8208e55be7b9a3063c4ec7232b4699ef.ppt
- Количество слайдов: 39

EUropean Best Information through Regional Outcomes in Diabetes The BIRO System Fabrizio Carinci Technical Coordinator EUBIROD Project The BIRO Academy 2 nd Residential Course Brussels, 23 rd-25 th January 2011

EU Council Conclusions June 2006 EU Ministers of Health adopted a set of Health Council Conclusions on the Promotion of Healthy Lifestyles and Prevention of Type 2 diabetes, agreeing that Member States should: • Develop and implement national diabetes framework plans • Improve the collection and reporting of diabetes epidemiological and economic data • Adopt a multi-sectoral, multi-disciplinary approach to managing diabetes • Develop comprehensive diabetes training for all healthcare professionals. • The Conclusions also called upon the European Commission to prioritise diabetes, to promote best practice through networking & exchange between Member States and to facilitate and support European diabetes research.
![Quality of Care in Diabetes [IDF Diabetes Atlas, Fourth Edition, 2009] 2004 -2008: >1, Quality of Care in Diabetes [IDF Diabetes Atlas, Fourth Edition, 2009] 2004 -2008: >1,](https://present5.com/presentation/8208e55be7b9a3063c4ec7232b4699ef/image-3.jpg)
Quality of Care in Diabetes [IDF Diabetes Atlas, Fourth Edition, 2009] 2004 -2008: >1, 500 publications on quality of care • Multicentric data in a single country • Analysis on a single centre • Only N=3 studies comparing quality across countries 1999 -2003: sample of 50% papers: • N=5 internazional studies OECD “Health Care Quality Indicators Project” N=9 diabetes indicators originally identified • N=2 computed: – Annual eye examination, Amputation rates
![Why are international comparisons so difficult? [IDF Diabetes Atlas, Fourth Edition, 2009] “So, why Why are international comparisons so difficult? [IDF Diabetes Atlas, Fourth Edition, 2009] “So, why](https://present5.com/presentation/8208e55be7b9a3063c4ec7232b4699ef/image-4.jpg)
Why are international comparisons so difficult? [IDF Diabetes Atlas, Fourth Edition, 2009] “So, why is it that there is a large number of studies of diabetes care within countries, many based on multiple sites, yet so few international comparisons? The simple answer is lack of consistently applied standards that would enable international comparisons. Standard systems and definitions, applied to comparable populations result in data that can be collected and compared relatively easily. The more unified systems are, the easier these comparisons become. ”
