1bbe391d0d94acaa087e34cf93085911.ppt
- Количество слайдов: 27

EUROpean network of transnational collaborative RTD projects in the field of NANOMEDicine 1 www. euronanomed. net

What are the societal needs? - the challenge for Europe Improve public health and welfare – Ageing population – Efficiency of current medical solutions – Emergence of new disease and old diseases are back – Personalized medicine future directions www. euronanomed. net 2

The global challenge • Therapeutic solutions for only 1/3 known diseases (WHO) • Infections: HIV, TB, malaria, risk of pandemia • Need to increase capability and innovation of medical industry – Rapid and accurate diagnosis for early detection, even prevention – Novel, more efficient therapies with less side effects www. euronanomed. net 3

Nano from research to patient and industry Nanomedicine: the application of nanotechnology to achieve breakthroughs in healthcare Foto: kaibara 87/www. flickr. com www. euronanomed. net 4

Emerging nanomedicine ses a Dise tious 009 Infec l 9, 2 t ance ue, vo L ril iss Ap www. euronanomed. net 5
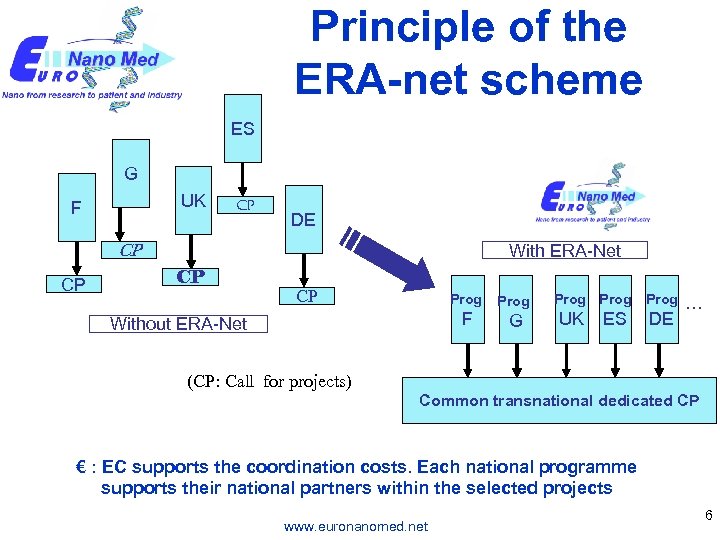
Principle of the ERA-net scheme ES G UK F CP DE CP CP With ERA-Net CP CP Prog F Without ERA-Net G Prog UK ES Prog DE … (CP: Call for projects) Common transnational dedicated CP € : EC supports the coordination costs. Each national programme supports their national partners within the selected projects www. euronanomed. net 6
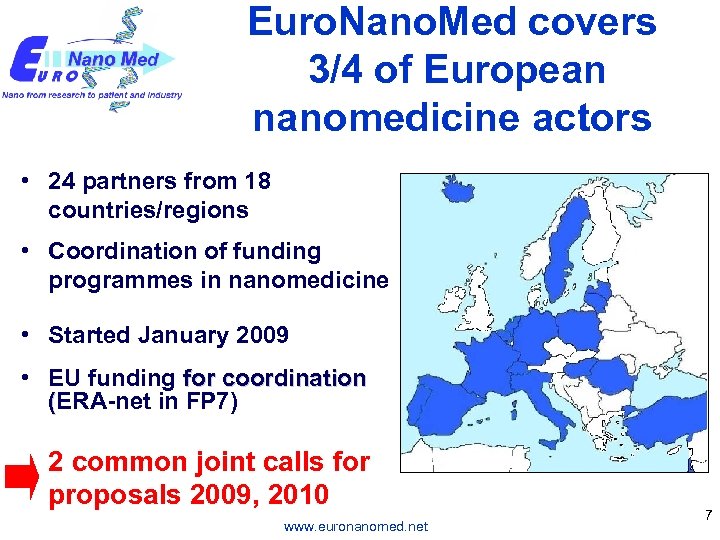
Euro. Nano. Med covers 3/4 of European nanomedicine actors • 24 partners from 18 countries/regions • Coordination of funding programmes in nanomedicine • Started January 2009 • EU funding for coordination (ERA-net in FP 7) 2 common joint calls for proposals 2009, 2010 www. euronanomed. net 7

