770cf82a1f6a725a0ab479f48a66ef52.ppt
- Количество слайдов: 17
 ® Ethyol Non-Small Cell Lung Cancer Indication ODAC Meeting March 12, 2003 Med. Immune Oncology, Inc.
® Ethyol Non-Small Cell Lung Cancer Indication ODAC Meeting March 12, 2003 Med. Immune Oncology, Inc.
 Outline of Presentation n n 2 Amifostine Accelerated approval in NSCLC Outcome of Phase III study in NSCLC Continuing post-accelerated approval obligation Med. Immune Oncology, Inc.
Outline of Presentation n n 2 Amifostine Accelerated approval in NSCLC Outcome of Phase III study in NSCLC Continuing post-accelerated approval obligation Med. Immune Oncology, Inc.
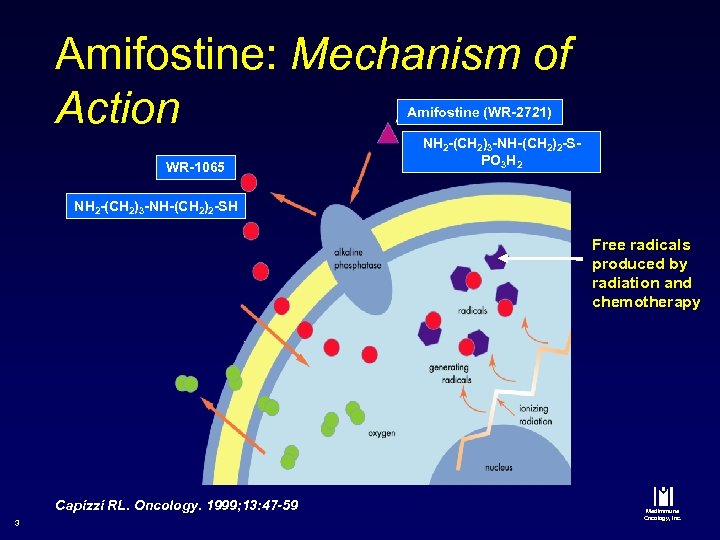 Amifostine: Mechanism of Action Amifostine (WR-2721) WR-1065 NH 2 -(CH 2)3 -NH-(CH 2)2 -SPO 3 H 2 NH 2 -(CH 2)3 -NH-(CH 2)2 -SH Free radicals produced by radiation and chemotherapy Capizzi RL. Oncology. 1999; 13: 47 -59 3 Med. Immune Oncology, Inc.
Amifostine: Mechanism of Action Amifostine (WR-2721) WR-1065 NH 2 -(CH 2)3 -NH-(CH 2)2 -SPO 3 H 2 NH 2 -(CH 2)3 -NH-(CH 2)2 -SH Free radicals produced by radiation and chemotherapy Capizzi RL. Oncology. 1999; 13: 47 -59 3 Med. Immune Oncology, Inc.
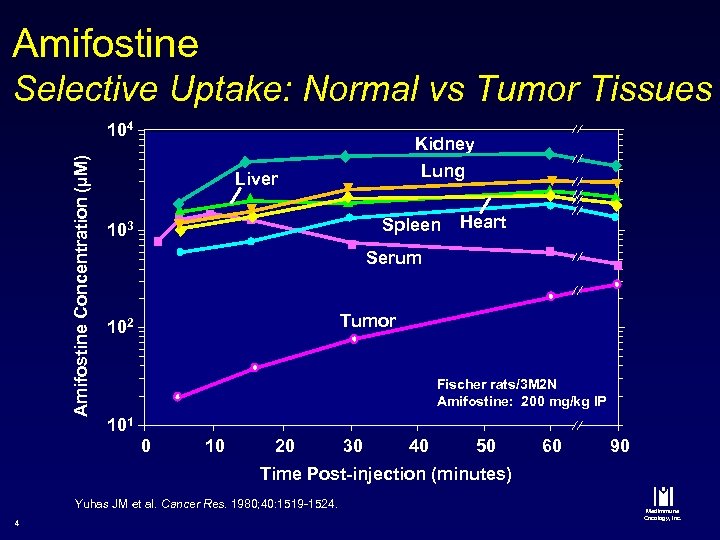 Amifostine Selective Uptake: Normal vs Tumor Tissues Amifostine Concentration (µM) 104 Kidney Lung Liver Spleen 103 Heart Serum Tumor 102 Fischer rats/3 M 2 N Amifostine: 200 mg/kg IP 101 0 10 20 30 40 50 60 90 Time Post-injection (minutes) Yuhas JM et al. Cancer Res. 1980; 40: 1519 -1524. 4 Med. Immune Oncology, Inc.