Diabetes Registers: different fruits
Types of Registers “Population-based” “Disease Management” “Specialistic”

Unified model: cathedral or bazaar?

Best Information through Regional Outcomes (BIRO) • Three year project in diabetes funded by the Health Information Strand, Public Health Program, DG-SANCO, European Commission • Start: 1 st December 2005 • Total cost: 1. 2 Mn€ - Total EU contribution: 715, 000€ • Aim: “to provide European health systems with an ad hoc, evidence and population-based diabetes information system” • Seven partners from academia and governmental institutions, sharing an extensive experience in diabetes research/chronic care management
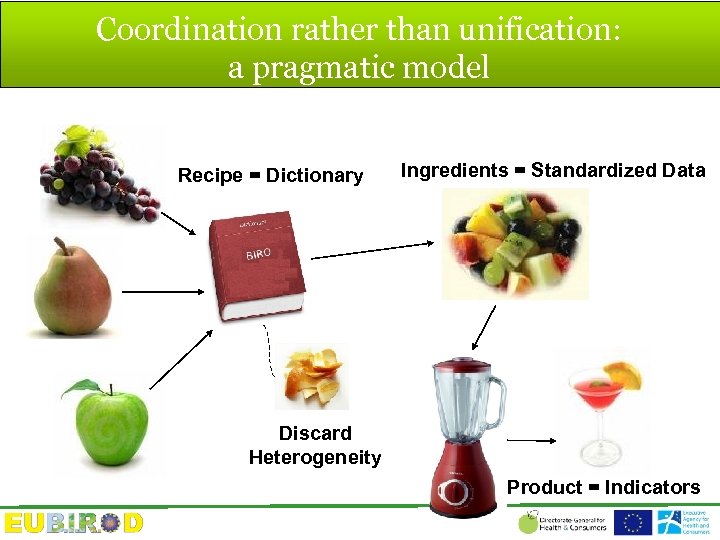
Coordination rather than unification: a pragmatic model Recipe = Dictionary Ingredients = Standardized Data Discard Heterogeneity Product = Indicators
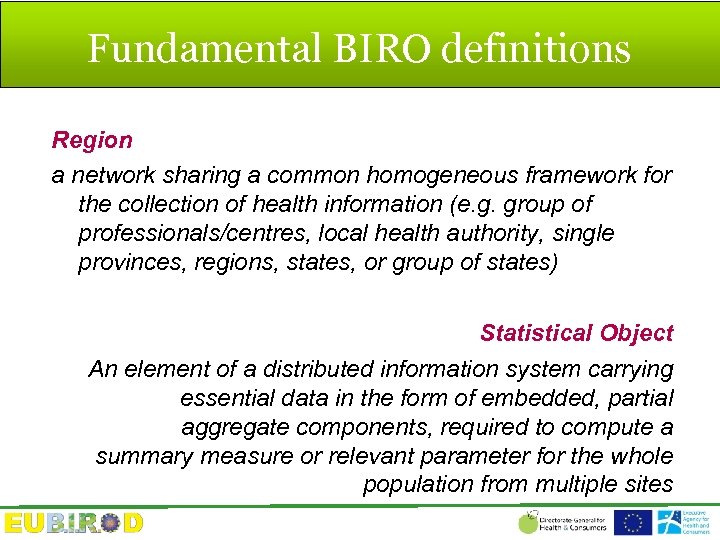
Fundamental BIRO definitions Region a network sharing a common homogeneous framework for the collection of health information (e. g. group of professionals/centres, local health authority, single provinces, regions, states, or group of states) Statistical Object An element of a distributed information system carrying essential data in the form of embedded, partial aggregate components, required to compute a summary measure or relevant parameter for the whole population from multiple sites

