tube; Diarrhea; Malabsorption Not entered school; Separation anxiety 10 Alopecia; Eczema; Impetigo √ √ √; Reactive airway; Fibrosis √ Esophageal stenosis; Chronic colitis Frequent school absences 11 Flat warts; Nail infections √ √ √; Bronchiectasis; Reactive airway √ 19 Flat warts √; Atelectasis; Fibrosis √ Diarrhea; Hepatomegaly Frequent school absences Ear Infections Sinusitis Molluscum √ 7 Eczema 8 Age (yrs) Skin 5 Psychosocial factors
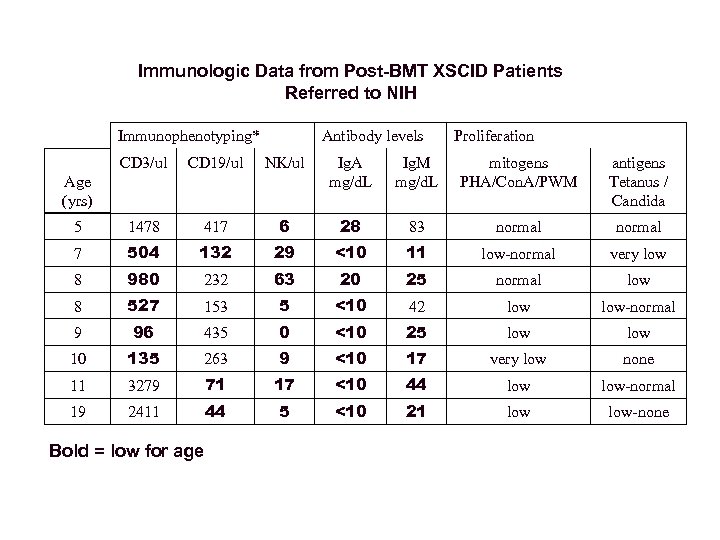 Immunologic Data from Post-BMT XSCID Patients Referred to NIH Immunophenotyping* Antibody levels Proliferation CD 3/ul CD 19/ul NK/ul Ig. A mg/d. L Ig. M mg/d. L mitogens PHA/Con. A/PWM antigens Tetanus / Candida 5 1478 417 6 28 83 normal 7 504 132 29 <10 11 low-normal very low 8 980 232 63 20 25 normal low 8 527 153 5 <10 42 low-normal 9 96 435 0 <10 25 low 10 135 263 9 <10 17 very low none 11 3279 71 17 <10 44 low-normal 19 2411 44 5 <10 21 low-none Age (yrs) Bold = low for age
Immunologic Data from Post-BMT XSCID Patients Referred to NIH Immunophenotyping* Antibody levels Proliferation CD 3/ul CD 19/ul NK/ul Ig. A mg/d. L Ig. M mg/d. L mitogens PHA/Con. A/PWM antigens Tetanus / Candida 5 1478 417 6 28 83 normal 7 504 132 29 <10 11 low-normal very low 8 980 232 63 20 25 normal low 8 527 153 5 <10 42 low-normal 9 96 435 0 <10 25 low 10 135 263 9 <10 17 very low none 11 3279 71 17 <10 44 low-normal 19 2411 44 5 <10 21 low-none Age (yrs) Bold = low for age
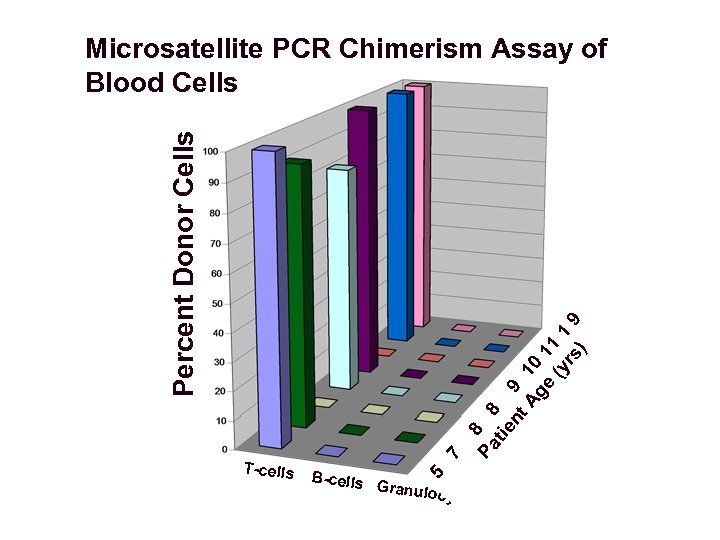 B-cells Pa 5 T-cells 7 8 tie 8 nt 9 Ag 10 e 11 (y 1 rs 9 ) Percent Donor Cells Microsatellite PCR Chimerism Assay of Blood Cells Granulo c ytes
B-cells Pa 5 T-cells 7 8 tie 8 nt 9 Ag 10 e 11 (y 1 rs 9 ) Percent Donor Cells Microsatellite PCR Chimerism Assay of Blood Cells Granulo c ytes
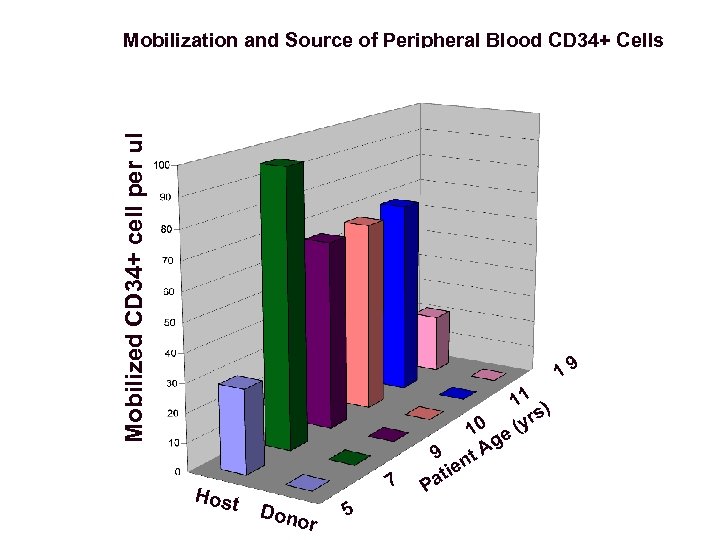 Mobilized CD 34+ cell per ul Mobilization and Source of Peripheral Blood CD 34+ Cells 19 Host 7 Dono r 5 9 e ati P 11 ) s 0 (yr 1 e g t. A n
Mobilized CD 34+ cell per ul Mobilization and Source of Peripheral Blood CD 34+ Cells 19 Host 7 Dono r 5 9 e ati P 11 ) s 0 (yr 1 e g t. A n