Amifostine Selective Uptake: Normal vs Tumor Tissues Amifostine Concentration (µM) 104 Kidney Lung Liver Spleen 103 Heart Serum Tumor 102 Fischer rats/3 M 2 N Amifostine: 200 mg/kg IP 101 0 10 20 30 40 50 60 90 Time Post-injection (minutes) Yuhas JM et al. Cancer Res. 1980; 40: 1519 -1524. 4 Med. Immune Oncology, Inc.
 Ethyol Indications n n 5 Prevention of xerostomia from radiation therapy in post-operative patients with Head and Neck cancer Reduction of cumulative renal toxicity associated with cisplatin in advanced ovarian cancer Med. Immune Oncology, Inc.
Ethyol Indications n n 5 Prevention of xerostomia from radiation therapy in post-operative patients with Head and Neck cancer Reduction of cumulative renal toxicity associated with cisplatin in advanced ovarian cancer Med. Immune Oncology, Inc.
 Ethyol Accelerated Approval n Prevention of cisplatin nephrotoxicity in NSCLC (1996) è n Phase II (n=25) Phase III trial required for full approval Demonstration of nephroprotection è Lack of tumor protection è 6 Med. Immune Oncology, Inc.
Ethyol Accelerated Approval n Prevention of cisplatin nephrotoxicity in NSCLC (1996) è n Phase II (n=25) Phase III trial required for full approval Demonstration of nephroprotection è Lack of tumor protection è 6 Med. Immune Oncology, Inc.
 Post-Approval Study (WR-0053) n n Phase III randomized controlled trial Stage IIIB or IV NSCLC (n=366) Cisplatin and Vinblastine ± Amifostine Endpoint: è 7 No reduction in anti-tumor efficacy with a reduction in cisplatin-related nephrotoxicity Med. Immune Oncology, Inc.
Post-Approval Study (WR-0053) n n Phase III randomized controlled trial Stage IIIB or IV NSCLC (n=366) Cisplatin and Vinblastine ± Amifostine Endpoint: è 7 No reduction in anti-tumor efficacy with a reduction in cisplatin-related nephrotoxicity Med. Immune Oncology, Inc.
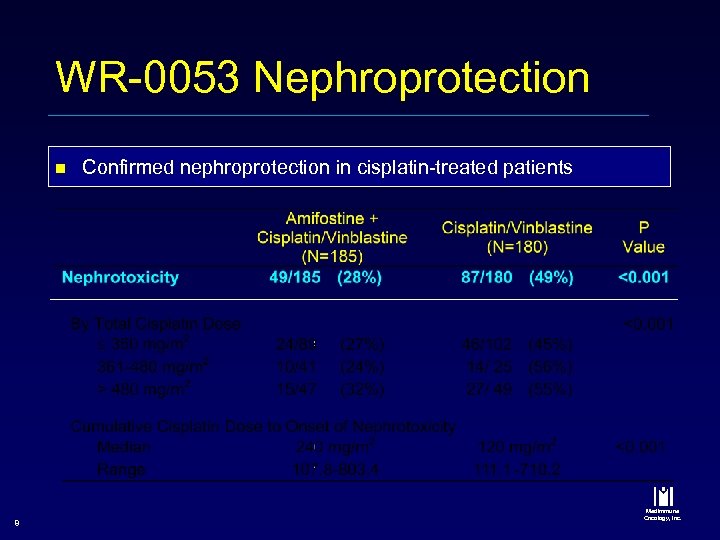 WR-0053 Nephroprotection n 8 Confirmed nephroprotection in cisplatin-treated patients Med. Immune Oncology, Inc.