BIRO Infrastructure: “Privacy by Design” DI IORIO CT et al, J Med Ethics. 2009 Dec; 35(12): 753 -61.
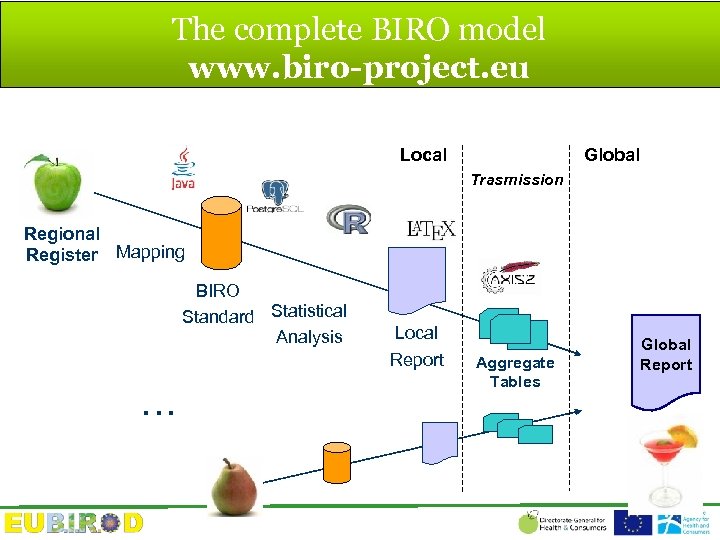
The complete BIRO model www. biro-project. eu Local Global Trasmission Regional Register Mapping BIRO Standard Statistical Analysis . . . Local Report Aggregate Tables Global Report
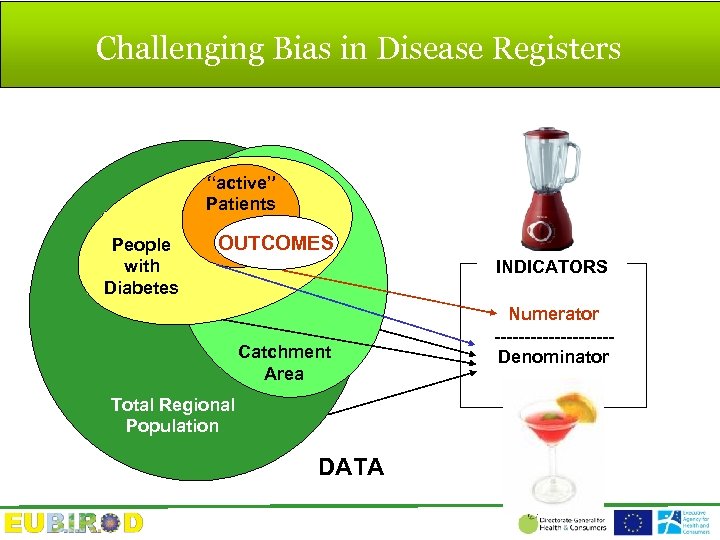
Challenging Bias in Disease Registers “active” Patients People with Diabetes OUTCOMES INDICATORS Catchment Area Total Regional Population DATA Numerator ----------Denominator
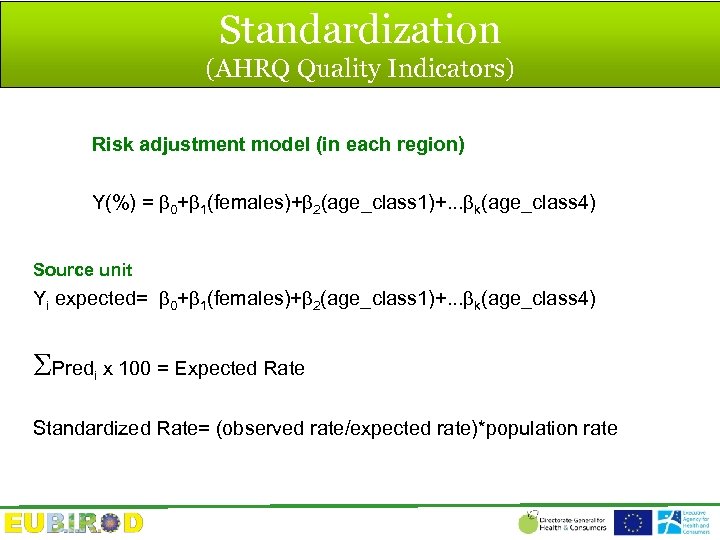
Standardization (AHRQ Quality Indicators) Risk adjustment model (in each region) Y(%) = 0+ 1(females)+ 2(age_class 1)+. . . k(age_class 4) Source unit Yi expected= 0+ 1(females)+ 2(age_class 1)+. . . k(age_class 4) Predi x 100 = Expected Rate Standardized Rate= (observed rate/expected rate)*population rate
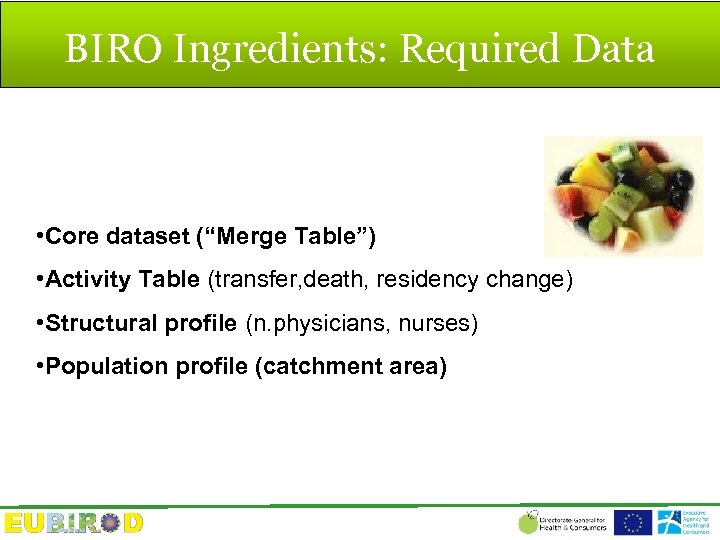
BIRO Ingredients: Required Data • Core dataset (“Merge Table”) • Activity Table (transfer, death, residency change) • Structural profile (n. physicians, nurses) • Population profile (catchment area)

