4bd281a014e12a8b4c72c921720ec6d3.ppt
- Количество слайдов: 50

EU Hygiene Regulations Food & Drug Administration Bangkok, Thailand 3 rd April 2007 Dorothy Guina-Dornan © 2005

Overview • New legislative framework • • • Part II Regulation (EC) 852/2004: General Hygiene Rules Regulation (EC) 2073/2005: Microbiological criteria • Regulation (EC) 853/2004: Hygiene Rules – Animal origin • Supporting documentation • Key elements for the Food & Drug Administration, Thailand © 2005
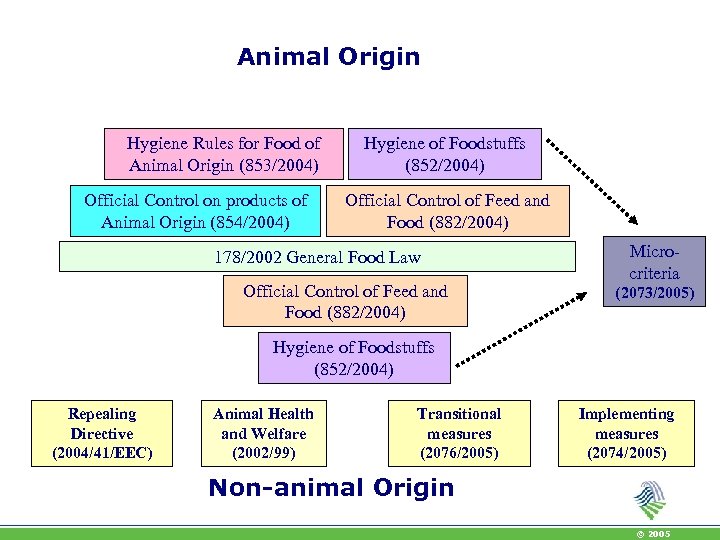
Animal Origin Hygiene Rules for Food of Animal Origin (853/2004) Official Control on products of Animal Origin (854/2004) Hygiene of Foodstuffs (852/2004) Official Control of Feed and Food (882/2004) 178/2002 General Food Law Official Control of Feed and Food (882/2004) Microcriteria (2073/2005) Hygiene of Foodstuffs (852/2004) Repealing Directive (2004/41/EEC) Animal Health and Welfare (2002/99) Transitional measures (2076/2005) Implementing measures (2074/2005) Non-animal Origin © 2005
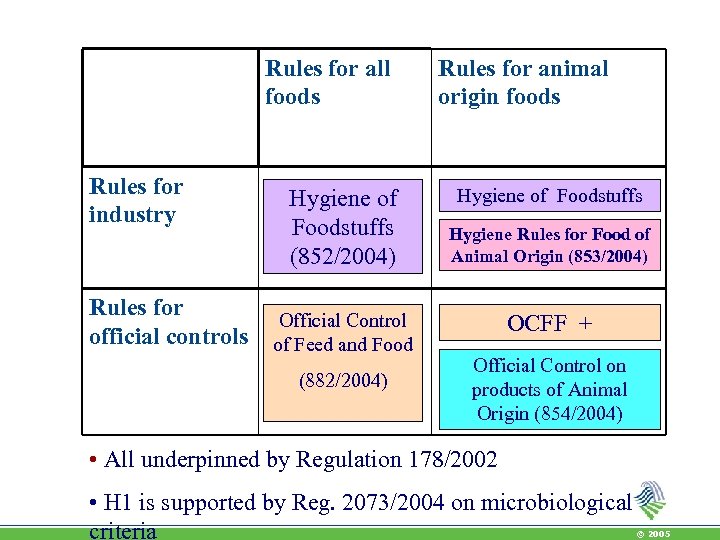
Rules for all foods Rules for industry Rules for official controls Hygiene of Foodstuffs (852/2004) Official Control of Feed and Food (882/2004) Rules for animal origin foods Hygiene of Foodstuffs Hygiene Rules for Food of Animal Origin (853/2004) OCFF + Official Control on products of Animal Origin (854/2004) • All underpinned by Regulation 178/2002 • H 1 is supported by Reg. 2073/2004 on microbiological criteria © 2005
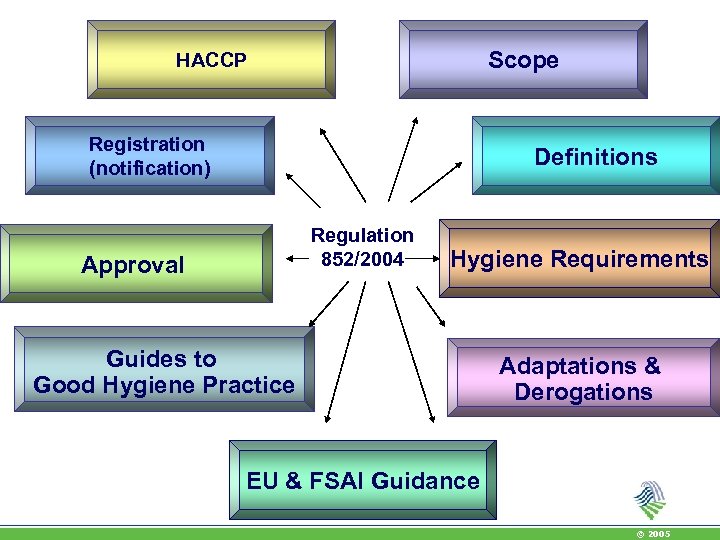
Scope HACCP Registration (notification) Definitions Regulation 852/2004 Approval Hygiene Requirements Guides to Good Hygiene Practice Adaptations & Derogations EU & FSAI Guidance © 2005