WR-0053 Nephroprotection n 8 Confirmed nephroprotection in cisplatin-treated patients Med. Immune Oncology, Inc.
 WR-0053 Results n 9 No difference observed in Response Rate and Progression Free Survival in Amifostine patients Med. Immune Oncology, Inc.
WR-0053 Results n 9 No difference observed in Response Rate and Progression Free Survival in Amifostine patients Med. Immune Oncology, Inc.
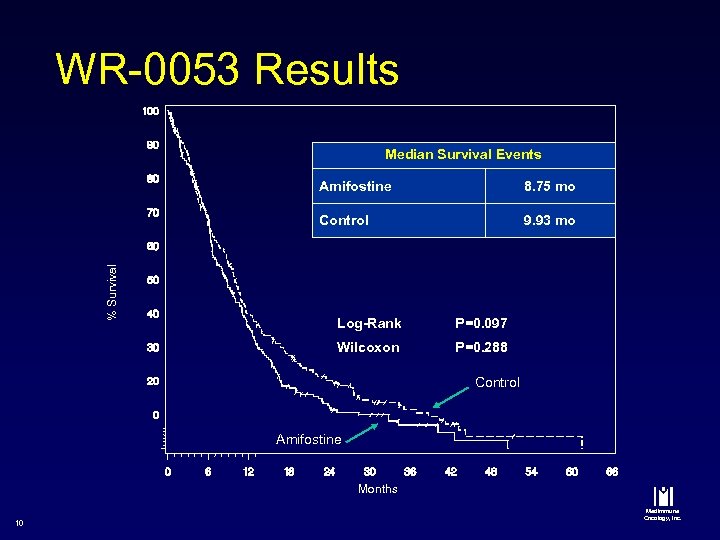 WR-0053 Results Median Survival Events 8. 75 mo Control % Survival Amifostine 9. 93 mo Log-Rank P=0. 097 Wilcoxon P=0. 288 Control Amifostine Months 10 Med. Immune Oncology, Inc.
WR-0053 Results Median Survival Events 8. 75 mo Control % Survival Amifostine 9. 93 mo Log-Rank P=0. 097 Wilcoxon P=0. 288 Control Amifostine Months 10 Med. Immune Oncology, Inc.
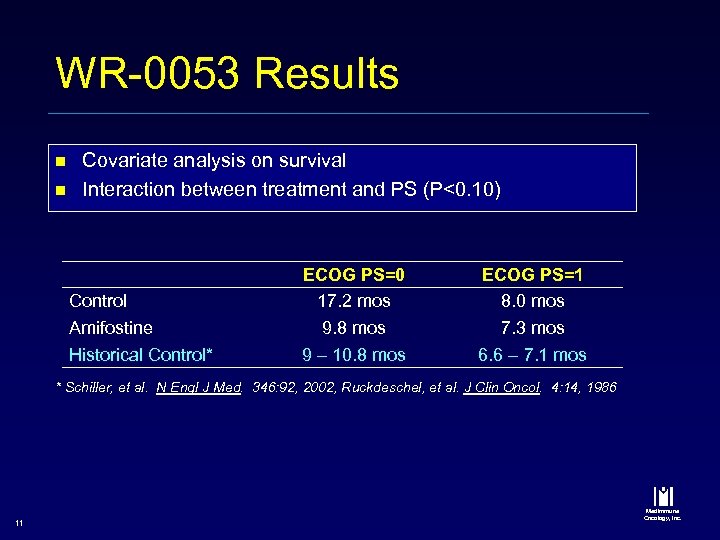 WR-0053 Results n n Covariate analysis on survival Interaction between treatment and PS (P<0. 10) Control Amifostine Historical Control* ECOG PS=0 17. 2 mos 9. 8 mos 9 – 10. 8 mos ECOG PS=1 8. 0 mos 7. 3 mos 6. 6 – 7. 1 mos * Schiller, et al. N Engl J Med. 346: 92, 2002, Ruckdeschel, et al. J Clin Oncol. 4: 14, 1986 11 Med. Immune Oncology, Inc.
