c4341997e17ff96d89284729093f6a5c.ppt
- Количество слайдов: 61
 Evolving Xolair Health Outcomes Data: What Does (or Should) it Mean to Patients, Clinicians and Payors Allan T. Luskin, MD Associate Clinical Professor of Medicine, University of Wisconsin Director, Respiratory Institute, Dean Medical Center Madison, Wisconsin Past Chair, Patient and Public Education Committee, NAEPP Past Co-Chair, Managed Care Liaison, NAEPP Committee on Asthma Measures, AMA Asthma Expert Panel, JCAHO Respiratory Measurement Advisory Panel, HEDIS/NCQA
Evolving Xolair Health Outcomes Data: What Does (or Should) it Mean to Patients, Clinicians and Payors Allan T. Luskin, MD Associate Clinical Professor of Medicine, University of Wisconsin Director, Respiratory Institute, Dean Medical Center Madison, Wisconsin Past Chair, Patient and Public Education Committee, NAEPP Past Co-Chair, Managed Care Liaison, NAEPP Committee on Asthma Measures, AMA Asthma Expert Panel, JCAHO Respiratory Measurement Advisory Panel, HEDIS/NCQA
 Agenda • Outcomes and variability of disease and response to Rx and lack of correlation between outcomes • HRQOL with particular attention to newest Xolair analysis • Pharmacoeconomics: basics, specifics and what current data does and doesn’t tell us
Agenda • Outcomes and variability of disease and response to Rx and lack of correlation between outcomes • HRQOL with particular attention to newest Xolair analysis • Pharmacoeconomics: basics, specifics and what current data does and doesn’t tell us
 Asthma is a syndrome, not a disease syndrome • The Asthma phenotype is highly variable (clinically, pathologically and physiologically) • Response to ALL therapy is highly variable BHR and Reversible airflow obstruction does not predict response to therapy • Outcomes do not necessarily correlate with each other • There are Outcome phenotypes
Asthma is a syndrome, not a disease syndrome • The Asthma phenotype is highly variable (clinically, pathologically and physiologically) • Response to ALL therapy is highly variable BHR and Reversible airflow obstruction does not predict response to therapy • Outcomes do not necessarily correlate with each other • There are Outcome phenotypes
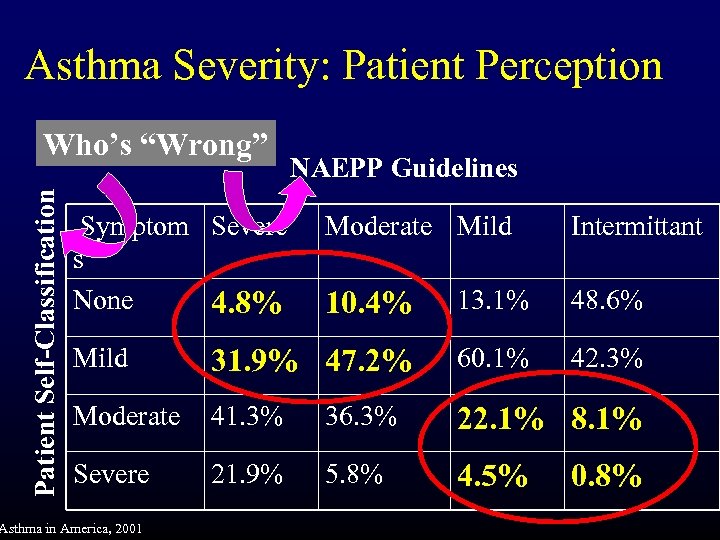 Asthma Severity: Patient Perception Patient Self-Classification Who’s “Wrong” Symptom Severe s None 4. 8% NAEPP Guidelines Moderate Mild Intermittant 10. 4% 13. 1% 48. 6% Mild 31. 9% 47. 2% 60. 1% 42. 3% Moderate 41. 3% 36. 3% 22. 1% 8. 1% Severe 21. 9% 5. 8% 4. 5% Asthma in America, 2001 0. 8%
Asthma Severity: Patient Perception Patient Self-Classification Who’s “Wrong” Symptom Severe s None 4. 8% NAEPP Guidelines Moderate Mild Intermittant 10. 4% 13. 1% 48. 6% Mild 31. 9% 47. 2% 60. 1% 42. 3% Moderate 41. 3% 36. 3% 22. 1% 8. 1% Severe 21. 9% 5. 8% 4. 5% Asthma in America, 2001 0. 8%
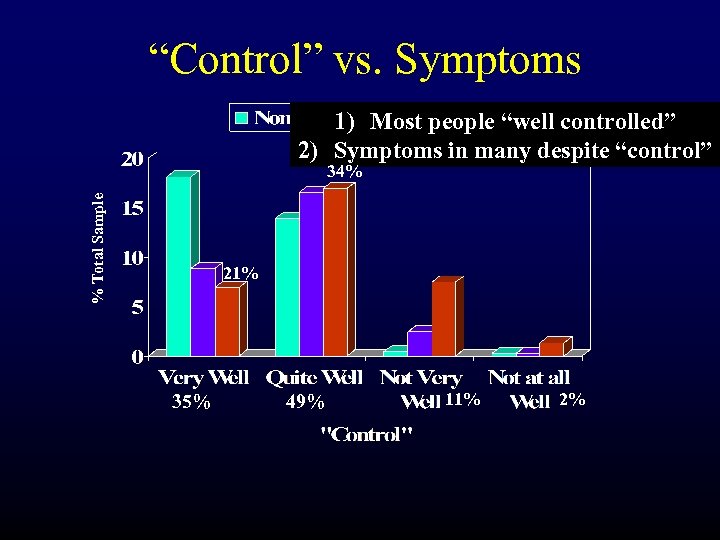 “Control” vs. Symptoms 1) Most people “well controlled” 2) Symptoms in many despite “control” % Total Sample 34% 21% 35% 49% 11% 2%
“Control” vs. Symptoms 1) Most people “well controlled” 2) Symptoms in many despite “control” % Total Sample 34% 21% 35% 49% 11% 2%
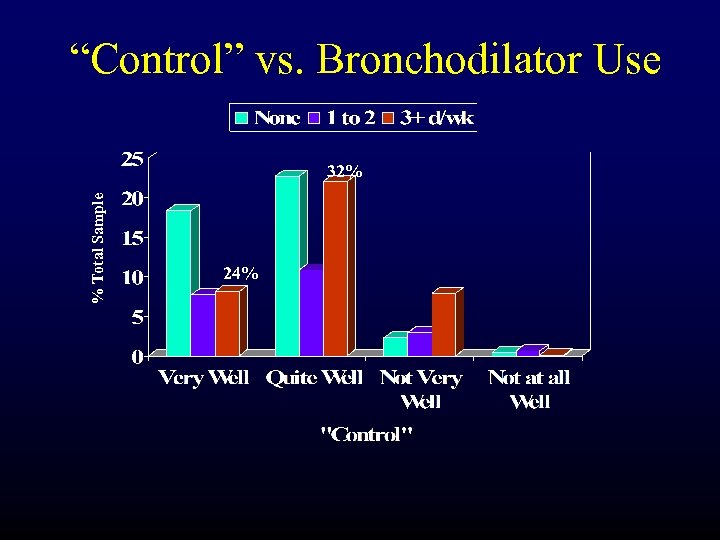 “Control” vs. Bronchodilator Use % Total Sample 32% 24%
“Control” vs. Bronchodilator Use % Total Sample 32% 24%