Regulation 852/2004: Hygiene of Foodstuffs • All food safety practitioners will enforce the Regulation on the Hygiene of Foodstuffs • Applies to all stages of food and feed chain • Food business operators obligations • Guides to good hygiene practice • HACCP – all non primary producers, all principles • Registration of all premises, approval of some • Microbiological criteria, fixing maximum levels • Temperature control requirements • Import control - 178/2002 • Annexes with specific hygiene rules © 2005

Regulation 852/2004 • Prerequisites: Articles 3 and 4 and Annex II • HACCP: Article 5 • Registration: Article 6 • Guides to good hygiene practice: Article 7 • EU guidance on certain provisions of Regulation 852/2004 • EU guidance on Implementation procedures based on the HACCP principles © 2005
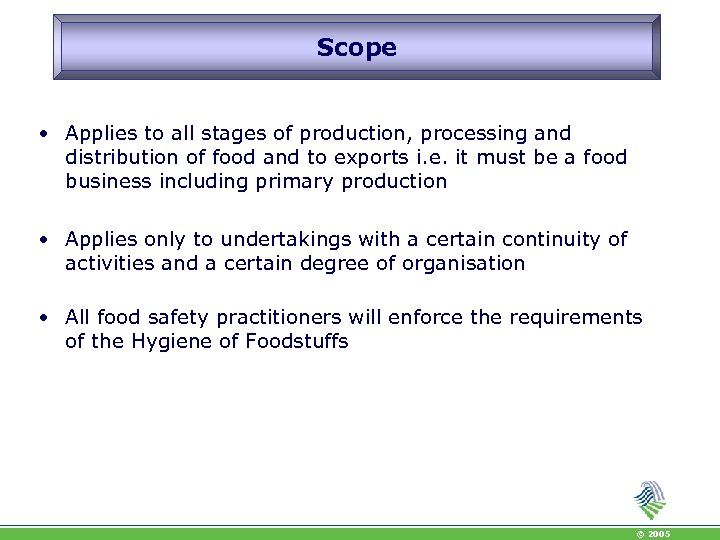
Scope • Applies to all stages of production, processing and distribution of food and to exports i. e. it must be a food business including primary production • Applies only to undertakings with a certain continuity of activities and a certain degree of organisation • All food safety practitioners will enforce the requirements of the Hygiene of Foodstuffs © 2005

Regulation 852/2004 • Exemptions • Occasional preparation of food i. e. where there is no “certain continuity of activities” or “certain degree of organisation” (Note: 178/2002 applies) • Primary production for private domestic use • Domestic preparation, handling or storage for private domestic use • • Direct supply, by the producer, of small quantities of primary products to the final consumer or to local retail establishments © 2005

Hygiene Requirements: Prerequisites Article 3: Food business operators’ obligations • Food business operators must ensure that all stages of production, processing and distribution of food under their control satisfy the relevant hygiene requirements laid down in this Regulation © 2005