 Conclusions About Post-BMT XSCID Patients Referred to NIH • Some XSCID patients have persistent immune deficiency despite one or more prior haploidentical T-depleted BMTs. • These patients have immune defects, poor growth, and chronic medical conditions. • Engraftment of donor T cells was detected in 6 of 8 patients, but no patient had any donor B cells, granulocytes, or monocytes. • 6 of 6 patients mobilized with G-CSF to collect CD 34+ cells as part of a clinical evaluation protocol had no CD 34+ cells of donor origin.
Conclusions About Post-BMT XSCID Patients Referred to NIH • Some XSCID patients have persistent immune deficiency despite one or more prior haploidentical T-depleted BMTs. • These patients have immune defects, poor growth, and chronic medical conditions. • Engraftment of donor T cells was detected in 6 of 8 patients, but no patient had any donor B cells, granulocytes, or monocytes. • 6 of 6 patients mobilized with G-CSF to collect CD 34+ cells as part of a clinical evaluation protocol had no CD 34+ cells of donor origin.
 NIH XSCID Protocol Vector: GALV pseudotyped MFGS-gamma chain MFGS vector contains only the open reading frame of IL 2 RG (common gamma chain of the IL 2 receptor). MFGS differs from MFG by only 3 nucleotides in the truncated gag region, further reducing the potential for production of gag peptide through recombination events. Our replication incompetent vector was packaged by the PG 13 cell line, and supernatant for clinical use was collected from confluent cultures of a stable, highly characterized producer clone.
NIH XSCID Protocol Vector: GALV pseudotyped MFGS-gamma chain MFGS vector contains only the open reading frame of IL 2 RG (common gamma chain of the IL 2 receptor). MFGS differs from MFG by only 3 nucleotides in the truncated gag region, further reducing the potential for production of gag peptide through recombination events. Our replication incompetent vector was packaged by the PG 13 cell line, and supernatant for clinical use was collected from confluent cultures of a stable, highly characterized producer clone.
 NIH XSCID Protocol Ex Vivo Transduction 1 -10 x 106 autologous mobilized peripheral blood CD 34+ cells will be subjected to 4 daily transductions ex vivo with GALV pseudotyped, replication-defective MFGS-gc. Transductions will occur in flexible gas-permeable plastic containers using serum-free medium with 1% human serum albumin and 5 recombinant growth factors (50 ng/ml Flt 3 -L, 50 ng/ml SCF, 50 ng/ml TPO, 25 ng/ml IL-6, and 5 ng/ml IL-3). Expected transduction efficiency is 40 -60%, based on testing of the clinical vector.
