7378d4ce2cecf2e31307331a33fae25e.ppt
- Количество слайдов: 14
 Evaluation of Apparent Diffusion Coefficient (ADC) in Prion Encephalopathies REYES GARCÍA DE EULATE RADIOLOGY DEPARTMENT
Evaluation of Apparent Diffusion Coefficient (ADC) in Prion Encephalopathies REYES GARCÍA DE EULATE RADIOLOGY DEPARTMENT
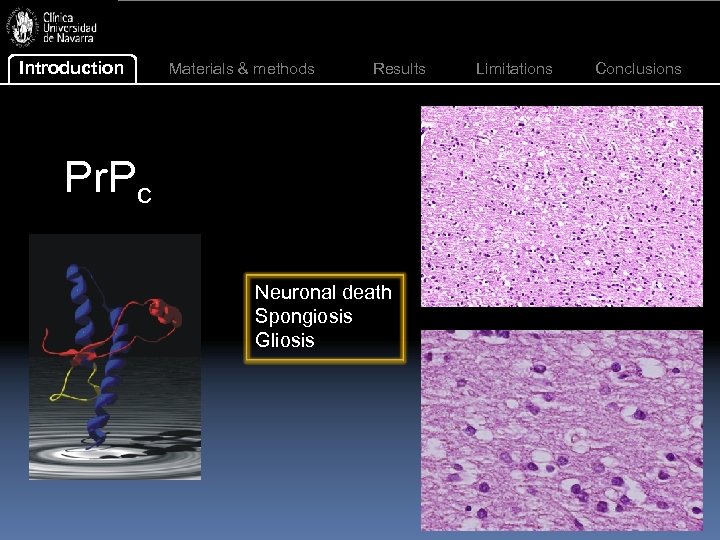 Introduction Materials & methods Results Pr. Pc Neuronal death Spongiosis Gliosis Limitations Conclusions
Introduction Materials & methods Results Pr. Pc Neuronal death Spongiosis Gliosis Limitations Conclusions
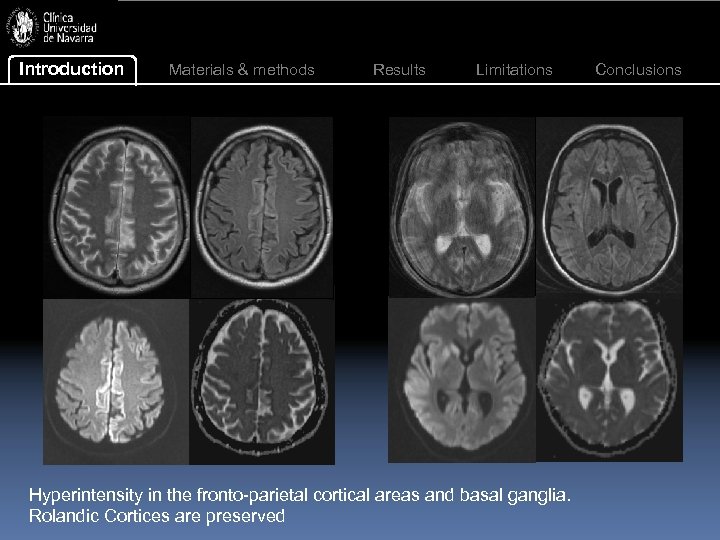 Introduction Materials & methods Results Limitations Hyperintensity in the fronto-parietal cortical areas and basal ganglia. Rolandic Cortices are preserved Conclusions
Introduction Materials & methods Results Limitations Hyperintensity in the fronto-parietal cortical areas and basal ganglia. Rolandic Cortices are preserved Conclusions
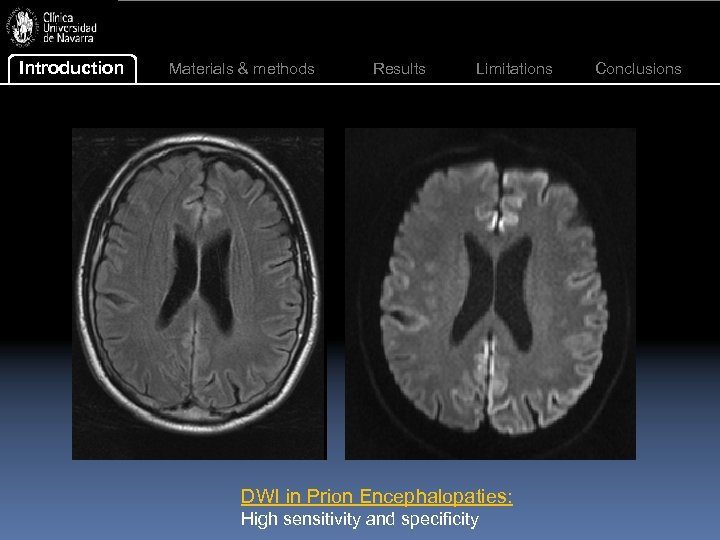 Introduction Materials & methods Results Limitations DWI in Prion Encephalopaties: High sensitivity and specificity Conclusions
Introduction Materials & methods Results Limitations DWI in Prion Encephalopaties: High sensitivity and specificity Conclusions
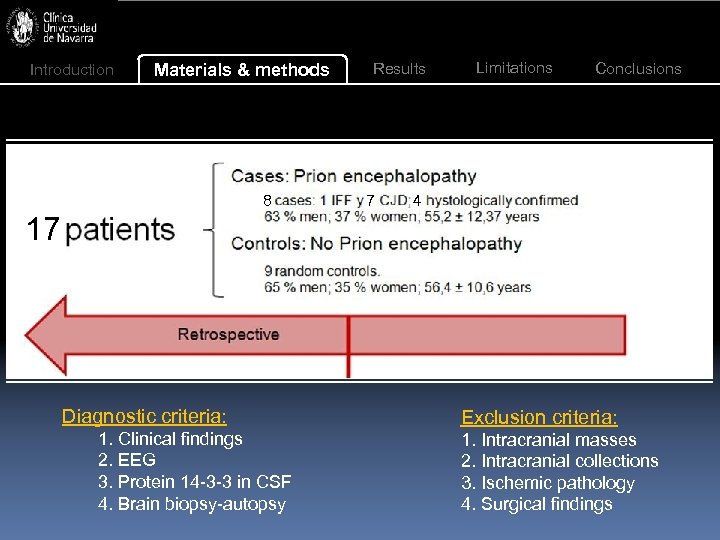 Introduction Materials & methods 8 17 17 Results Limitations Conclusions 4 Study 7 Hypotheses: 1. Quantitative ADC assessment is a valuable diagnosis technique for PD 2. It is a reliable technique: findings of different radiologists are consistent Diagnostic criteria: 1. Clinical findings 2. EEG 3. Protein 14 -3 -3 in CSF 4. Brain biopsy-autopsy Exclusion criteria: 1. Intracranial masses 2. Intracranial collections 3. Ischemic pathology 4. Surgical findings