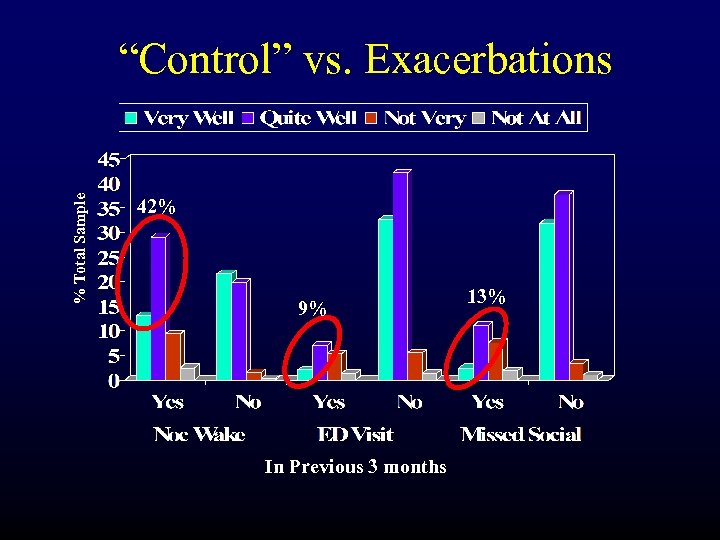 % Total Sample “Control” vs. Exacerbations 42% 9% In Previous 3 months 13%
% Total Sample “Control” vs. Exacerbations 42% 9% In Previous 3 months 13%
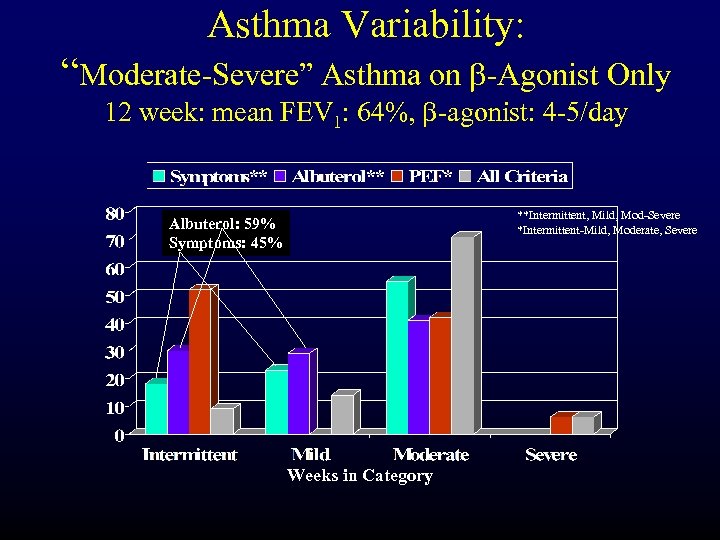 Asthma Variability: “Moderate-Severe” Asthma on b-Agonist Only 12 week: mean FEV 1: 64%, b-agonist: 4 -5/day **Intermittent, Mild, Mod-Severe *Intermittent-Mild, Moderate, Severe Albuterol: 59% Symptoms: 45% Weeks in Category
Asthma Variability: “Moderate-Severe” Asthma on b-Agonist Only 12 week: mean FEV 1: 64%, b-agonist: 4 -5/day **Intermittent, Mild, Mod-Severe *Intermittent-Mild, Moderate, Severe Albuterol: 59% Symptoms: 45% Weeks in Category
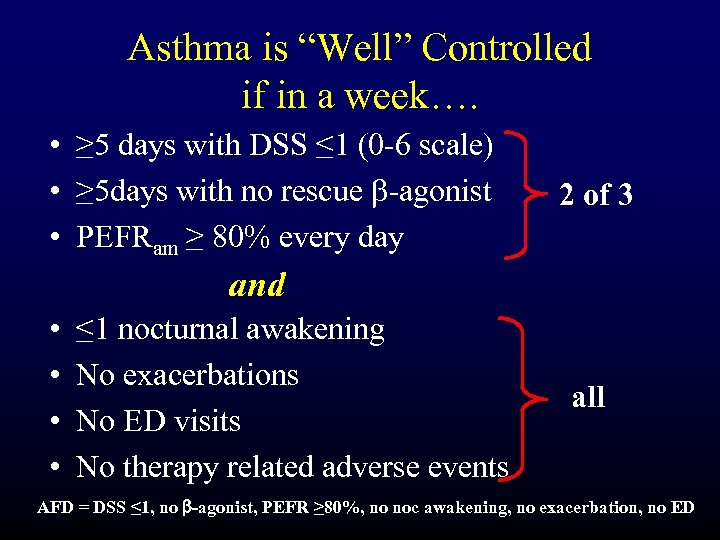 Asthma is “Well” Controlled if in a week…. • ≥ 5 days with DSS ≤ 1 (0 -6 scale) • ≥ 5 days with no rescue b-agonist • PEFRam ≥ 80% every day 2 of 3 and • • ≤ 1 nocturnal awakening No exacerbations No ED visits No therapy related adverse events all AFD = DSS ≤ 1, no b-agonist, PEFR ≥ 80%, no noc awakening, no exacerbation, no ED
Asthma is “Well” Controlled if in a week…. • ≥ 5 days with DSS ≤ 1 (0 -6 scale) • ≥ 5 days with no rescue b-agonist • PEFRam ≥ 80% every day 2 of 3 and • • ≤ 1 nocturnal awakening No exacerbations No ED visits No therapy related adverse events all AFD = DSS ≤ 1, no b-agonist, PEFR ≥ 80%, no noc awakening, no exacerbation, no ED
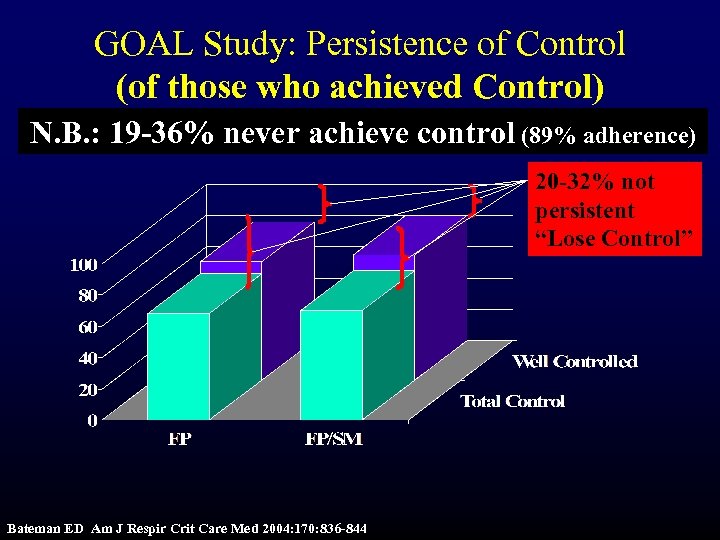 GOAL Study: Persistence of Control (of those who achieved Control) N. B. : 19 -36% never achieve control (89% adherence) 20 -32% not persistent “Lose Control” Bateman ED Am J Respir Crit Care Med 2004: 170: 836 -844
GOAL Study: Persistence of Control (of those who achieved Control) N. B. : 19 -36% never achieve control (89% adherence) 20 -32% not persistent “Lose Control” Bateman ED Am J Respir Crit Care Med 2004: 170: 836 -844
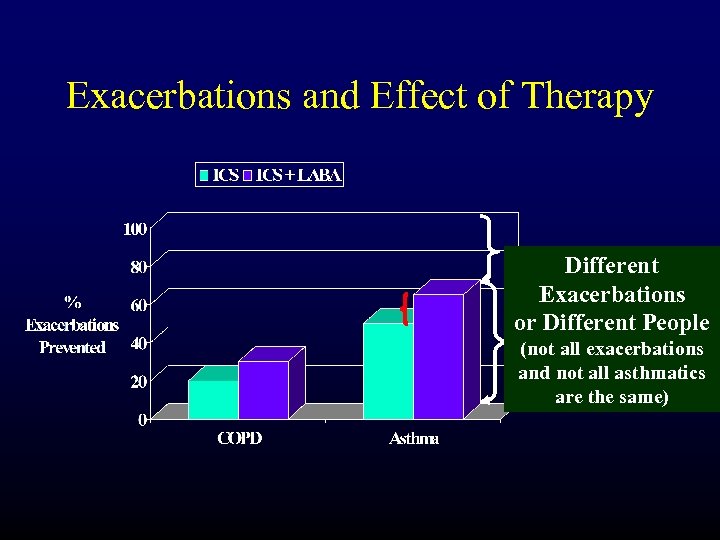 Exacerbations and Effect of Therapy Different Exacerbations or Different People (not all exacerbations and not all asthmatics are the same)
Exacerbations and Effect of Therapy Different Exacerbations or Different People (not all exacerbations and not all asthmatics are the same)
 Dimensions of Control How the Disease Affects the Organism • • • Physiology Symptoms (nocturnal, exercise) Quality of life and Activities of Daily Living Medications (adverse events, adherence) Health Care Utilization (function of exacerbations) • Comorbidities
Dimensions of Control How the Disease Affects the Organism • • • Physiology Symptoms (nocturnal, exercise) Quality of life and Activities of Daily Living Medications (adverse events, adherence) Health Care Utilization (function of exacerbations) • Comorbidities
 Outcomes • Functional – Symptoms/Medication Use – Exacerbation – Global: QOL, ADL • Physiologic – Lung function/BHR • Progression • Pathologic (Inflammation) – Sputum eos/ e. NO • Economic – Direct and indirect
Outcomes • Functional – Symptoms/Medication Use – Exacerbation – Global: QOL, ADL • Physiologic – Lung function/BHR • Progression • Pathologic (Inflammation) – Sputum eos/ e. NO • Economic – Direct and indirect
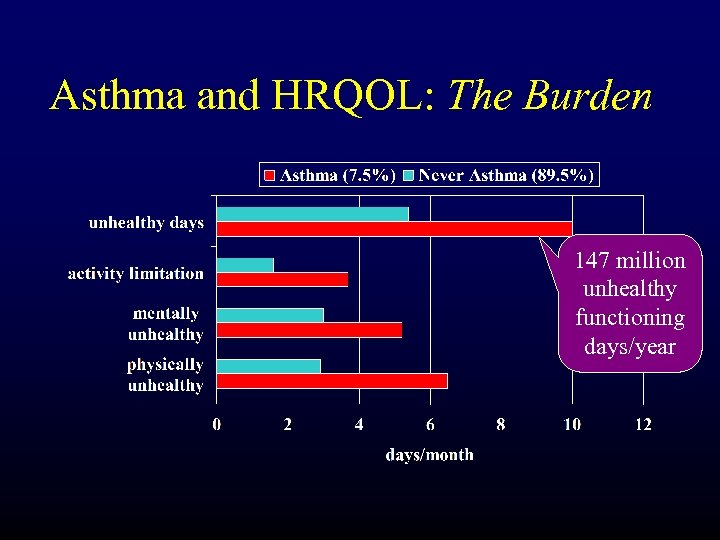 Asthma and HRQOL: The Burden 147 million unhealthy functioning days/year