Hygiene Requirements: Prerequisites Article 4: General and specific hygiene requirements • Primary producers must comply with Annex I and specific requirements of Regulation 853/2004 • All other food business operators must comply with the general hygiene requirements laid down in Annex II • Microbiological criteria • Targets set to achieve objectives • Temperature control requirements • Maintenance of the cold chain • Sampling and analysis © 2005
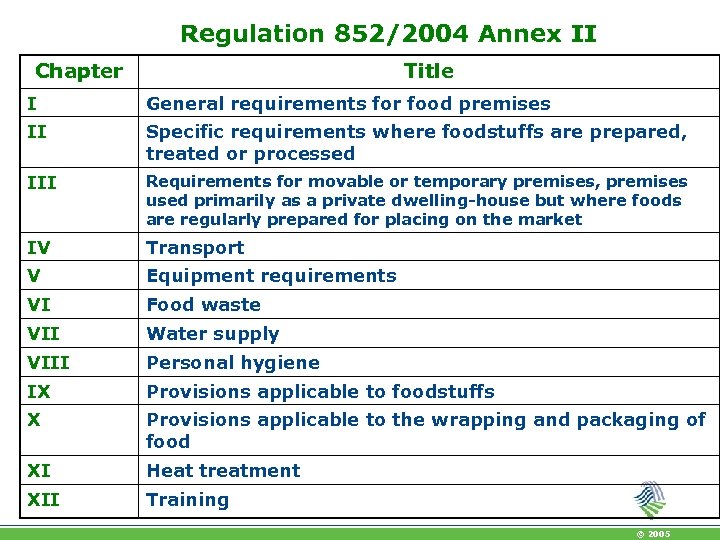
Regulation 852/2004 Annex II Chapter Title I General requirements for food premises II Specific requirements where foodstuffs are prepared, treated or processed III Requirements for movable or temporary premises, premises used primarily as a private dwelling-house but where foods are regularly prepared for placing on the market IV Transport V Equipment requirements VI Food waste VII Water supply VIII Personal hygiene IX Provisions applicable to foodstuffs X Provisions applicable to the wrapping and packaging of food XI Heat treatment XII Training © 2005
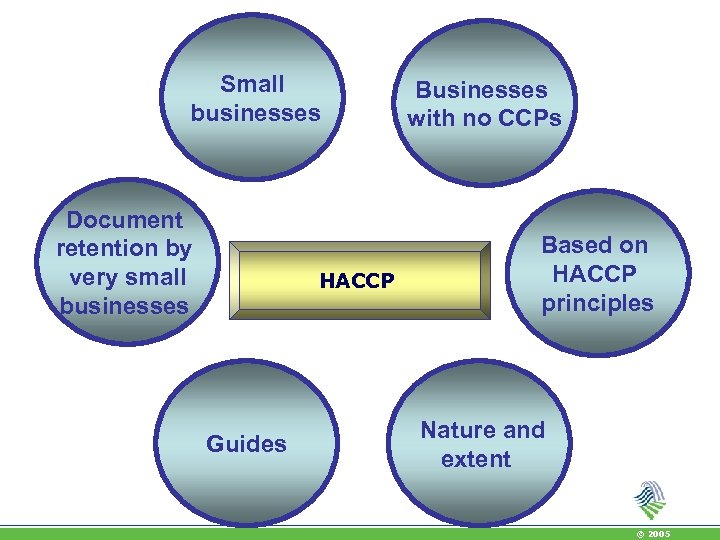
Small businesses Document retention by very small businesses HACCP Guides Businesses with no CCPs Based on HACCP principles Nature and extent © 2005

HACCP Requirement: Article 5 (1) ‘Food business operators shall put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles. ’ = shall have a system based on HACCP principles © 2005

7 HACCP Principles Simplified • Conduct Hazard Analysis • Identify critical control points • Set critical control limits • Monitor critical control points • What in my food could harm my customers? • Which steps are most important to ensure I prevent the harm? • What are the key things to control in these important steps? • How do I know they are controlled? • Establish corrective actions • What do I do if they are not in control? • Verify the HACCP plan • Document the HACCP plan • Will the plan prevent harm to my customers? • How do I show an inspector that I am being proactive and how do I introduce new staff to the plan? © 2005

Additionally Article 5 (2) emphasises the need to review ‘When any modification is made in the product, process, or any step, food business operators shall review the procedure and make the necessary changes to it. ’ © 2005