NIH XSCID Protocol Ex Vivo Transduction 1 -10 x 106 autologous mobilized peripheral blood CD 34+ cells will be subjected to 4 daily transductions ex vivo with GALV pseudotyped, replication-defective MFGS-gc. Transductions will occur in flexible gas-permeable plastic containers using serum-free medium with 1% human serum albumin and 5 recombinant growth factors (50 ng/ml Flt 3 -L, 50 ng/ml SCF, 50 ng/ml TPO, 25 ng/ml IL-6, and 5 ng/ml IL-3). Expected transduction efficiency is 40 -60%, based on testing of the clinical vector.
 NIH XSCID Protocol Treatment and Follow up Subjects will receive a single infusion of transduced autologous CD 34+ cells. Subjects will be monitored for: • gene marking in blood cell lineages • changes in numbers of T-, B- and NK cells • changes in T-, B-, and NK cell function. • changes in clinical status (growth, chronic conditions and quality of life).
NIH XSCID Protocol Treatment and Follow up Subjects will receive a single infusion of transduced autologous CD 34+ cells. Subjects will be monitored for: • gene marking in blood cell lineages • changes in numbers of T-, B- and NK cells • changes in T-, B-, and NK cell function. • changes in clinical status (growth, chronic conditions and quality of life).
 NIH XSCID Protocol Safety Considerations The first 3 subjects will be treated at least one month apart and there must be appearance of gene marked T-cells in at least one of these 3 patients before additional patients can be enrolled. Safety studies include: • monitoring for replication competent virus in blood cells. • evaluation of hematologic, kidney, liver, neurologic and other organ function.
NIH XSCID Protocol Safety Considerations The first 3 subjects will be treated at least one month apart and there must be appearance of gene marked T-cells in at least one of these 3 patients before additional patients can be enrolled. Safety studies include: • monitoring for replication competent virus in blood cells. • evaluation of hematologic, kidney, liver, neurologic and other organ function.
 Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Subjects: Further limit enrollment to patients with immune defects, growth impairment, and recurrent infections post -haploidentical transplant who have no detectable engraftment in B-cells, NK cells, myeloid cells or CD 34+ cells. Exclude subjects with a history of leukemia or childhood cancers in first degree relatives.
Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Subjects: Further limit enrollment to patients with immune defects, growth impairment, and recurrent infections post -haploidentical transplant who have no detectable engraftment in B-cells, NK cells, myeloid cells or CD 34+ cells. Exclude subjects with a history of leukemia or childhood cancers in first degree relatives.
 Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Informed consent: Full disclosure in the informed consent document of everything known about the severe adverse event in the French study.
Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Informed consent: Full disclosure in the informed consent document of everything known about the severe adverse event in the French study.
 Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Insert site analysis: Following appearance of marked cells in peripheral blood, determine insertion sites by inverse PCR and sequencing at 6 month intervals, from blood lineages and from clones of T-cells grown ex vivo. Using that information, quantify the persistence and changes in relative proportion of particular inserts in different lineages over time. Insert site analysis in the bulk ex vivo tranduced CD 34+ cells is not proposed as this is highly unlikely to reveal the predominant clones in the rare cells which actually engraft long term.
Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Insert site analysis: Following appearance of marked cells in peripheral blood, determine insertion sites by inverse PCR and sequencing at 6 month intervals, from blood lineages and from clones of T-cells grown ex vivo. Using that information, quantify the persistence and changes in relative proportion of particular inserts in different lineages over time. Insert site analysis in the bulk ex vivo tranduced CD 34+ cells is not proposed as this is highly unlikely to reveal the predominant clones in the rare cells which actually engraft long term.
 Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Analysis of T-cell subsets: Closely follow numbers of gamma/delta T-cells and other subtypes including T-cell receptor analyses
Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Analysis of T-cell subsets: Closely follow numbers of gamma/delta T-cells and other subtypes including T-cell receptor analyses
 Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Infection treatment: Early interventional treatment of all infections, particularly virus infections with specific therapies where approved therapies are available (antibiotics, anti-virus agents and/or specific immune globulin where appropriate).
Proposed Modifications of NIH XSCID Protocol in Response to the Adverse Event in the French Study Infection treatment: Early interventional treatment of all infections, particularly virus infections with specific therapies where approved therapies are available (antibiotics, anti-virus agents and/or specific immune globulin where appropriate).
 The subset of patients with XSCID who have persistent immune defects and chronic medical problems despite allogeneic bone marrow transplantation do not have other treatment options and may benefit from ex vivo gene therapy. The risk/benefit assessment for gene therapy treatment of these patients should take into account the degree of impairment of their immune function and quality of life, and the lack of alternative therapies.
The subset of patients with XSCID who have persistent immune defects and chronic medical problems despite allogeneic bone marrow transplantation do not have other treatment options and may benefit from ex vivo gene therapy. The risk/benefit assessment for gene therapy treatment of these patients should take into account the degree of impairment of their immune function and quality of life, and the lack of alternative therapies.