Introduction Materials & methods 8 17 17 Results Limitations Conclusions 4 Study 7 Hypotheses: 1. Quantitative ADC assessment is a valuable diagnosis technique for PD 2. It is a reliable technique: findings of different radiologists are consistent Diagnostic criteria: 1. Clinical findings 2. EEG 3. Protein 14 -3 -3 in CSF 4. Brain biopsy-autopsy Exclusion criteria: 1. Intracranial masses 2. Intracranial collections 3. Ischemic pathology 4. Surgical findings
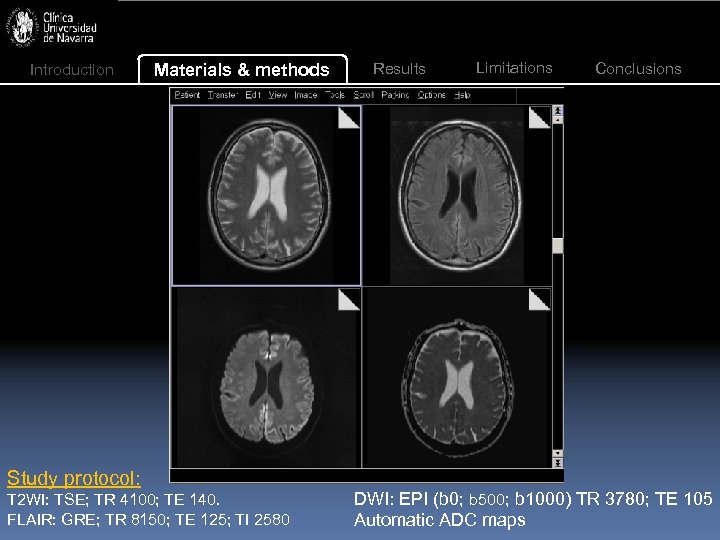 Introduction Materials & methods Study protocol: T 2 WI: TSE; TR 4100; TE 140. FLAIR: GRE; TR 8150; TE 125; TI 2580 Results Limitations Conclusions DWI: EPI (b 0; b 500; b 1000) TR 3780; TE 105 Automatic ADC maps
Introduction Materials & methods Study protocol: T 2 WI: TSE; TR 4100; TE 140. FLAIR: GRE; TR 8150; TE 125; TI 2580 Results Limitations Conclusions DWI: EPI (b 0; b 500; b 1000) TR 3780; TE 105 Automatic ADC maps
 Introduction Materials & methods Results Limitations Conclusions
Introduction Materials & methods Results Limitations Conclusions
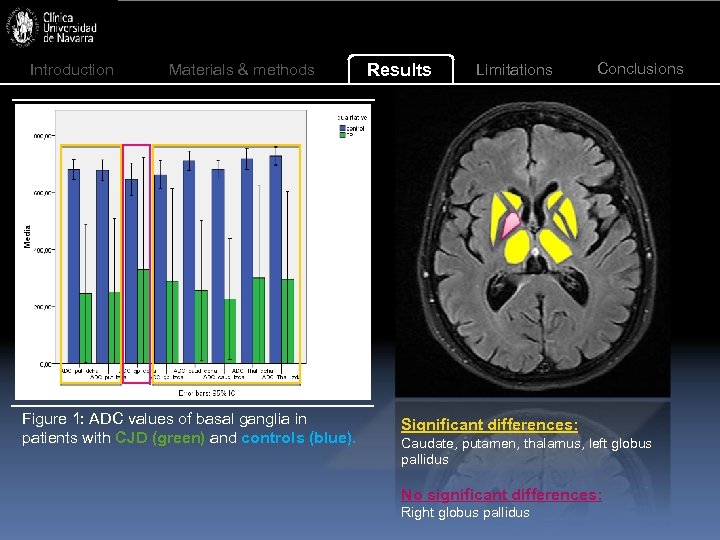 Introduction Materials & methods Figure 1: ADC values of basal ganglia in patients with CJD (green) and controls (blue). Results Limitations Conclusions Significant differences: Caudate, putamen, thalamus, left globus pallidus No significant differences: Right globus pallidus
Introduction Materials & methods Figure 1: ADC values of basal ganglia in patients with CJD (green) and controls (blue). Results Limitations Conclusions Significant differences: Caudate, putamen, thalamus, left globus pallidus No significant differences: Right globus pallidus
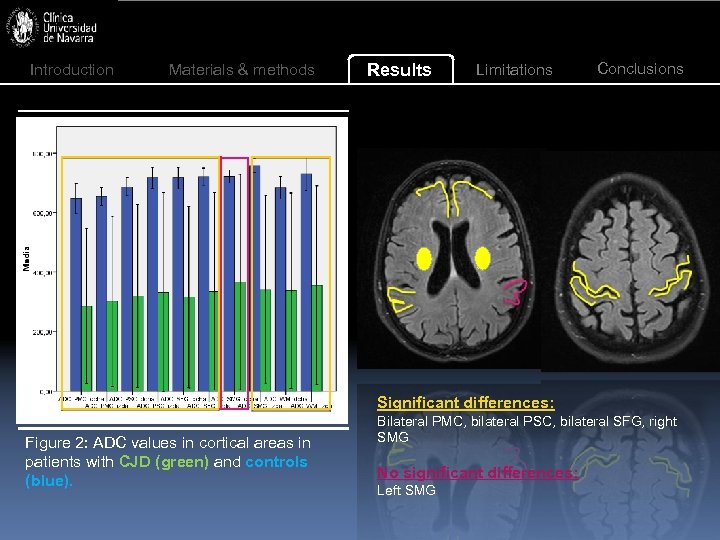 Introduction Materials & methods Results Limitations Conclusions Significant differences: Figure 2: ADC values in cortical areas in patients with CJD (green) and controls (blue). Bilateral PMC, bilateral PSC, bilateral SFG, right SMG No significant differences: Left SMG