BIRO Core EU Dataset N=48 1. ID Patient 2. ID Centre 3. Type of Diabetes 4. Sex 5. Date of Birth 6. Date of Diagnosis 7. Episode Date 8. Smoking Status 9. N. Cigarettes (x day) 10. Alcohol Intake (g/x day) 11. Weight 12. Height 13. BMI 14. Systolic Blood Pressure 15. Dyastolic Blood Pressure 16. Hb. A 1 c 17. Creatinine 18. Microalbumin 19. Total Cholesterol 20. HDL 21. Tryglicerides 22. Eye Examination 23. Retinopathy Status 24. Maculopathy Status 25. Foot Examination 26. Foot Pulses 27. Foot vibration 28. End Stage Renal Failure 29. Renal Dyalisis 30. Renal Transplant 31. Stroke 32. Foot Ulceration 33. Acute Myocardial Infarction 34. Laser 35. Hypertension 36. Blindness 37. Amputation 38. Antihypertensive Medication 39. Hypoglicemic Drug Therapy 40. Oral Drug Therapy 41. Pump Therapy 42. Nasal Therapy 43. Average Injections (x day) 44. Self monitoring 45. Diabetes Specific Education 46. Lipid Lowering Therapy 47. Anti-platelet Therapy 48. Patient enrollment in DMP for diabetes
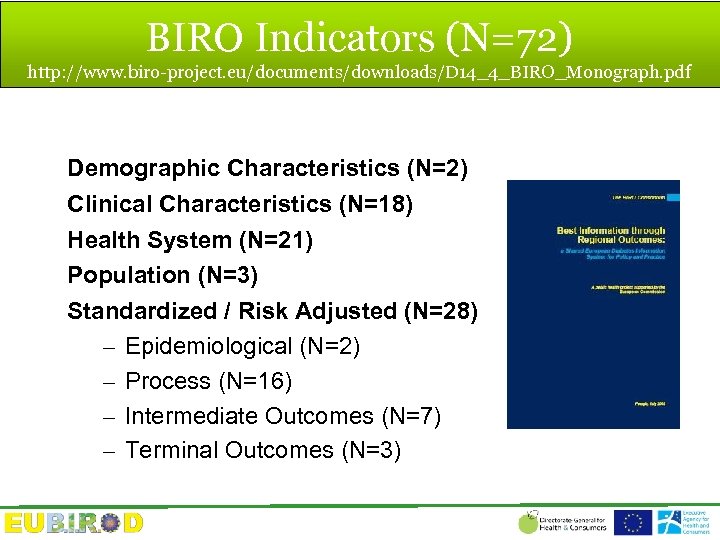
BIRO Indicators (N=72) http: //www. biro-project. eu/documents/downloads/D 14_4_BIRO_Monograph. pdf Demographic Characteristics (N=2) Clinical Characteristics (N=18) Health System (N=21) Population (N=3) Standardized / Risk Adjusted (N=28) – Epidemiological (N=2) – Process (N=16) – Intermediate Outcomes (N=7) – Terminal Outcomes (N=3)