Exemption for primary producers: Article 5 (3) ‘Paragraph 1 shall apply only to food business operators carrying out any stage of production, processing and distribution of food after primary production and those associated operations listed in Annex I. ’ = HACCP does not apply to primary producers and related activities listed in Annex I © 2005

Evidence of compliance: Article 5 (4) Food business operators must: (a) provide evidence of their compliance with paragraph 1 in the manner that the competent authority requires, allowing for the nature and size of the food business (b) ensure documents describing the procedures developed are up-to-date at all times; (c) retain any other documents and records for an appropriate period. © 2005
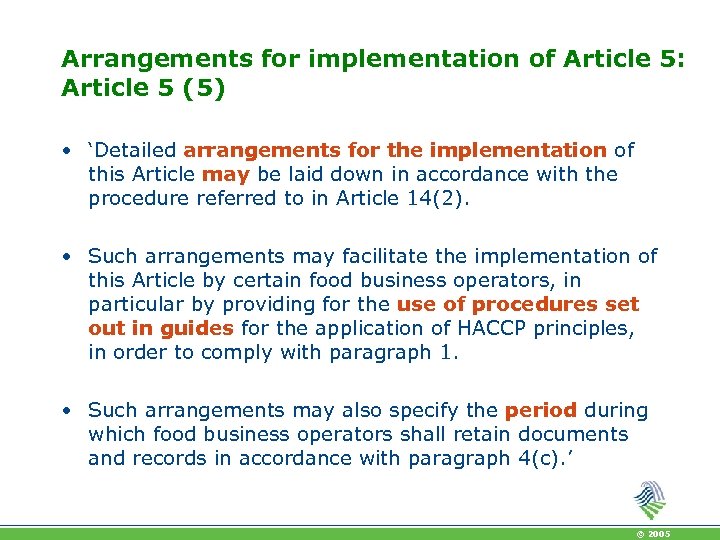
Arrangements for implementation of Article 5: Article 5 (5) • ‘Detailed arrangements for the implementation of this Article may be laid down in accordance with the procedure referred to in Article 14(2). • Such arrangements may facilitate the implementation of this Article by certain food business operators, in particular by providing for the use of procedures set out in guides for the application of HACCP principles, in order to comply with paragraph 1. • Such arrangements may also specify the period during which food business operators shall retain documents and records in accordance with paragraph 4(c). ’ © 2005

© 2005

http: //www. fsai. ie/legislation/food/eu_docs/Food_hygiene/EU_Guidance_HACCP. pdf © 2005

The Scenarios/Options: 1. Businesses where hazards are controlled by good hygiene practices (GHP)/prerequisite hygiene requirements (PRPs) i. ‘Presumed’ to be the case due to the nature of the business or ii. Demonstrated by the business’ own Hazard Analysis 2. Businesses which following a sector specific guide to good practice which has already applied the principles of HACCP 3. Businesses which develop own HACCP system © 2005

Hazard Analysis • Formal hazard analysis not needed where it is obvious that GHP is sufficient to control hazards • Such businesses should be advised to follow a guide to good practice • For certain categories of businesses the hazard analysis may be done for them in generic HACCP guides, i. e. in businesses where: • there is a lot of commonality • the process is linear • the hazard prevalence is high • E. g. pasteurisation of liquid food or freezing/quick freezing of food © 2005

Businesses where hazards are controlled by GHP (prerequisites) alone • In particular in food businesses where there is no preparation, manufacturing or processing of food • Simple food preparation operations • (such as the slicing of food, bar, small shop) • Where necessary, it must be ensured that the necessary monitoring and verification (and possibly record keeping) are carried out • e. g where the cold chain must be maintained. © 2005

Guides to good practice • May help food businesses demonstrate compliance • Applied by any food sector, where procedures that are well known are used such as: • Restaurants, including food handling facilities on board means of transport such as vessels, • Catering sectors dispatching prepared food from a central establishment, • The bakery and confectionary sector, • Retail shops, including butcher shops. to control hazards and © 2005

Guides to good practice • It may suffice that the guides describe in a practical and simple way the methods to control hazards • Cover all significant hazards in a business and define procedures to control these hazards and the corrective action to be taken. • Highlight the possible hazards linked to certain food (e. g. possible presence of Salmonella in raw eggs ), and • Methods to control food contamination (e. g. the purchase of raw eggs from a reliable source and time/temperature combinations for processing). © 2005