Introduction Materials & methods Results Limitations Conclusions Significant differences: Figure 2: ADC values in cortical areas in patients with CJD (green) and controls (blue). Bilateral PMC, bilateral PSC, bilateral SFG, right SMG No significant differences: Left SMG
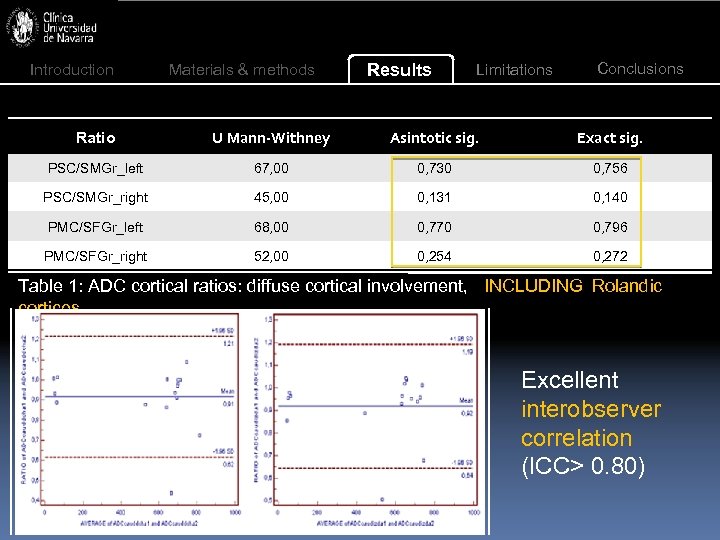 Introduction Materials & methods Results Limitations Conclusions Ratio U Mann-Withney Asintotic sig. Exact sig. PSC/SMGr_left 67, 00 0, 730 0, 756 PSC/SMGr_right 45, 00 0, 131 0, 140 PMC/SFGr_left 68, 00 0, 770 0, 796 PMC/SFGr_right 52, 00 0, 254 0, 272 Table 1: ADC cortical ratios: diffuse cortical involvement, INCLUDING Rolandic cortices. Excellent interobserver correlation (ICC> 0. 80)
Introduction Materials & methods Results Limitations Conclusions Ratio U Mann-Withney Asintotic sig. Exact sig. PSC/SMGr_left 67, 00 0, 730 0, 756 PSC/SMGr_right 45, 00 0, 131 0, 140 PMC/SFGr_left 68, 00 0, 770 0, 796 PMC/SFGr_right 52, 00 0, 254 0, 272 Table 1: ADC cortical ratios: diffuse cortical involvement, INCLUDING Rolandic cortices. Excellent interobserver correlation (ICC> 0. 80)
 Introduction Materials & methods Results Limitations Conclusions Low number of patients (non parametric tests) Absence of histological confirmation in some patients
Introduction Materials & methods Results Limitations Conclusions Low number of patients (non parametric tests) Absence of histological confirmation in some patients
 Introduction Materials & methods Results Limitations Conclusions Quantitative ADC measurement demostrates diffuse affection This is a reproducible technique for any radiologist, regarless of his or her experience Quantitative ADC measurement provides a proper diagnosis as well as sensitive, reliable and applicable in daily clinical practice
Introduction Materials & methods Results Limitations Conclusions Quantitative ADC measurement demostrates diffuse affection This is a reproducible technique for any radiologist, regarless of his or her experience Quantitative ADC measurement provides a proper diagnosis as well as sensitive, reliable and applicable in daily clinical practice