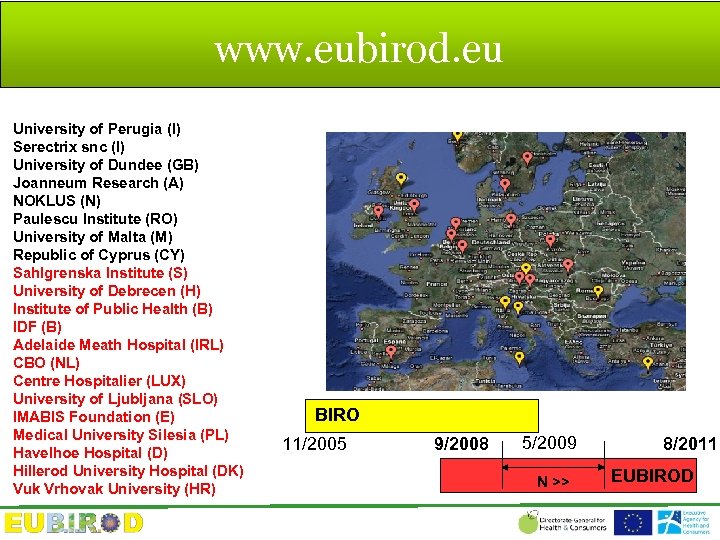
www. eubirod. eu University of Perugia (I) Serectrix snc (I) University of Dundee (GB) Joanneum Research (A) NOKLUS (N) Paulescu Institute (RO) University of Malta (M) Republic of Cyprus (CY) Sahlgrenska Institute (S) University of Debrecen (H) Institute of Public Health (B) IDF (B) Adelaide Meath Hospital (IRL) CBO (NL) Centre Hospitalier (LUX) University of Ljubljana (SLO) IMABIS Foundation (E) Medical University Silesia (PL) Havelhoe Hospital (D) Hillerod University Hospital (DK) Vuk Vrhovak University (HR) BIRO 11/2005 9/2008 5/2009 N >> 8/2011 EUBIROD

EUBIROD: complete, refine, measure, disseminate http: //www. eubirod. eu/academy
Privacy Performance Self-Evaluation

e. learning platform
BIROX: Ubuntu Linux Virtualized distribution
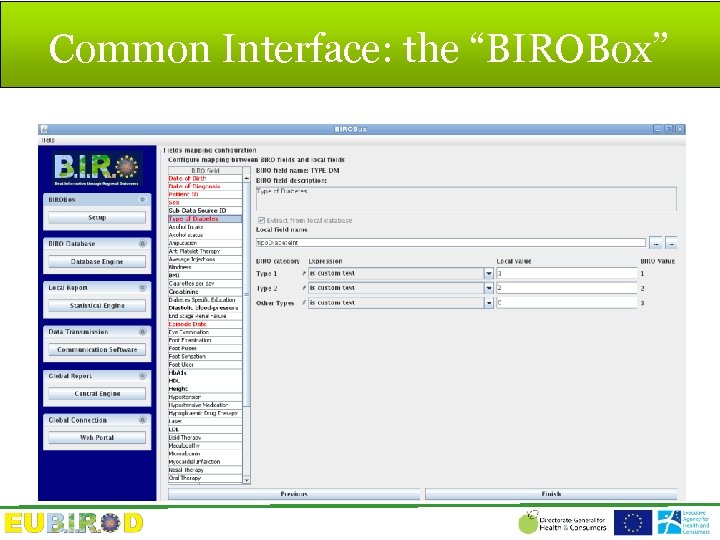
Common Interface: the “BIROBox”
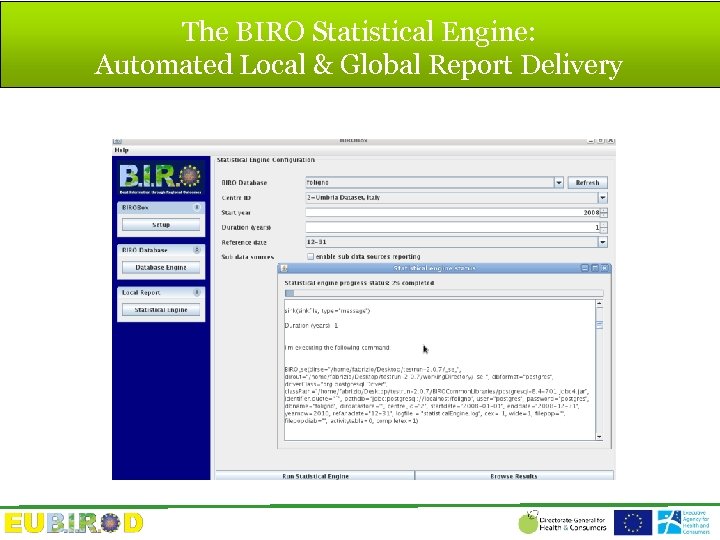
The BIRO Statistical Engine: Automated Local & Global Report Delivery

Report Delivery • Outputs are produced in html and pdf formats, together with a very large number of component files that can be conveniently reused in customized web portals

Structure of the Report
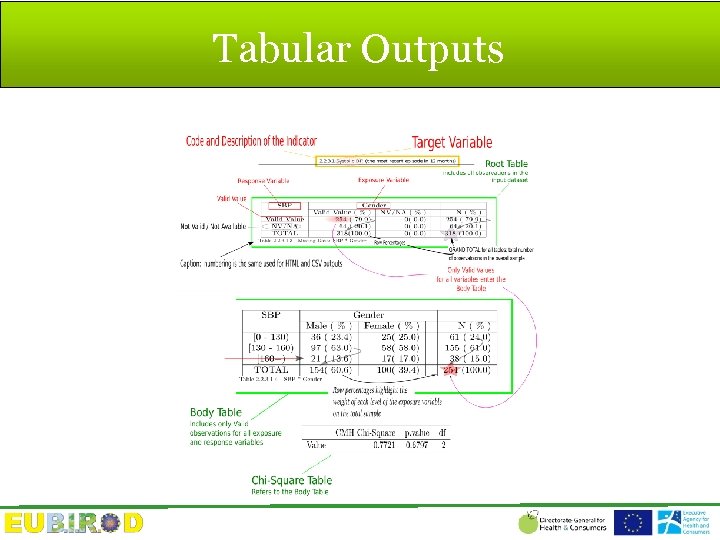
Tabular Outputs