Euro. Nano. Med: an asset to bridge the gap Aims of the Calls Develop innovative diagnostic and therapeutic solutions for the patient Solving unmet medical needs Enhancing the competitiveness of the European health industry Maturation of the Nanomedicine field in Europe Cross-border collaboration between the best researchers / teams in Nanomedicine, bringing together teams from Ø academic / public research Ø clinical / public health Ø industry www. euronanomed. net 8

Why now? What’s the urgency? • Health industry: a key for future growth • European countries invest substantially in nano industry and basic nano research • Nano technologies: a promising field for innovation and patient application Despite these efforts, Europe is lagging behind the US in an increasing competition : Europe needs to act now! www. euronanomed. net 9

Expected outcome • To move innovation from knowledge to industrial technology and health care applications • Opening the national programmes to support transnational RTD partnerships Long term vision: formula for an Europeanwide integrated programme with a coordinated funding www. euronanomed. net 10

Contacts • Coordinator and Executive Board Chair Dr. Virginie Sivan Commissariat à l’Energie Atomique (CEA) Virginie. sivan@cea. fr (+33) 1 69 08 87 99 • Network Steering Committee chair Dr. Olaf Rotthaus VDI Technologiezentrum Gmb. H (VDI) rotthaus@vdi. de (+49) 211 6214 -233 www. euronanomed. net 11

Euro. Nano. Med: 1 st Joint Transnational Call open from May 25 th to September 1 st 2009 Evaluation by Peer Review Panel in September 2009 Call Steering Committee decision in October 2009 8 Projects selected for funding (40 Partners, 14 Countries /Regions) Selected projects have recently started to work or are about to start. Total costs of funded projects: 16. 9 M € Total requested budget 9. 4 M € www. euronanomed. net 12
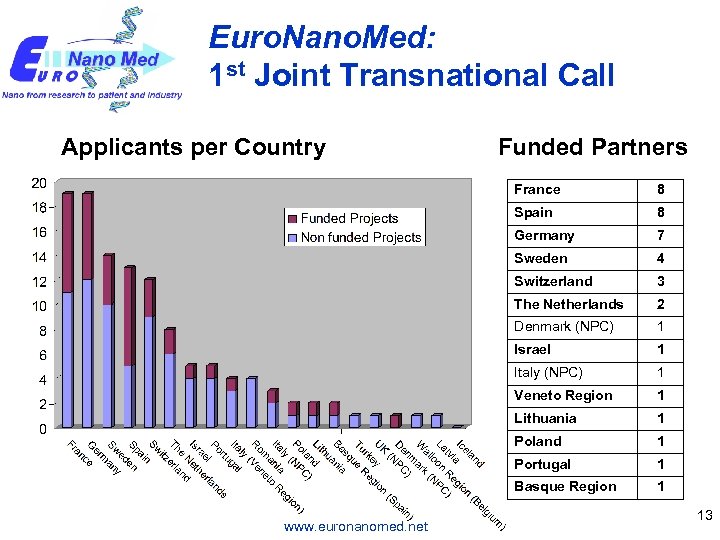
Euro. Nano. Med: 1 st Joint Transnational Call Applicants per Country Funded Partners France Spain 8 Germany 7 Sweden 4 Switzerland 3 The Netherlands 2 Denmark (NPC) 1 Israel 1 Italy (NPC) 1 Veneto Region 1 Lithuania 1 Poland 1 Portugal 1 Basque Region www. euronanomed. net 8 1 13
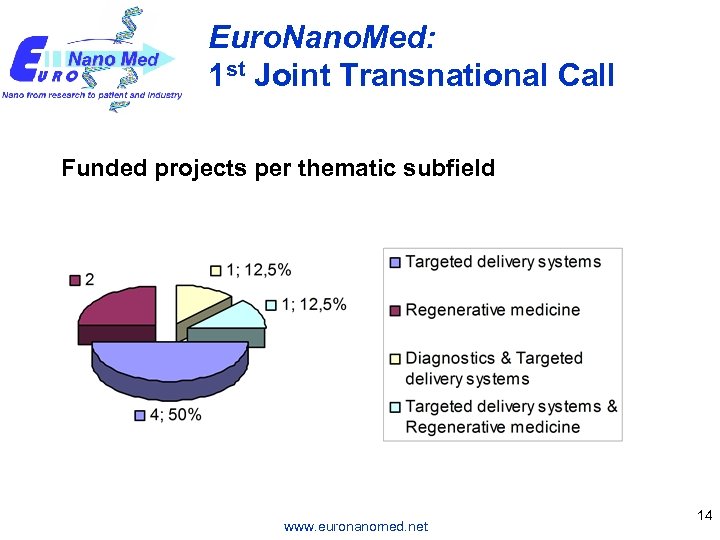
Euro. Nano. Med: 1 st Joint Transnational Call Funded projects per thematic subfield www. euronanomed. net 14