Critical Limits • Can be established on the basis of • Experience (best practice) • International documentation for a number of operations, e. g. canning of food, pasteurisation of liquids etc. for which internationally accepted standards (Codex Alimentarius) exist • In a guide to good practice • Do not need to be numerical e. g. • boiling of liquid food • the change of physical properties of food during processing (e. g. cooking) © 2005

Alternative Critical Limits • Use of alternative critical limits to those in the approved national guides is permitted providing the business can demonstrate that the alternative approach ensures the same level of food safety • A business can demonstrate by: • Obtaining critical limit from a reputable source • Conducting own experiments © 2005
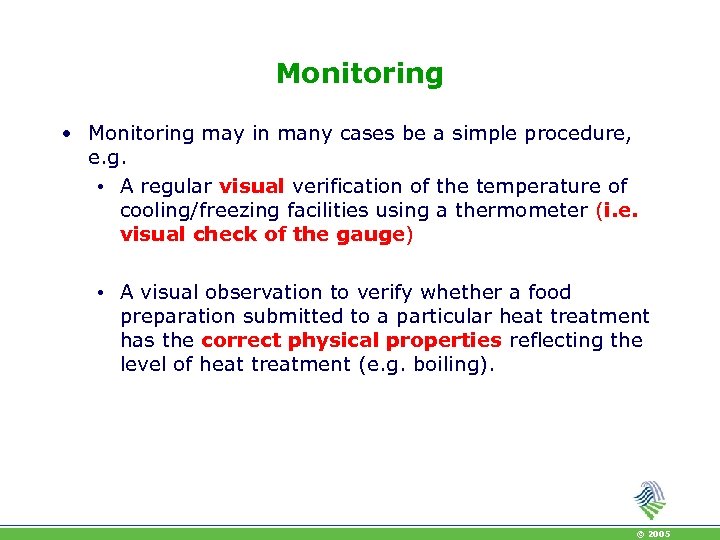
Monitoring • Monitoring may in many cases be a simple procedure, e. g. • A regular visual verification of the temperature of cooling/freezing facilities using a thermometer (i. e. visual check of the gauge) • A visual observation to verify whether a food preparation submitted to a particular heat treatment has the correct physical properties reflecting the level of heat treatment (e. g. boiling). © 2005
Monitoring • Certain foods may sometimes be processed in a standard way using standard calibrated equipment, e. g. certain cooking operations, roasting chicken etc. • In such cases the cooking temperature of the product need not be systematically measured as long as it is ensured that • the equipment is functioning properly, • the required time/temperature combination is respected and • the necessary controls for that purpose are carried out (and corrective action taken where necessary). • In restaurants, food is prepared in accordance with well established culinary procedures. This implies that measurements (e. g. food temperature measurements) need not be carried out systematically as long as the established procedures are followed. © 2005

© 2005

Registration of a food business • All food business operators must notify the competent authority of each establishment under its control • Provide the competent authority with up to date information including any significant change in activity and any closure of an existing establishment © 2005

Approval • Provision set out in 852/2004 and 882/2004 • A risk-based approval system may be introduced under national regulations • On receipt of an application an on-site visit is required • Approval is contingent on compliance with food law • Conditional approval • subject to infrastructure and equipment requirements being met • 2 x 3 months • Suspension and revocation © 2005

Guides to Good Hygiene Practice • Member States must encourage the development and use of guides to good hygiene practice • Voluntary • Developed and disseminated by the food business sectors • In consultation with all stakeholders • Have regard to Codex • May be developed through national standards institutes • Member States must assess the guides and notify the European Commission who will set up a register of guides © 2005

Essential considerations when developing a guide • • • All stakeholders should be involved Consensus achieved Guides should be practical and user friendly Address compliance with local food safety requirements Account should be taken of practical concerns of small business • Account should be taken of local and cultural customs and practices • Review periodically, when new significant hazards emerge or changes in legislation • Possibly 3 rd party certification © 2005
Regulation 853/2004 • Exemptions • Composite products • Food containing both products of plant origin and processed products of animal origin • Retail • Activities involving direct sale or supply by producers of small quantities of food of animal origin to the final consumer • Wholesale • Storage and transport only, where temperature requirements in 853/20004 will apply • Local, restricted, marginalised • EU Guidance on the implementation of certain provisions of Regulation 853/2004 © 2005