 1. Collins S, Boyd A, Fletcher A, Gonzales MF, Mc. Lean CA, Masters CL. Recent advances in the pre-mortem diagnosis of Creutzfeld-Jakob disease. J Clin Neurosci 2000; 7 (3): 195 -202. 2. Johnson RT, Gibbs Jr CJ. Creutzfeld-Jakob disease and related transmissible spongiform encefalopathies. N Engl J Med 1998; 339(27): 1994 -2004. 3. Prusiner SB. Prions. Proc Natl Acad Sci U. S. A 1998; 95(23): 13363 -13383. 4. Fulbright RK, Kingsley PB, Guo X, Hoffmann C, Kahana E, Chapman JC, et al. The imaging appearance of Creutzfeld-Jakob disease caused bu E 200 K mutation. Magn Reson Imaging 2006; 24: 1121 -1129. 5. World health Organization. Global surveillance, diagnosis, and therapy of human transmissible forms of prion encefalopathies: report of WHO consultation. . In: Global surveillance, diangosis, and therapy of human transmissible Spongiform encefalopathies. Geneva: World Health Organisation 1998: 1 - 29. 6. Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, Wong S, et al. Diffusion–weighted and fluid attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: Hgh sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol 2005; 26: 1551 -1562. 7. Gertz HJ, Henkes H, Cervs-Navarro J. Creutzfeldt-Jakob disesase: correlation of MRI and neuropathologic findings. Neurology 1988; 38: 1481 -1482. 8. Urbach H. Creutfeldt-Jakob disease: analysis of MRI signal. Neuroreport 2000; 11: L 5 -6. 9. Demaerel P, Baert AL, Vanopdenbosch L, Roberecht W, Dom R. Diffusion-weighted magnetic resonance imaging in Creutzfeldt-Jakob disease. Lancet 1997; 349: 847 -848. 10. Murata T, Shiga Y, Higano S, Takahashi S, Mugikura S. Conspicuity and evolution of lesions in Creutzfeldt-Jakob disease at diffusion witghted imaging. AJNR Am J Neuroradiol 2002; 23: 1164 -1172. 