Asthma and HRQOL: The Burden 147 million unhealthy functioning days/year
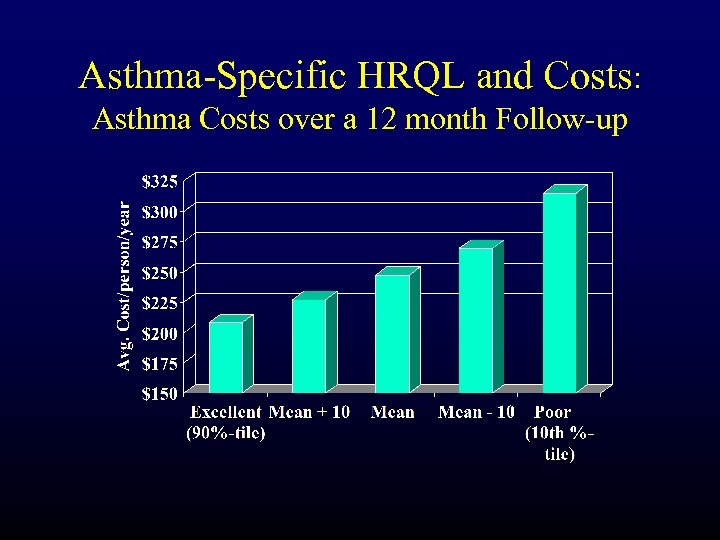 Asthma-Specific HRQL and Costs: Asthma Costs over a 12 month Follow-up
Asthma-Specific HRQL and Costs: Asthma Costs over a 12 month Follow-up
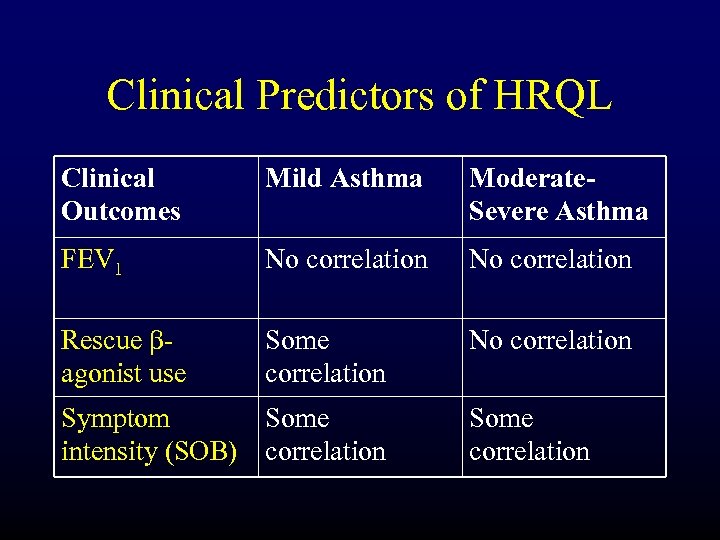 Clinical Predictors of HRQL Clinical Outcomes Mild Asthma Moderate. Severe Asthma FEV 1 No correlation Rescue bagonist use Some correlation No correlation Symptom Some intensity (SOB) correlation Some correlation
Clinical Predictors of HRQL Clinical Outcomes Mild Asthma Moderate. Severe Asthma FEV 1 No correlation Rescue bagonist use Some correlation No correlation Symptom Some intensity (SOB) correlation Some correlation
 The ATAQ Questionnaire: Scoring • 1 barrier each if: – NO or UNSURE to “did you feel your asthma was well-controlled” – YES or UNSURE to “missed work/school/activities” in past 4 weeks or 12 months – YES or UNSURE to “waking at night” in past 4 weeks or 12 months – Used 9 or more puffs of quick relief inhaler • Total: 0 to 4 barriers
The ATAQ Questionnaire: Scoring • 1 barrier each if: – NO or UNSURE to “did you feel your asthma was well-controlled” – YES or UNSURE to “missed work/school/activities” in past 4 weeks or 12 months – YES or UNSURE to “waking at night” in past 4 weeks or 12 months – Used 9 or more puffs of quick relief inhaler • Total: 0 to 4 barriers
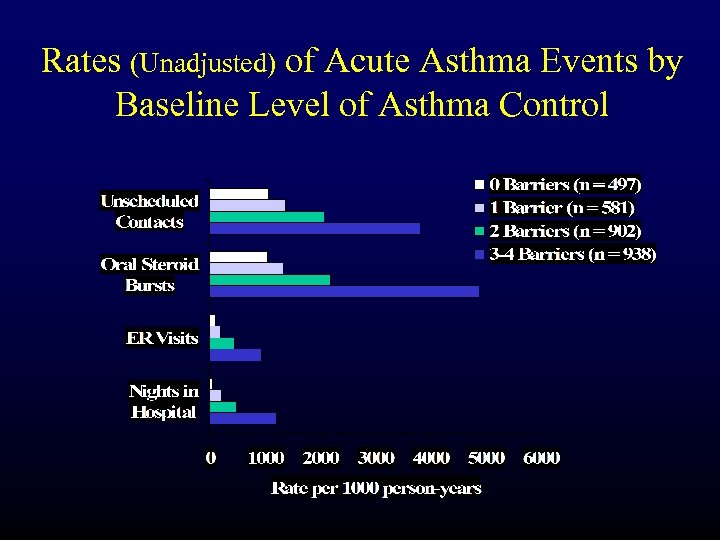 Rates (Unadjusted) of Acute Asthma Events by Baseline Level of Asthma Control
Rates (Unadjusted) of Acute Asthma Events by Baseline Level of Asthma Control
 “. . . the Asthma Is Controlled!” I can. . . • No inflammation • Good lung function • No urgent visits • Low costs I can. . . • Play ball • Stay at my friend’s who has a dog • Forget my medicine • Go out for a drink • Do work around the house • Fool around with my wife • Forget my medicine
“. . . the Asthma Is Controlled!” I can. . . • No inflammation • Good lung function • No urgent visits • Low costs I can. . . • Play ball • Stay at my friend’s who has a dog • Forget my medicine • Go out for a drink • Do work around the house • Fool around with my wife • Forget my medicine
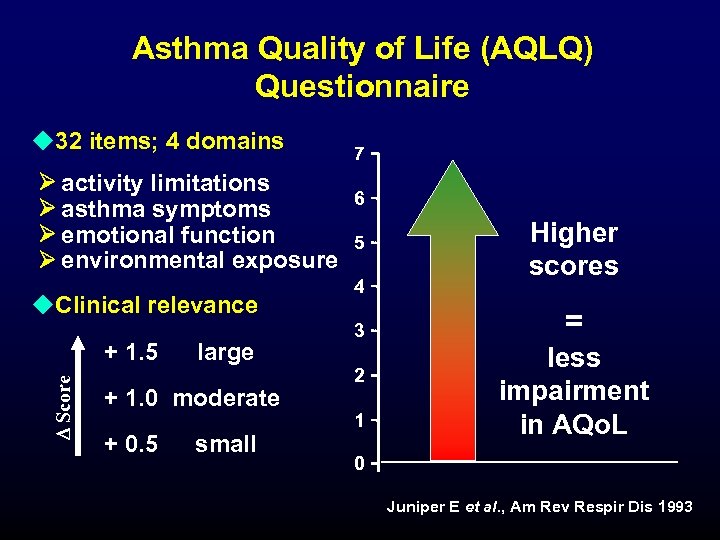 Asthma Quality of Life (AQLQ) Questionnaire u 32 items; 4 domains activity limitations asthma symptoms emotional function environmental exposure u. Clinical relevance D Score + 1. 5 large + 1. 0 moderate + 0. 5 small 7 6 5 4 3 2 1 Higher scores = less impairment in AQo. L 0 Juniper E et al. , Am Rev Respir Dis 1993
Asthma Quality of Life (AQLQ) Questionnaire u 32 items; 4 domains activity limitations asthma symptoms emotional function environmental exposure u. Clinical relevance D Score + 1. 5 large + 1. 0 moderate + 0. 5 small 7 6 5 4 3 2 1 Higher scores = less impairment in AQo. L 0 Juniper E et al. , Am Rev Respir Dis 1993
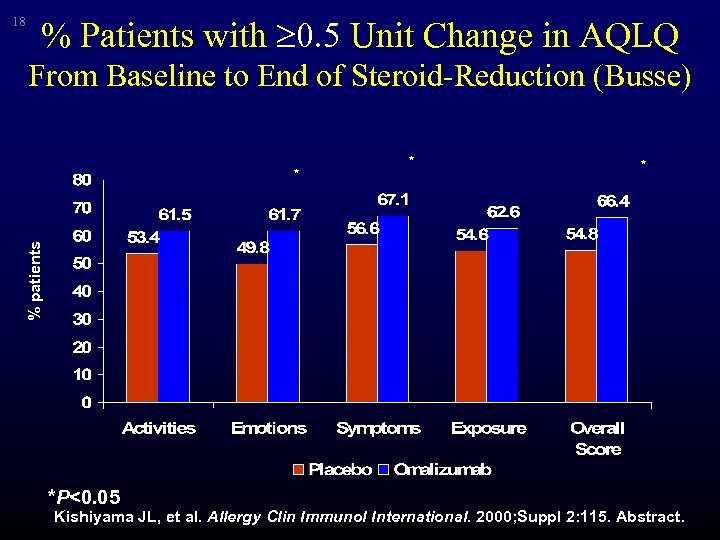 18 % Patients with 0. 5 Unit Change in AQLQ From Baseline to End of Steroid-Reduction (Busse) * * % patients * *P<0. 05 Kishiyama JL, et al. Allergy Clin Immunol International. 2000; Suppl 2: 115. Abstract.
18 % Patients with 0. 5 Unit Change in AQLQ From Baseline to End of Steroid-Reduction (Busse) * * % patients * *P<0. 05 Kishiyama JL, et al. Allergy Clin Immunol International. 2000; Suppl 2: 115. Abstract.