Hygiene of Foods of Animal Origin These are in addition to H 1 • Specific rules for certain foods i. e. meat, meat products, fish and dairy products • Defines “unprocessed” and “processed” products • Approval of premises and approval number to ensure only approved premises are allowed to place product on the market • Health and identification marking • Approval number to follow product through food chain • Food business operators obligations • Trade, import control © 2005

Hygiene of Foods of Animal Origin • Annexes – definitions, requirements concerning several products of animal origin • Specific rules – meat, poultry, farmed game, wild game, minced meat and meat preparations, MSM, meat products, live bivalve molluscs, fishery products, raw milk and dairy products, eggs and egg products, frogs legs and snails, rendered animal fats and greaves, treated stomachs, bladders and intestines and gelatine © 2005

Regulation 2073/2005 on Micro - criteria • Regulation on microbiological criteria for foodstuffs • Food business operators own checks • Official control • Maximum limits for undesirable micro-organisms in foodstuffs after risk assessment • Lays down micro-criteria referred to in Regulation 852/2004 • Linked to Regulation 178/2002 • Safety of foodstuffs is mainly ensured through good hygiene practice and HACCP • Micro criteria may be used to validate control measures and verify the correct functioning of these procedures © 2005
Microbiological Criterion Defines the acceptability of a product, a batch of foodstuffs or a process based on: absence, presence or a number of microorganisms and/or quantity of their toxins/metabolites per unit of mass, volume, area or batch © 2005

Types of criteria 1. Food safety criteria • Defines the acceptability of product or a batch of foodstuffs • Applicable to products placed on the market • Unsatisfactory results: product recall / withdrawal 2. Process hygiene criteria • Indicates the acceptable functioning of the production process • Applicable to products at a specified stages during their production process • Unsatisfactory results: Improvements in hygiene practices © 2005
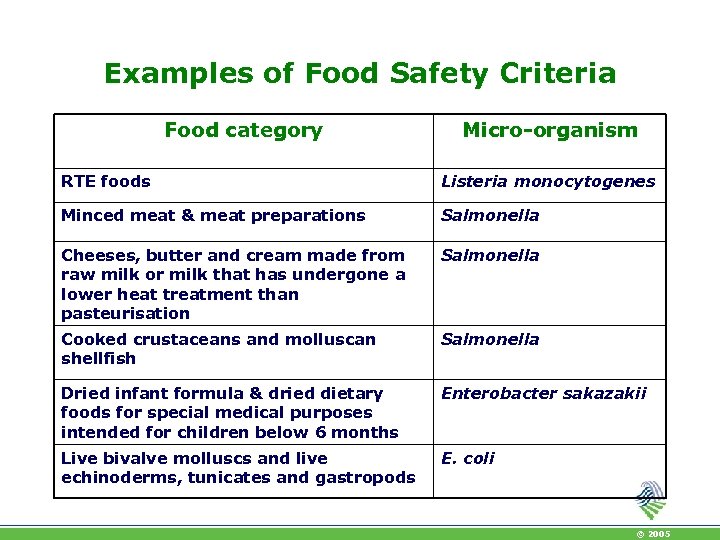
Examples of Food Safety Criteria Food category Micro-organism RTE foods Listeria monocytogenes Minced meat & meat preparations Salmonella Cheeses, butter and cream made from raw milk or milk that has undergone a lower heat treatment than pasteurisation Salmonella Cooked crustaceans and molluscan shellfish Salmonella Dried infant formula & dried dietary foods for special medical purposes intended for children below 6 months Enterobacter sakazakii Live bivalve molluscs and live echinoderms, tunicates and gastropods E. coli © 2005
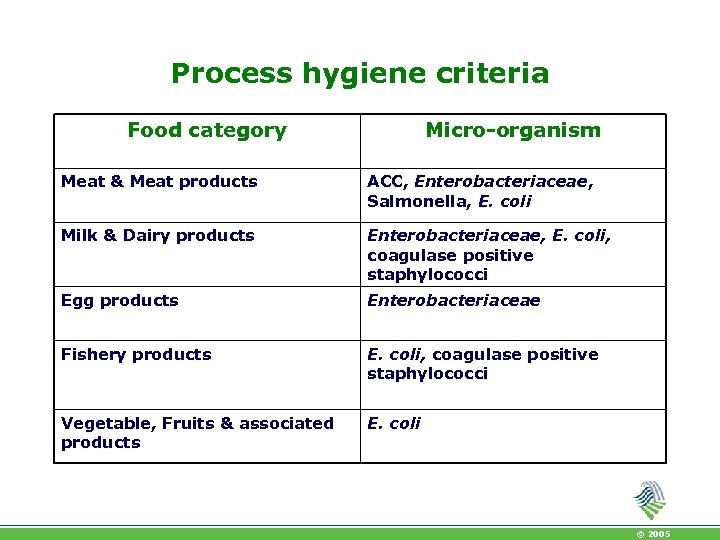
Process hygiene criteria Food category Micro-organism Meat & Meat products ACC, Enterobacteriaceae, Salmonella, E. coli Milk & Dairy products Enterobacteriaceae, E. coli, coagulase positive staphylococci Egg products Enterobacteriaceae Fishery products E. coli, coagulase positive staphylococci Vegetable, Fruits & associated products E. coli © 2005