11. Bahn MM, Parchi P. Abnormal diffusion weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol 1999; 56: 557 -583. 12. Schwaninger M, Winter R, Hacke W, et al. Macnetic resonance imaging in Creutzfeldt-Jakob disease: evidence of focal involvement of cortex. J Neurol Neurosurg Psychiatry 1997; 63: 408 -409. 13. Montagna P, Gambetti P, Cortelli P, Lugaresi E. Familial and sporadic fatal insomnia. Lancet neurology 2002; 2: 167 -176. 14. Kralovicova S, Fontaine SN, Alderton A, Alderman J, Ragnarsdottir KV, Collins SJ et al. The effects of prion protein expression on metal metabolism. Moll Cell Neurosci 2009; 41: 135 -147. 15. Finkenstaedt M, Szudra A, Zerr I, et al. MR imaging of Creutzfeldt-Jakob disease. Radiology 1996; 199(3): 793 -798. 16. Mao-Drayer Y, Braff SP, Nagle KJ, Pendlebury W, Penar PL, Shapiro RE. Emerging patterns of diffusion-weighted MR imaging in Creutzfeldt-Jakob disease: case report and review of the literature. AJNR Am J Neuroradiol 2002; 23(4): 550 -556. 17. Matoba M, Tonami H, Miyaji H, Yokota H, Yamamoto I. Creutzfeldt-Jakob disease: serial changes on diffusion-weighted MRI. J Comput Assist Tomogr 2001; 25(2): 274 -277. 18. Na DL, Suh CK, Choi SH, et al. Diffusion-weighted magnetic resonance imaging in probable Creutzfeldt-Jakob disease: a clinical anatomic correlation. Arch Neurol 1999; 56(8): 951 -957. 19. Samman I, Schultz-Schaeffer WJ, Whorle JC, Sommer A, Kretzschmar HA, Hennerici M. Clinical range and MRI in Creutzfeldt-Jakob disease with heterozygosity at codon 129 and prion protein type 2. J Neurol Neurosurg Psychiatry 1999; 67(5): 678 -681. 20. Ukisu R, Kushibashi T, Kitanosono T et al. Serial diffusion weighted MRI of Creutzfeldt-Jakob disease. Am J Roentgenol 2005; 184(2): 560 -566. 21. Lin Y-R, Young GS, Chen N-K, Dillon WP, Wong S. Creutzfeldt-Jakob disease involvement of rolandic cortex: a quantitative apparent diffusion coefficient evaluation. AJNR Am J Neuroradiol 2006; 27: 1755 -1759.