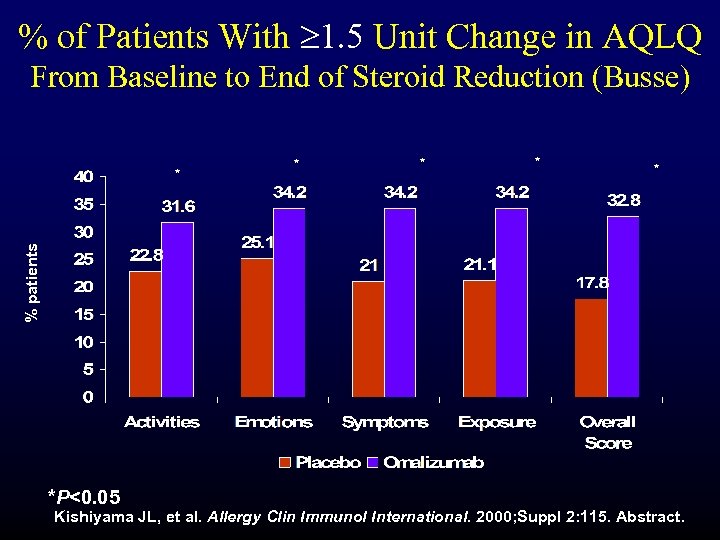 % of Patients With 1. 5 Unit Change in AQLQ From Baseline to End of Steroid Reduction (Busse) * * % patients * *P<0. 05 Kishiyama JL, et al. Allergy Clin Immunol International. 2000; Suppl 2: 115. Abstract.
% of Patients With 1. 5 Unit Change in AQLQ From Baseline to End of Steroid Reduction (Busse) * * % patients * *P<0. 05 Kishiyama JL, et al. Allergy Clin Immunol International. 2000; Suppl 2: 115. Abstract.
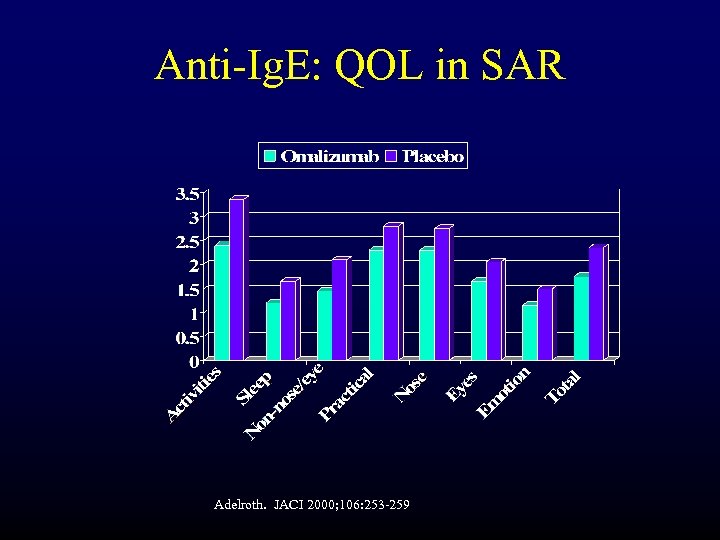 Anti-Ig. E: QOL in SAR Adelroth. JACI 2000; 106: 253 -259
Anti-Ig. E: QOL in SAR Adelroth. JACI 2000; 106: 253 -259
 AQLQ: Symptom Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
AQLQ: Symptom Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
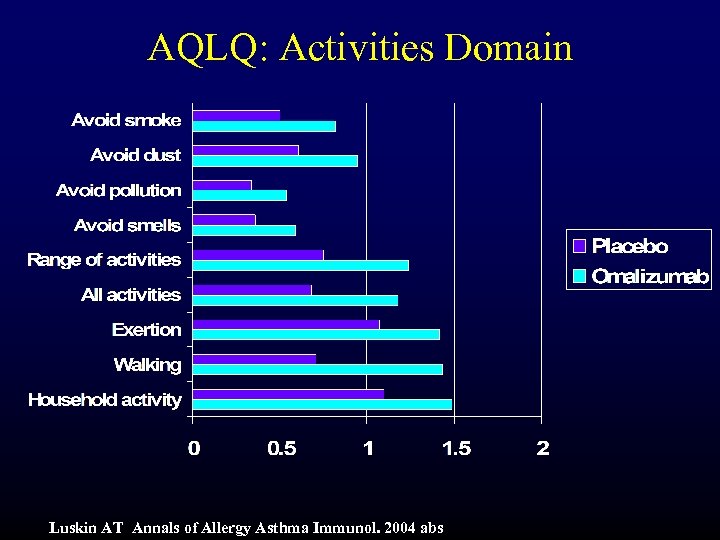 AQLQ: Activities Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
AQLQ: Activities Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
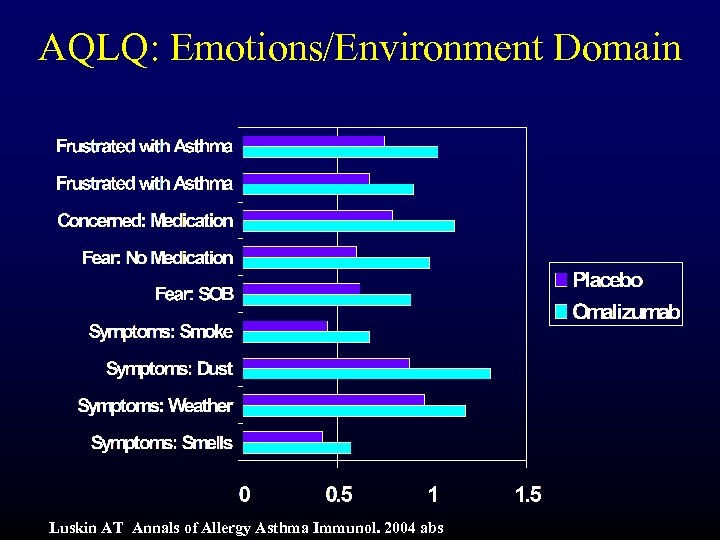 AQLQ: Emotions/Environment Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
AQLQ: Emotions/Environment Domain Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
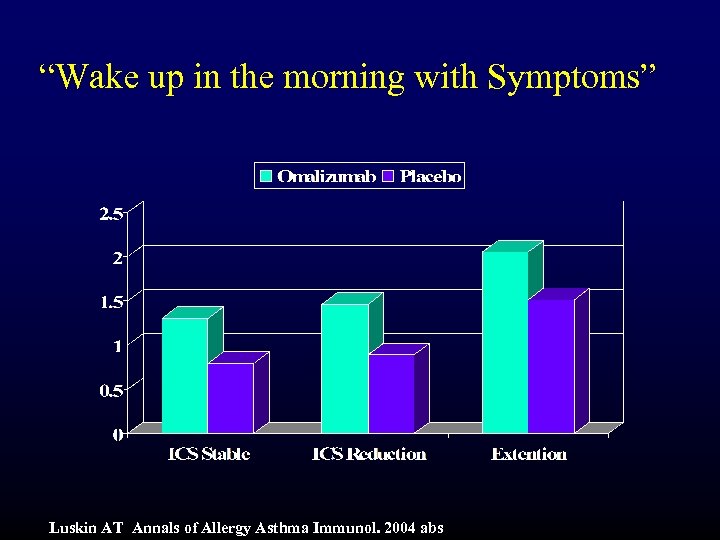 “Wake up in the morning with Symptoms” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
“Wake up in the morning with Symptoms” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
 “Overall Range of Activities” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
“Overall Range of Activities” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
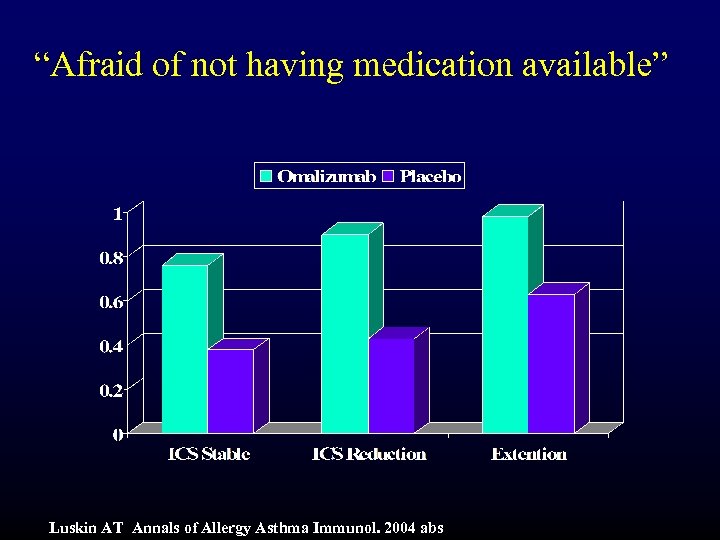 “Afraid of not having medication available” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
“Afraid of not having medication available” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
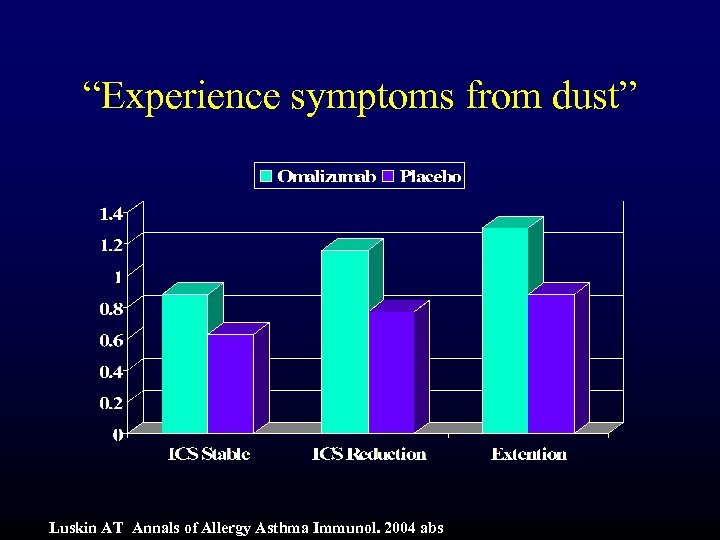 “Experience symptoms from dust” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
“Experience symptoms from dust” Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
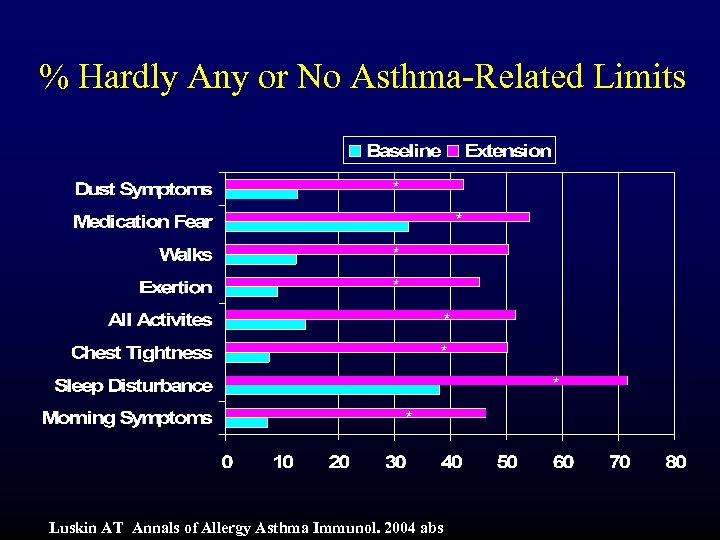 % Hardly Any or No Asthma-Related Limits * * * * Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
% Hardly Any or No Asthma-Related Limits * * * * Luskin AT Annals of Allergy Asthma Immunol. 2004 abs
 Summary and Conclusions • Consistent and positive impact of omalizumab on AQLQ overall and domain scores (p<0. 05) • Specific drivers of improvement in each of the domains were noted • Correlations between AQLQ and other clinical outcomes were low-moderate – r=0. 14 to r=0. 60
Summary and Conclusions • Consistent and positive impact of omalizumab on AQLQ overall and domain scores (p<0. 05) • Specific drivers of improvement in each of the domains were noted • Correlations between AQLQ and other clinical outcomes were low-moderate – r=0. 14 to r=0. 60
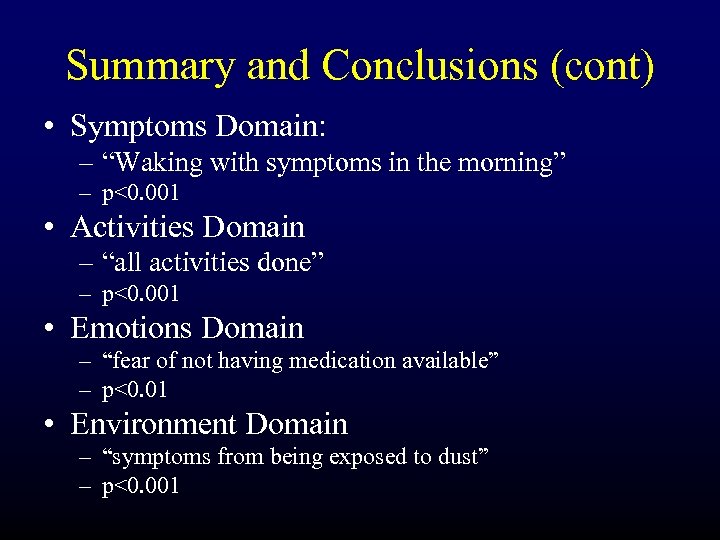 Summary and Conclusions (cont) • Symptoms Domain: – “Waking with symptoms in the morning” – p<0. 001 • Activities Domain – “all activities done” – p<0. 001 • Emotions Domain – “fear of not having medication available” – p<0. 01 • Environment Domain – “symptoms from being exposed to dust” – p<0. 001
Summary and Conclusions (cont) • Symptoms Domain: – “Waking with symptoms in the morning” – p<0. 001 • Activities Domain – “all activities done” – p<0. 001 • Emotions Domain – “fear of not having medication available” – p<0. 01 • Environment Domain – “symptoms from being exposed to dust” – p<0. 001
 Summary and Conclusions (cont) • ARQL assessment provides non-overlapping information on clinical benefit distinct from other outcomes • Examination of variability in mean scores reveals item-level responses strongly influence symptom and activity improvement • Symptoms likely to be important to patients are significantly improved by omalizumab compared to placebo in patients with mod-severe asthma