In Regulation 2073/2005 microbiological criteria are specified for the following food categories: 1. Meat & meat products 2. 3. 4. 5. 6. Milk & dairy products Fish & fishery products Egg products Vegetables, fruits & associated products Ready-To-Eat (RTE) Foods © 2005

Who must comply with the Regulation? Food Business Operators (FBOs): i. e. all FBOs involved in the: • processing • manufacturing • handling and • distribution of food (this includes retailers and caterers) Legal basis: Regulation 852/2004 (H 1) Competent Authorities: Must verify that FBOs are complying with the Regulation Legal basis: Regulation 882/2004 © 2005

Testing against the criteria * Traditional Approach: End product testing * New Approach: Validation & verification of HACCP based procedures * FBOs must ensure that: Process hygiene criteria & Food safety criteria are met © 2005
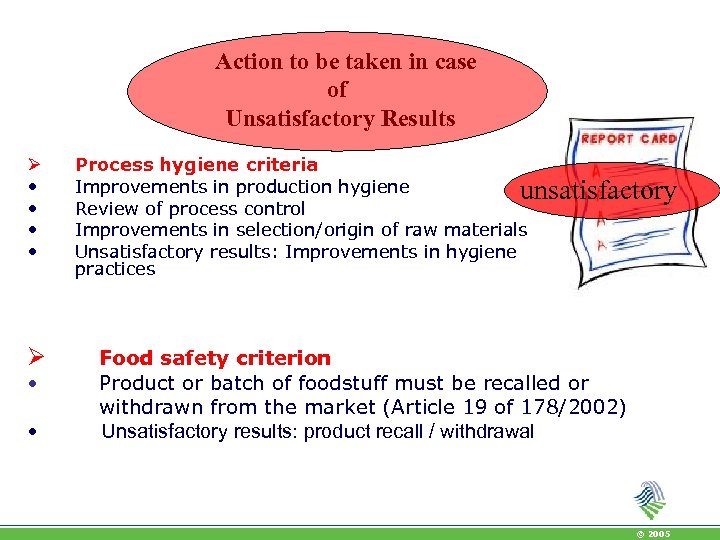
Action to be taken in case of Unsatisfactory Results Ø • • Process hygiene criteria Improvements in production hygiene unsatisfactory Review of process control Improvements in selection/origin of raw materials Unsatisfactory results: Improvements in hygiene practices Food safety criterion Product or batch of foodstuff must be recalled or withdrawn from the market (Article 19 of 178/2002) Unsatisfactory results: product recall / withdrawal © 2005
Analysis of trend Environmental sampling Shelf life studies © 2005

How can compliance be verified? • Sampling & testing NOTE: Sampling & testing is only one means of verifying compliance • Monitoring & surveillance • Audits & inspections © 2005

EU Guidance on … 1. 2. 3. 4. 5. 6. Sampling plan and frequency Transport of samples, storage and starting analysis Requirements for official laboratories Methods of analysis Interpretation of test results Samples for supplementary opinion 7. EU Guidance Document on official controls under Regulation (EC) No 882/2004 concerning microbiological sampling and testing of foodstuffs © 2005
4bd281a014e12a8b4c72c921720ec6d3.ppt