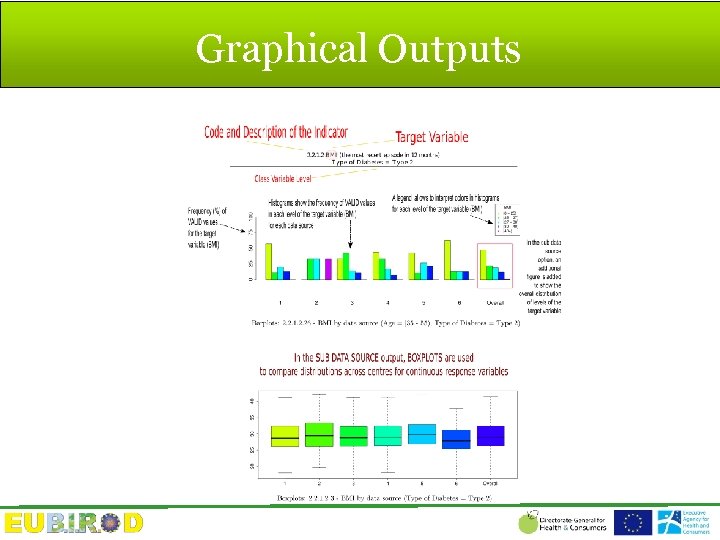
Graphical Outputs
Statistical Objects Data
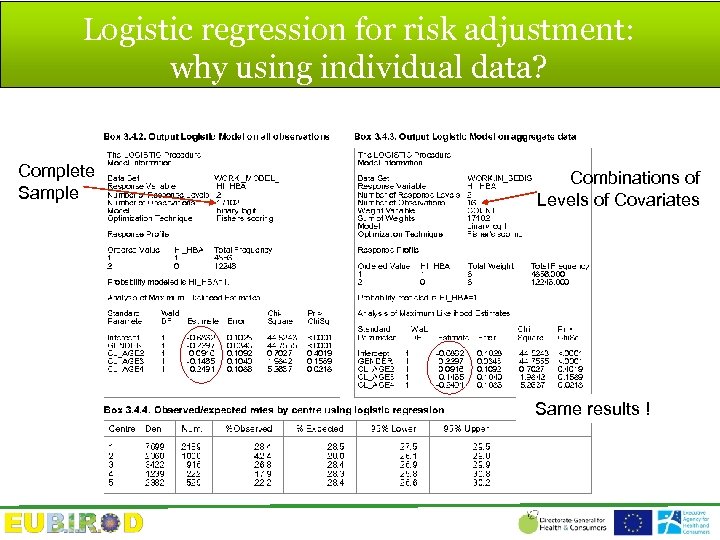
Logistic regression for risk adjustment: why using individual data? Complete Sample Combinations of Levels of Covariates Same results !
Standardization outputs

European Diabetes Report 2010 Data Sources from 17 countries; N=138, 153
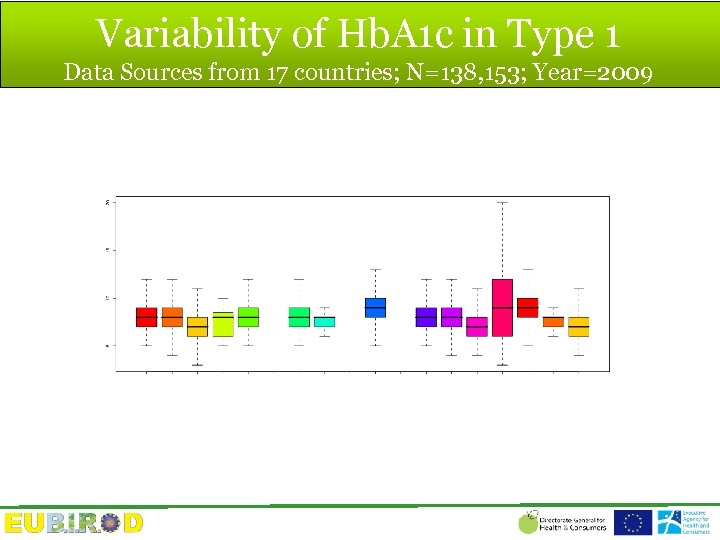
Variability of Hb. A 1 c in Type 1 Data Sources from 17 countries; N=138, 153; Year=2009
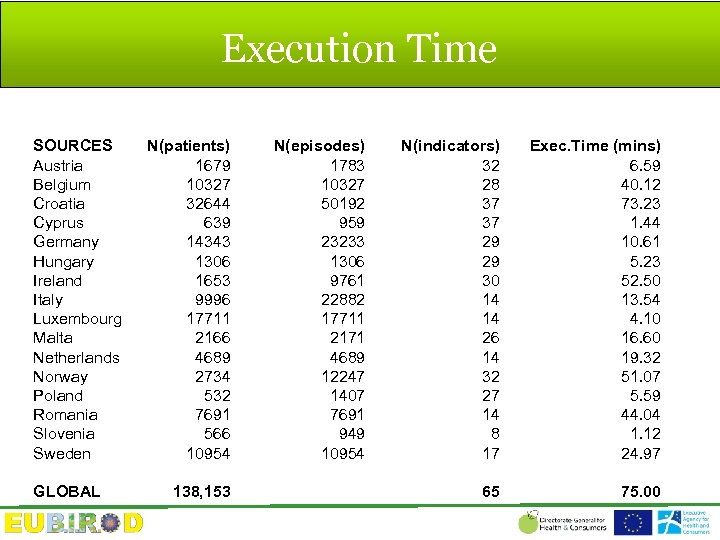
Execution Time SOURCES Austria Belgium Croatia Cyprus Germany Hungary Ireland Italy Luxembourg Malta Netherlands Norway Poland Romania Slovenia Sweden GLOBAL N(patients) 1679 10327 32644 639 14343 1306 1653 9996 17711 2166 4689 2734 532 7691 566 10954 138, 153 N(episodes) 1783 10327 50192 959 23233 1306 9761 22882 17711 2171 4689 12247 1407 7691 949 10954 N(indicators) 32 28 37 37 29 29 30 14 14 26 14 32 27 14 8 17 Exec. Time (mins) 6. 59 40. 12 73. 23 1. 44 10. 61 5. 23 52. 50 13. 54 4. 10 16. 60 19. 32 51. 07 5. 59 44. 04 1. 12 24. 97 65 75. 00
Web Portal

The BIRO System - Dissemination • • • Privacy protective infrastructure published in the Journal of Medical Ethics 2009 Ranked among the six best European Public Health projects at European Health Forum Gastein 2009 Podium Presentation at the IDF World Congress Montreal 2009, Academy Health Research Meeting Boston 2010, European Public Health Conference Amsterdam 2010