1. Collins S, Boyd A, Fletcher A, Gonzales MF, Mc. Lean CA, Masters CL. Recent advances in the pre-mortem diagnosis of Creutzfeld-Jakob disease. J Clin Neurosci 2000; 7 (3): 195 -202. 2. Johnson RT, Gibbs Jr CJ. Creutzfeld-Jakob disease and related transmissible spongiform encefalopathies. N Engl J Med 1998; 339(27): 1994 -2004. 3. Prusiner SB. Prions. Proc Natl Acad Sci U. S. A 1998; 95(23): 13363 -13383. 4. Fulbright RK, Kingsley PB, Guo X, Hoffmann C, Kahana E, Chapman JC, et al. The imaging appearance of Creutzfeld-Jakob disease caused bu E 200 K mutation. Magn Reson Imaging 2006; 24: 1121 -1129. 5. World health Organization. Global surveillance, diagnosis, and therapy of human transmissible forms of prion encefalopathies: report of WHO consultation. . In: Global surveillance, diangosis, and therapy of human transmissible Spongiform encefalopathies. Geneva: World Health Organisation 1998: 1 - 29. 6. Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, Wong S, et al. Diffusion–weighted and fluid attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: Hgh sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol 2005; 26: 1551 -1562. 7. Gertz HJ, Henkes H, Cervs-Navarro J. Creutzfeldt-Jakob disesase: correlation of MRI and neuropathologic findings. Neurology 1988; 38: 1481 -1482. 8. Urbach H. Creutfeldt-Jakob disease: analysis of MRI signal. Neuroreport 2000; 11: L 5 -6. 9. Demaerel P, Baert AL, Vanopdenbosch L, Roberecht W, Dom R. Diffusion-weighted magnetic resonance imaging in Creutzfeldt-Jakob disease. Lancet 1997; 349: 847 -848. 10. Murata T, Shiga Y, Higano S, Takahashi S, Mugikura S. Conspicuity and evolution of lesions in Creutzfeldt-Jakob disease at diffusion witghted imaging. AJNR Am J Neuroradiol 2002; 23: 1164 -1172. 11. Bahn MM, Parchi P. Abnormal diffusion weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol 1999; 56: 557 -583. 12. Schwaninger M, Winter R, Hacke W, et al. Macnetic resonance imaging in Creutzfeldt-Jakob disease: evidence of focal involvement of cortex. J Neurol Neurosurg Psychiatry 1997; 63: 408 -409. 13. Montagna P, Gambetti P, Cortelli P, Lugaresi E. Familial and sporadic fatal insomnia. Lancet neurology 2002; 2: 167 -176. 14. Kralovicova S, Fontaine SN, Alderton A, Alderman J, Ragnarsdottir KV, Collins SJ et al. The effects of prion protein expression on metal metabolism. Moll Cell Neurosci 2009; 41: 135 -147. 15. Finkenstaedt M, Szudra A, Zerr I, et al. MR imaging of Creutzfeldt-Jakob disease. Radiology 1996; 199(3): 793 -798. 16. Mao-Drayer Y, Braff SP, Nagle KJ, Pendlebury W, Penar PL, Shapiro RE. Emerging patterns of diffusion-weighted MR imaging in Creutzfeldt-Jakob disease: case report and review of the literature. AJNR Am J Neuroradiol 2002; 23(4): 550 -556. 17. Matoba M, Tonami H, Miyaji H, Yokota H, Yamamoto I. Creutzfeldt-Jakob disease: serial changes on diffusion-weighted MRI. J Comput Assist Tomogr 2001; 25(2): 274 -277. 18. Na DL, Suh CK, Choi SH, et al. Diffusion-weighted magnetic resonance imaging in probable Creutzfeldt-Jakob disease: a clinical anatomic correlation. Arch Neurol 1999; 56(8): 951 -957. 19. Samman I, Schultz-Schaeffer WJ, Whorle JC, Sommer A, Kretzschmar HA, Hennerici M. Clinical range and MRI in Creutzfeldt-Jakob disease with heterozygosity at codon 129 and prion protein type 2. J Neurol Neurosurg Psychiatry 1999; 67(5): 678 -681. 20. Ukisu R, Kushibashi T, Kitanosono T et al. Serial diffusion weighted MRI of Creutzfeldt-Jakob disease. Am J Roentgenol 2005; 184(2): 560 -566. 21. Lin Y-R, Young GS, Chen N-K, Dillon WP, Wong S. Creutzfeldt-Jakob disease involvement of rolandic cortex: a quantitative apparent diffusion coefficient evaluation. AJNR Am J Neuroradiol 2006; 27: 1755 -1759.
 Radiology Department Gracias/Thank you mgeulate@unav. es
Radiology Department Gracias/Thank you mgeulate@unav. es