Summary and Conclusions (cont) • ARQL assessment provides non-overlapping information on clinical benefit distinct from other outcomes • Examination of variability in mean scores reveals item-level responses strongly influence symptom and activity improvement • Symptoms likely to be important to patients are significantly improved by omalizumab compared to placebo in patients with mod-severe asthma
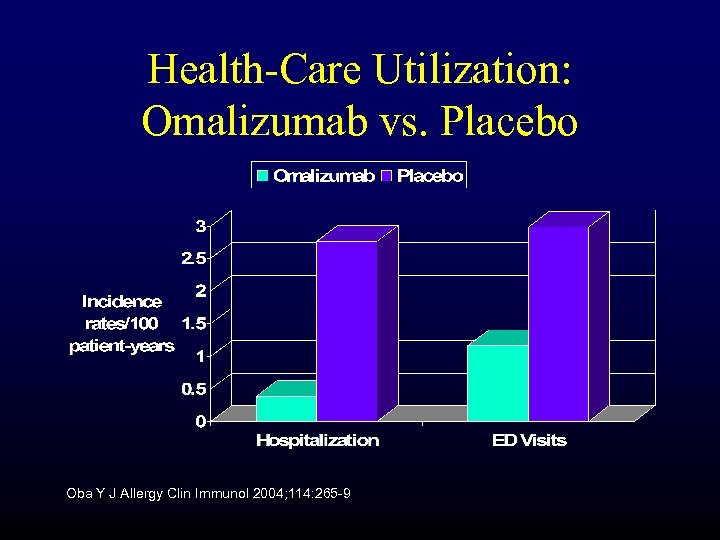 Health-Care Utilization: Omalizumab vs. Placebo Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
Health-Care Utilization: Omalizumab vs. Placebo Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
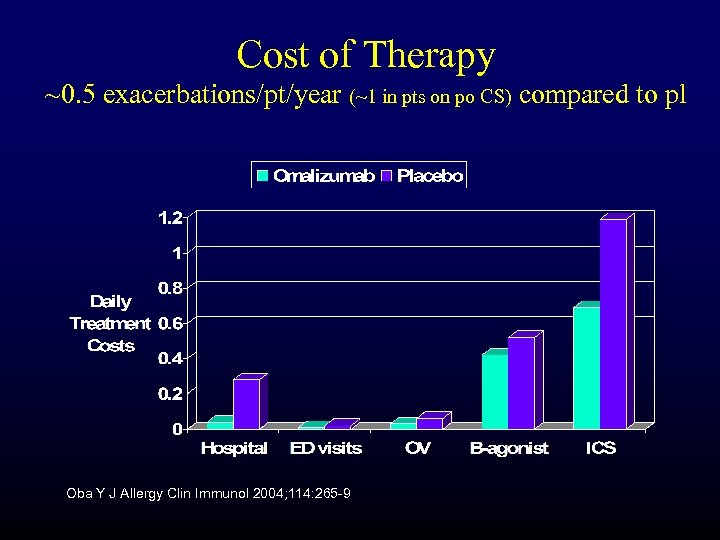 Cost of Therapy ~0. 5 exacerbations/pt/year (~1 in pts on po CS) compared to pl Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
Cost of Therapy ~0. 5 exacerbations/pt/year (~1 in pts on po CS) compared to pl Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
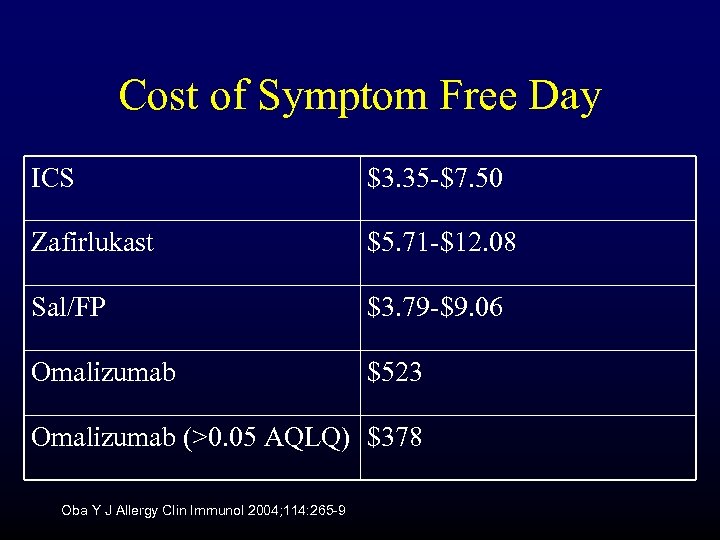 Cost of Symptom Free Day ICS $3. 35 -$7. 50 Zafirlukast $5. 71 -$12. 08 Sal/FP $3. 79 -$9. 06 Omalizumab $523 Omalizumab (>0. 05 AQLQ) $378 Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
Cost of Symptom Free Day ICS $3. 35 -$7. 50 Zafirlukast $5. 71 -$12. 08 Sal/FP $3. 79 -$9. 06 Omalizumab $523 Omalizumab (>0. 05 AQLQ) $378 Oba Y J Allergy Clin Immunol 2004; 114: 265 -9
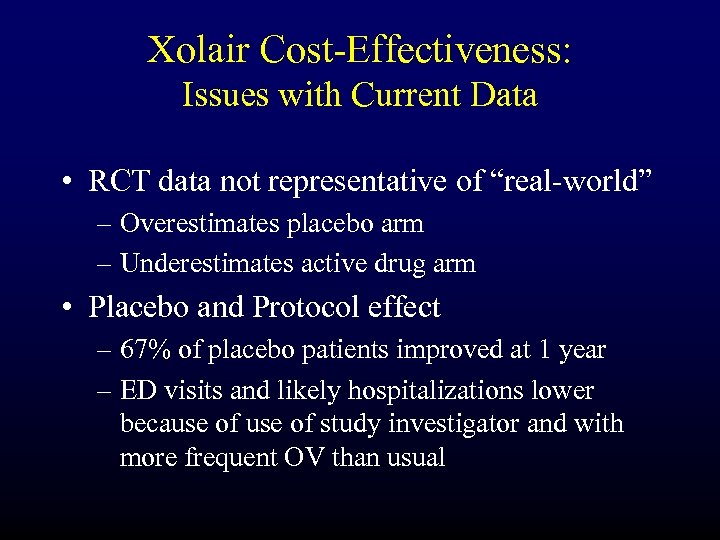 Xolair Cost-Effectiveness: Issues with Current Data • RCT data not representative of “real-world” – Overestimates placebo arm – Underestimates active drug arm • Placebo and Protocol effect – 67% of placebo patients improved at 1 year – ED visits and likely hospitalizations lower because of study investigator and with more frequent OV than usual
Xolair Cost-Effectiveness: Issues with Current Data • RCT data not representative of “real-world” – Overestimates placebo arm – Underestimates active drug arm • Placebo and Protocol effect – 67% of placebo patients improved at 1 year – ED visits and likely hospitalizations lower because of study investigator and with more frequent OV than usual
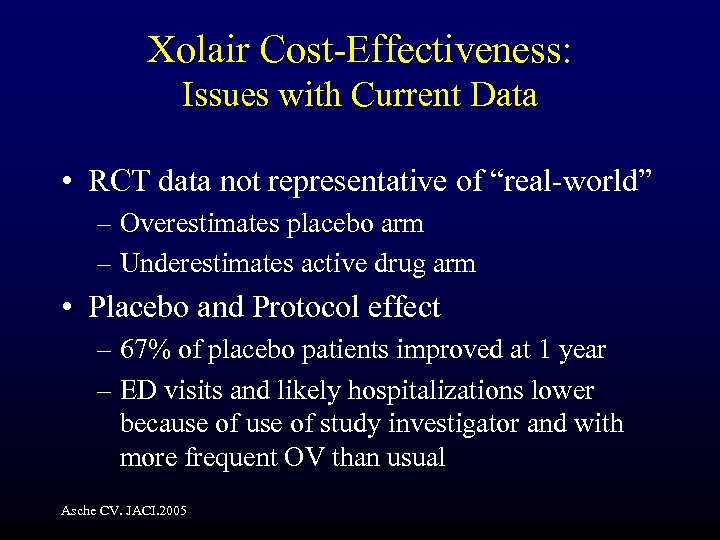 Xolair Cost-Effectiveness: Issues with Current Data • RCT data not representative of “real-world” – Overestimates placebo arm – Underestimates active drug arm • Placebo and Protocol effect – 67% of placebo patients improved at 1 year – ED visits and likely hospitalizations lower because of study investigator and with more frequent OV than usual Asche CV. JACI. 2005
Xolair Cost-Effectiveness: Issues with Current Data • RCT data not representative of “real-world” – Overestimates placebo arm – Underestimates active drug arm • Placebo and Protocol effect – 67% of placebo patients improved at 1 year – ED visits and likely hospitalizations lower because of study investigator and with more frequent OV than usual Asche CV. JACI. 2005
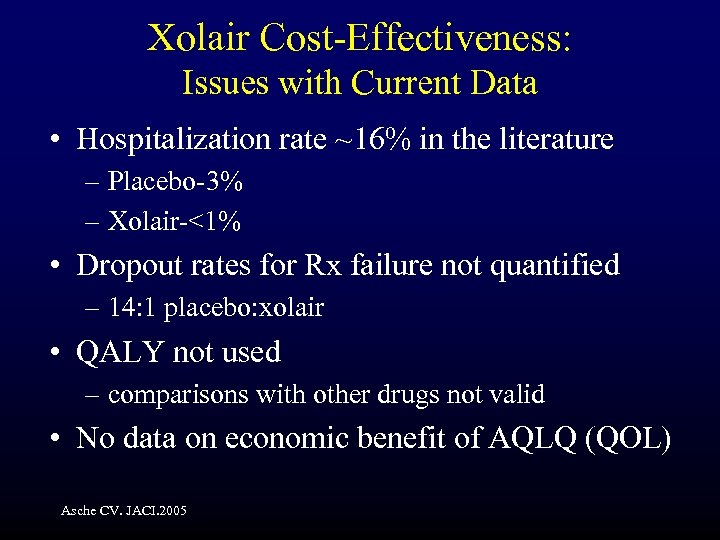 Xolair Cost-Effectiveness: Issues with Current Data • Hospitalization rate ~16% in the literature – Placebo-3% – Xolair-<1% • Dropout rates for Rx failure not quantified – 14: 1 placebo: xolair • QALY not used – comparisons with other drugs not valid • No data on economic benefit of AQLQ (QOL) Asche CV. JACI. 2005
Xolair Cost-Effectiveness: Issues with Current Data • Hospitalization rate ~16% in the literature – Placebo-3% – Xolair-<1% • Dropout rates for Rx failure not quantified – 14: 1 placebo: xolair • QALY not used – comparisons with other drugs not valid • No data on economic benefit of AQLQ (QOL) Asche CV. JACI. 2005