Conclusions (1) • • • The BIRO system is a multilevel initiative aimed at linking an improved capacity to collect high quality data with the direct ability of providing sustainable input for policy making Privacy by design and cutting edge information technology allow sharing information from very large databases without altering local practice The delivery of fully automated reports on quality and outcomes in diabetes paves the way for a whole range of applications, spanning from research to routine public health surveillance

Conclusions (2) • • The deployment of the BIRO system, fully open source and entirely available in the public domain, will allow international users to join European partners in the construction of collaborative networks BIRO may represent a valid instrument for the concrete construction of a global system of comparable quality indicators in diabetes
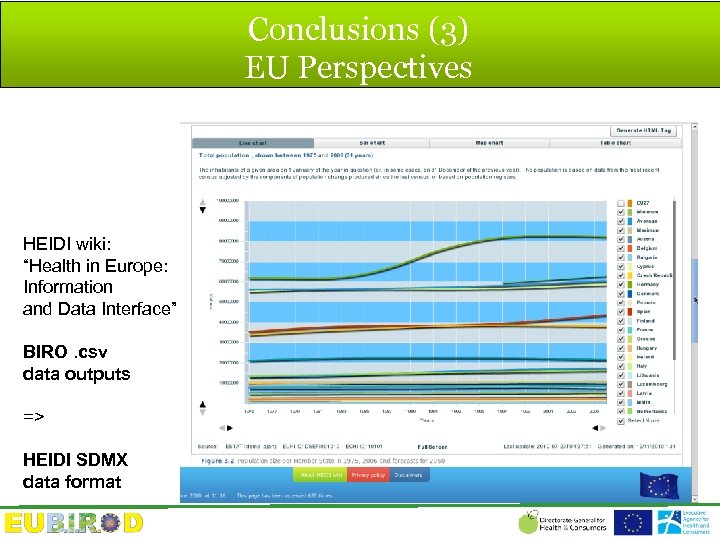
Conclusions (3) EU Perspectives HEIDI wiki: “Health in Europe: Information and Data Interface” BIRO. csv data outputs => HEIDI SDMX data format
8208e55be7b9a3063c4ec7232b4699ef.ppt