Euro. Nano. Med: open Call 2 nd Joint Transnational Call for Proposals 17 Partners from 16 Countries / Regions Deadline: June 11 www. euronanomed. net 15

Participating Funding Organisations Industry, Innovation, Trade and Tourism Department, Basque Government The French National Research Agency (ANR) VDI Technologiezentrum Gmb. H National Office for Research and Technology (NKTH) The Icelandic Centre for Research (RANNIS) The Chief Scientist Office, The Ministry of Health (CSO-MOH) The Latvian Academy of Sciences (LAS) Science Council of Lithuania (LSC) National Centre for Research and Development (NCBi. R) Fundação para a Ciência e a Tecnologia (FCT) National Center for Programme Management (CNMP) Instituto de Salud Carlos III (ISCIII) The Swedish Research Council (SRC) (Vetenskapsrådet) The Swedish Governmental Agency for Innovation Systems (VINNOVA ) Swiss National Science Foundation (SNSF) The Scientific and Technological Research Council of Turkey (TUBITAK) Ministry of the Walloon Region: Operational General Directorate for Economy, Employment, and Research (DGOEER) www. euronanomed. net BASQUE REGION (SPAIN) FRANCE GERMANY HUNGARY ICELAND ISRAEL LATVIA LITHUANIA POLAND PORTUGAL ROMANIA SPAIN SWEDEN SWITZERLAND TURKEY WALLOON REGION (BELGIUM) 16

Topics / Funding Topics Diagnostics (incl. Imaging) Targeted drug delivery Regenerative medicine Funding Indicative total funding 12 M € Duration: Up to 3 years Every project partner will receive the funding from his national agency www. euronanomed. net 17

Eligibility criteria Consortium composition 3 – 7 partners per consortium Partners from at least 3 different countries (with a majority of partners from funding member states/regions participating in the call) Partners from at least two of the three categories Ø academic / public research Ø clinical / public health Ø industry Eligibility of partners also depends on national regulation in his/her country! Read carefully the “Country specific information for applicants” Contact your national agency (contact person) www. euronanomed. net 18

Time lines Call open since: March 8 th 2010 Deadline for proposals: 2010, 17: 00 h (Brussels Time) Funding Decision: June 11 th October 2010 Start of Projects: January 2011 www. euronanomed. net 19

How to apply? 1. Euro. Nano. Med Website www. euronanomed. net 20

How to apply? 2. Download Call Text , Guidelines for applicants and Country specific Information www. euronanomed. net 21

How to apply? 3. Contact your national contact point (list in the Country specific Information) 4. Download and fill in the Proposal Template www. euronanomed. net 22
How to apply? 5. Online Submission using the Euro. Nano. Med Submission Tool www. nanomedsubmission. net www. euronanomed. net 23

How to apply? Register by clicking on the link ”to register” on the homepage http: //www. euronanomed. net/*; you will receive an email confirming your registration Accede to the Euro. Nano. Med Electronic Submission System* (Use the username and password you chose for the registration) fill in all the details and upload your proposal document (pdf format only), which you have previously filled in on your computer (see The Proposal Template)*. Submit your proposal : Click on “submit proposal” to confirm your submission; you will receive an email confirming its receipt. Modify your data at any time until the submission deadline. It is strongly recommended to transfer the final version in proper time before this date www. euronanomed. net 24

Expression of Interest Tool Present your project ideas Find partners www. nanomedsubmission. net www. euronanomed. net 25

For more information Euro. Nano. Med Call Office Veneto Nanotech s. c. p. a. Via San Crispino, 106 35129 Padova – Italy : +39 049 7705520 e-mail: calloffice@euronanomed. net National / Regional Contact Points www. euronanomed. net 26

Together we are building Nanomedicine for the future! 27 www. euronanomed. net
1bbe391d0d94acaa087e34cf93085911.ppt