 • Conclusions reflect studies that were designed to assess efficacy, rather than effectiveness – Conclusions dependent on key assumptions about dosing and efficacy in a controlled clinical setting--not actual clinical practice – Retrospective C-E analyses have limited generalizability to actual clinical practice – If the RCT underestimate benefits patients achieve in actual clinical practice, then C-E ratios for omalizumab are overestimated
• Conclusions reflect studies that were designed to assess efficacy, rather than effectiveness – Conclusions dependent on key assumptions about dosing and efficacy in a controlled clinical setting--not actual clinical practice – Retrospective C-E analyses have limited generalizability to actual clinical practice – If the RCT underestimate benefits patients achieve in actual clinical practice, then C-E ratios for omalizumab are overestimated
 • Without assessing cost and efficacy in the same patient population, direct comparisons of costeffectiveness are misleading – Incremental C-E ratios for other asthma therapies should only provide context: ICS, LTRAs, and ICS-LABA combination are indicated for different patient populations – Omalizumab is indicated for patients with moderate-to-severe persistent Ig. E-mediated asthma who have failed otherapy
• Without assessing cost and efficacy in the same patient population, direct comparisons of costeffectiveness are misleading – Incremental C-E ratios for other asthma therapies should only provide context: ICS, LTRAs, and ICS-LABA combination are indicated for different patient populations – Omalizumab is indicated for patients with moderate-to-severe persistent Ig. E-mediated asthma who have failed otherapy
 • Identifying eligible patients based on “breakeven” criteria for cost-effectiveness would exclude most patients the clinical benefit that a therapy like omalizumab can deliver – Omalizumab is intended to address the disease process to prevent exacerbations and related cascade of healthcare utilization – Patients with persistent Ig. E-mediated asthma who may benefit significantly from omalizumab therapy are likely to be excluded from receiving therapy
• Identifying eligible patients based on “breakeven” criteria for cost-effectiveness would exclude most patients the clinical benefit that a therapy like omalizumab can deliver – Omalizumab is intended to address the disease process to prevent exacerbations and related cascade of healthcare utilization – Patients with persistent Ig. E-mediated asthma who may benefit significantly from omalizumab therapy are likely to be excluded from receiving therapy
 Public Health Impact of Omalizumab in High-Risk Patients • Risk difference: omalizumab prevented exacerbations in about 17 additional patients for every 100 treated • Prevented fraction: 50% of potential exacerbations were prevented by treatment with omalizumab • Number needed to treat: 5. 7 patients needed to be treated with omalizumab to maintain 1 patient free of an exacerbation Holgate S, et al. Curr Med Res Opin. 2001; 17(4): 233 -240.
Public Health Impact of Omalizumab in High-Risk Patients • Risk difference: omalizumab prevented exacerbations in about 17 additional patients for every 100 treated • Prevented fraction: 50% of potential exacerbations were prevented by treatment with omalizumab • Number needed to treat: 5. 7 patients needed to be treated with omalizumab to maintain 1 patient free of an exacerbation Holgate S, et al. Curr Med Res Opin. 2001; 17(4): 233 -240.
 Societal Burden of Asthma • Calculating societal burden of asthma requires assessment of both direct and indirect costs • Direct costs include – Costs attributed to medical care (office visits, hospitalizations, emergency visits, medications, etc. ) • Indirect costs – Dollars expended by the patient, family, employer, and/or society because of illness (including loss of productivity and quality of life) • Can be determined using either a cost of illness or cost of wellness approach Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
Societal Burden of Asthma • Calculating societal burden of asthma requires assessment of both direct and indirect costs • Direct costs include – Costs attributed to medical care (office visits, hospitalizations, emergency visits, medications, etc. ) • Indirect costs – Dollars expended by the patient, family, employer, and/or society because of illness (including loss of productivity and quality of life) • Can be determined using either a cost of illness or cost of wellness approach Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
 Cost of Illness Approach • Traditional view of government and other third party payers – Determines costs by multiplying average medical costs for one person with asthma by the total number of expected patients in the population – Focused on direct cost of care – Minimal emphasis on prevention or longterm control Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
Cost of Illness Approach • Traditional view of government and other third party payers – Determines costs by multiplying average medical costs for one person with asthma by the total number of expected patients in the population – Focused on direct cost of care – Minimal emphasis on prevention or longterm control Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
 Wall Street Journal, July 18, 2001
Wall Street Journal, July 18, 2001
 Cost of Wellness Approach • Goal of wellness is to minimize expenses caused by treatment failures and enhance productivity – Direct costs targeted for preventative health care and use of effective controller medications • Indirect costs are used for environmental control, lifestyle changes, and other interventions that promote better health • On balance, an investment in wellness promotes – Enhanced disease control – Greater productivity at work or school – Improved quality of life Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
Cost of Wellness Approach • Goal of wellness is to minimize expenses caused by treatment failures and enhance productivity – Direct costs targeted for preventative health care and use of effective controller medications • Indirect costs are used for environmental control, lifestyle changes, and other interventions that promote better health • On balance, an investment in wellness promotes – Enhanced disease control – Greater productivity at work or school – Improved quality of life Stempel DA, et al. J Allergy Clin Immunol. 2003; 111: 1203 -4.
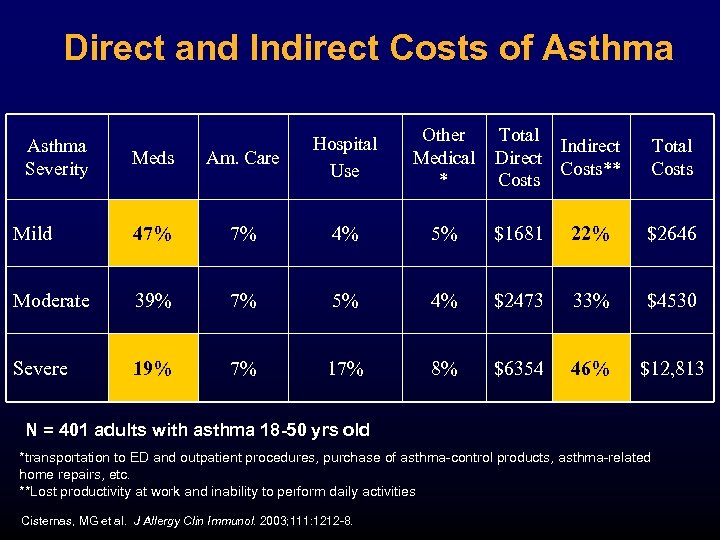 Direct and Indirect Costs of Asthma Other Medical * Total Indirect Direct Costs** Costs Total Costs Meds Am. Care Hospital Use Mild 47% 7% 4% 5% $1681 22% $2646 Moderate 39% 7% 5% 4% $2473 33% $4530 Severe 19% 7% 17% 8% $6354 46% $12, 813 Asthma Severity N = 401 adults with asthma 18 -50 yrs old *transportation to ED and outpatient procedures, purchase of asthma-control products, asthma-related home repairs, etc. **Lost productivity at work and inability to perform daily activities Cisternas, MG et al. J Allergy Clin Immunol. 2003; 111: 1212 -8.
Direct and Indirect Costs of Asthma Other Medical * Total Indirect Direct Costs** Costs Total Costs Meds Am. Care Hospital Use Mild 47% 7% 4% 5% $1681 22% $2646 Moderate 39% 7% 5% 4% $2473 33% $4530 Severe 19% 7% 17% 8% $6354 46% $12, 813 Asthma Severity N = 401 adults with asthma 18 -50 yrs old *transportation to ED and outpatient procedures, purchase of asthma-control products, asthma-related home repairs, etc. **Lost productivity at work and inability to perform daily activities Cisternas, MG et al. J Allergy Clin Immunol. 2003; 111: 1212 -8.
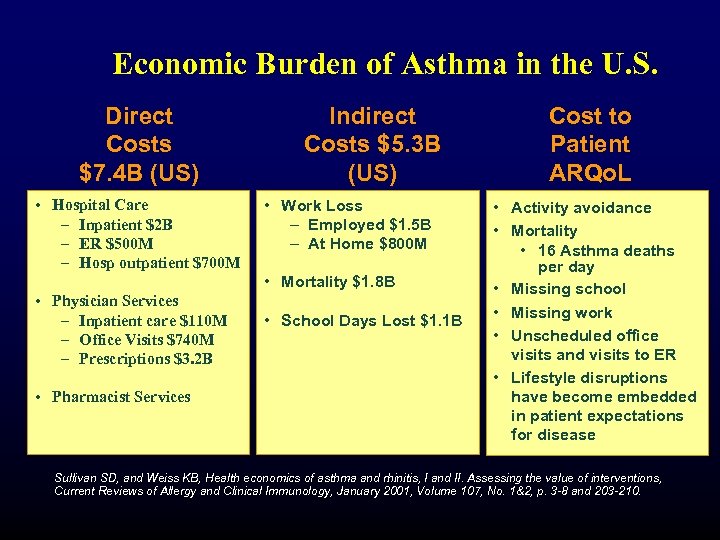 Economic Burden of Asthma in the U. S. Direct Costs $7. 4 B (US) • Hospital Care – Inpatient $2 B – ER $500 M – Hosp outpatient $700 M Indirect Costs $5. 3 B (US) • Work Loss – Employed $1. 5 B – At Home $800 M • Mortality $1. 8 B • Physician Services – Inpatient care $110 M – Office Visits $740 M – Prescriptions $3. 2 B • Pharmacist Services • School Days Lost $1. 1 B Cost to Patient ARQo. L • Activity avoidance • Mortality • 16 Asthma deaths per day • Missing school • Missing work • Unscheduled office visits and visits to ER • Lifestyle disruptions have become embedded in patient expectations for disease Sullivan SD, and Weiss KB, Health economics of asthma and rhinitis, I and II. Assessing the value of interventions, Current Reviews of Allergy and Clinical Immunology, January 2001, Volume 107, No. 1&2, p. 3 -8 and 203 -210.
Economic Burden of Asthma in the U. S. Direct Costs $7. 4 B (US) • Hospital Care – Inpatient $2 B – ER $500 M – Hosp outpatient $700 M Indirect Costs $5. 3 B (US) • Work Loss – Employed $1. 5 B – At Home $800 M • Mortality $1. 8 B • Physician Services – Inpatient care $110 M – Office Visits $740 M – Prescriptions $3. 2 B • Pharmacist Services • School Days Lost $1. 1 B Cost to Patient ARQo. L • Activity avoidance • Mortality • 16 Asthma deaths per day • Missing school • Missing work • Unscheduled office visits and visits to ER • Lifestyle disruptions have become embedded in patient expectations for disease Sullivan SD, and Weiss KB, Health economics of asthma and rhinitis, I and II. Assessing the value of interventions, Current Reviews of Allergy and Clinical Immunology, January 2001, Volume 107, No. 1&2, p. 3 -8 and 203 -210.
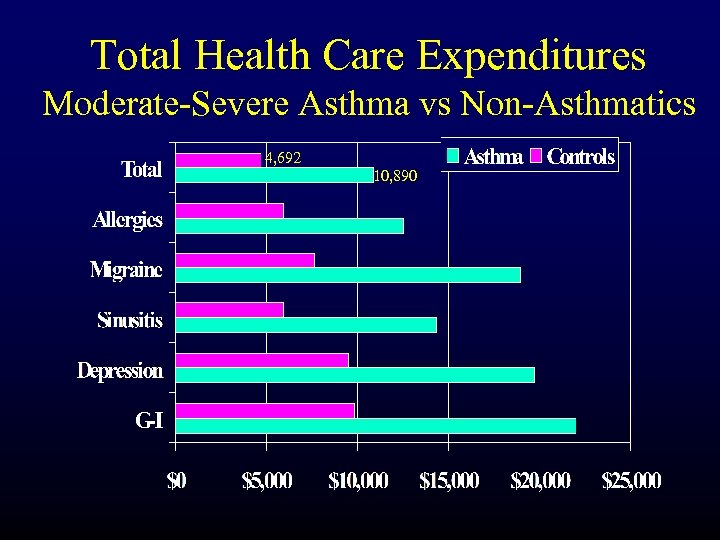 Total Health Care Expenditures Moderate-Severe Asthma vs Non-Asthmatics 4, 692 10, 890
Total Health Care Expenditures Moderate-Severe Asthma vs Non-Asthmatics 4, 692 10, 890
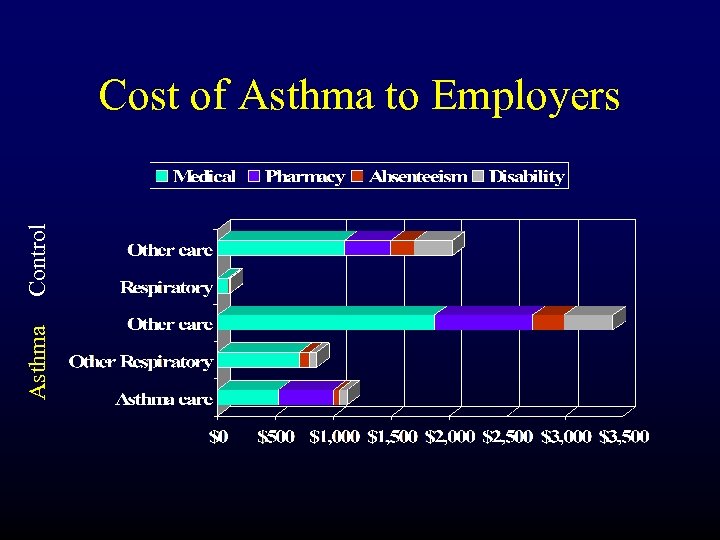 Asthma Control Cost of Asthma to Employers
Asthma Control Cost of Asthma to Employers
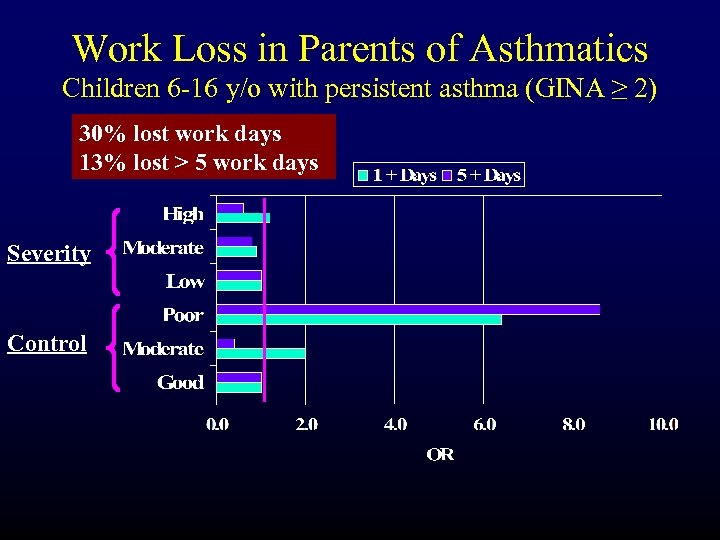 Work Loss in Parents of Asthmatics Children 6 -16 y/o with persistent asthma (GINA ≥ 2) 30% lost work days 13% lost > 5 work days Severity Control
Work Loss in Parents of Asthmatics Children 6 -16 y/o with persistent asthma (GINA ≥ 2) 30% lost work days 13% lost > 5 work days Severity Control
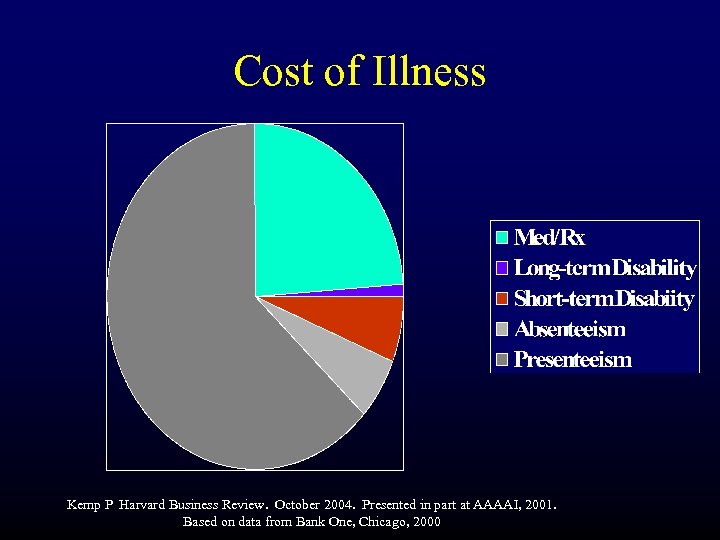 Cost of Illness Kemp P Harvard Business Review. October 2004. Presented in part at AAAAI, 2001. Based on data from Bank One, Chicago, 2000
Cost of Illness Kemp P Harvard Business Review. October 2004. Presented in part at AAAAI, 2001. Based on data from Bank One, Chicago, 2000
 Effect of Presenteeism
Effect of Presenteeism
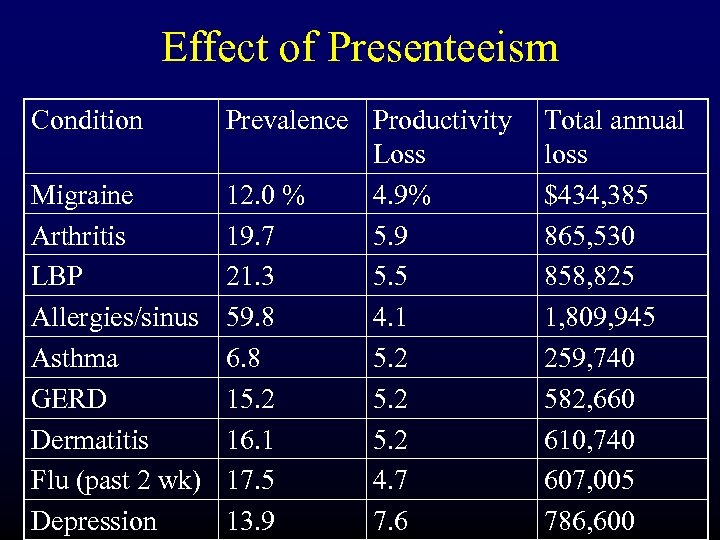 Effect of Presenteeism Condition Prevalence Productivity Loss Migraine 12. 0 % 4. 9% Arthritis 19. 7 5. 9 LBP 21. 3 5. 5 Allergies/sinus 59. 8 4. 1 Asthma 6. 8 5. 2 GERD 15. 2 Dermatitis 16. 1 5. 2 Flu (past 2 wk) 17. 5 4. 7 Depression 13. 9 7. 6 Total annual loss $434, 385 865, 530 858, 825 1, 809, 945 259, 740 582, 660 610, 740 607, 005 786, 600
Effect of Presenteeism Condition Prevalence Productivity Loss Migraine 12. 0 % 4. 9% Arthritis 19. 7 5. 9 LBP 21. 3 5. 5 Allergies/sinus 59. 8 4. 1 Asthma 6. 8 5. 2 GERD 15. 2 Dermatitis 16. 1 5. 2 Flu (past 2 wk) 17. 5 4. 7 Depression 13. 9 7. 6 Total annual loss $434, 385 865, 530 858, 825 1, 809, 945 259, 740 582, 660 610, 740 607, 005 786, 600
 Cost-Sharing • In an attempt to reduce costs, payors will shift costs to patients: – “consumer-driven health plans” – Utilization control and influence choice • This will demand a FULLY educated consumer • We will need to help patient evaluate the full cost-benefit (not just HCU but QOL)
Cost-Sharing • In an attempt to reduce costs, payors will shift costs to patients: – “consumer-driven health plans” – Utilization control and influence choice • This will demand a FULLY educated consumer • We will need to help patient evaluate the full cost-benefit (not just HCU but QOL)
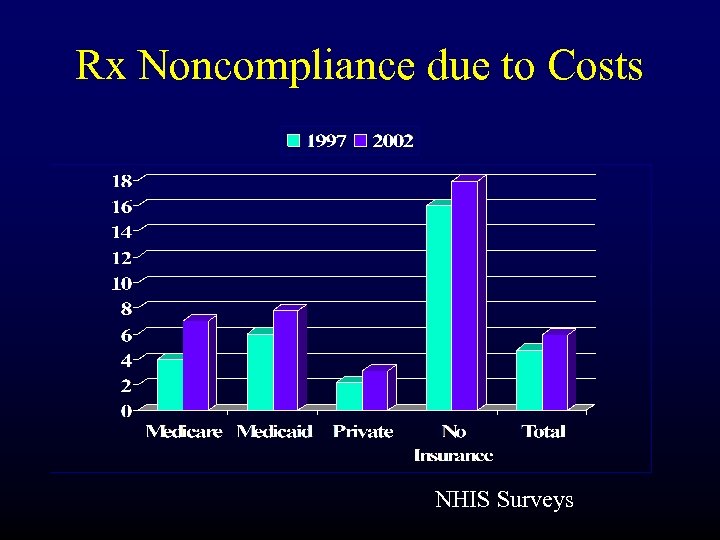 Rx Noncompliance due to Costs NHIS Surveys
Rx Noncompliance due to Costs NHIS Surveys

 Discussion Questions • Are the current outcomes that we consider in the treatment algorithm for asthma adequate? • If not, what else should we be considering? • What are the benefits and challenges of looking at these other outcomes? • What endpoints would help clarify and communicate the value proposition for Xolair?
Discussion Questions • Are the current outcomes that we consider in the treatment algorithm for asthma adequate? • If not, what else should we be considering? • What are the benefits and challenges of looking at these other outcomes? • What endpoints would help clarify and communicate the value proposition for Xolair?
 Discussion Questions • What indirect costs are most strongly associated with poor control of asthma symptoms? • With increasing focus on the concept of control, should we rethink the conventional cost-effectiveness approach for asthma interventions? – Is an outcome measure other than the symptom free-day warranted? – Should analyses take into account the significant burden associated with indirect costs that may be mitigated by therapies that reduce activity limitations?
Discussion Questions • What indirect costs are most strongly associated with poor control of asthma symptoms? • With increasing focus on the concept of control, should we rethink the conventional cost-effectiveness approach for asthma interventions? – Is an outcome measure other than the symptom free-day warranted? – Should analyses take into account the significant burden associated with indirect costs that may be mitigated by therapies that reduce activity limitations?