WR-0053 Results n n Covariate analysis on survival Interaction between treatment and PS (P<0. 10) Control Amifostine Historical Control* ECOG PS=0 17. 2 mos 9. 8 mos 9 – 10. 8 mos ECOG PS=1 8. 0 mos 7. 3 mos 6. 6 – 7. 1 mos * Schiller, et al. N Engl J Med. 346: 92, 2002, Ruckdeschel, et al. J Clin Oncol. 4: 14, 1986 11 Med. Immune Oncology, Inc.
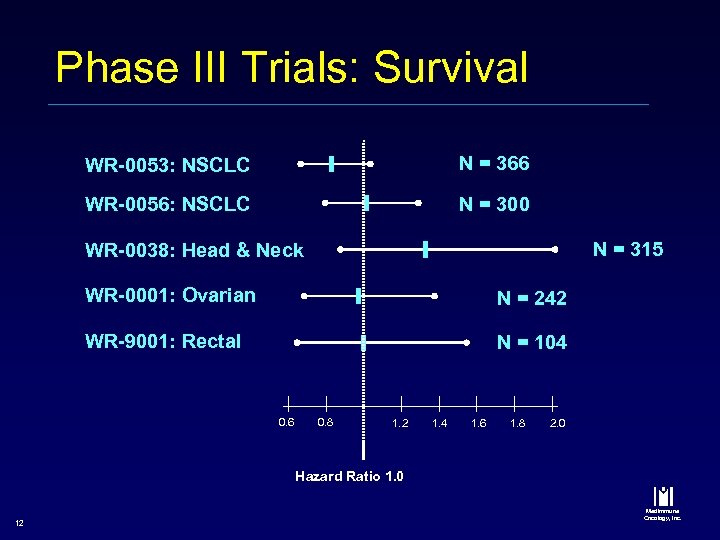 Phase III Trials: Survival WR-0053: NSCLC N = 366 WR-0056: NSCLC N = 300 N = 315 WR-0038: Head & Neck WR-0001: Ovarian N = 242 WR-9001: Rectal N = 104 0. 6 0. 8 1. 2 1. 4 1. 6 1. 8 2. 0 Hazard Ratio 1. 0 12 Med. Immune Oncology, Inc.
Phase III Trials: Survival WR-0053: NSCLC N = 366 WR-0056: NSCLC N = 300 N = 315 WR-0038: Head & Neck WR-0001: Ovarian N = 242 WR-9001: Rectal N = 104 0. 6 0. 8 1. 2 1. 4 1. 6 1. 8 2. 0 Hazard Ratio 1. 0 12 Med. Immune Oncology, Inc.
 Post-approval Study Conclusion n n Nephroprotection confirmed Anti-tumor efficacy endpoint No difference in Response Rate è No difference in Progression Free Survival è Median survival è ü ü 13 Amifostine Control = = 8. 75 months 9. 93 months Med. Immune Oncology, Inc.
Post-approval Study Conclusion n n Nephroprotection confirmed Anti-tumor efficacy endpoint No difference in Response Rate è No difference in Progression Free Survival è Median survival è ü ü 13 Amifostine Control = = 8. 75 months 9. 93 months Med. Immune Oncology, Inc.
 Continuing Obligation n n Completion of a new cisplatin-based study in NSCLC Co-primary endpoints Nephroprotection è Non-inferiority of survival or survival surrogate è 14 Med. Immune Oncology, Inc.
Continuing Obligation n n Completion of a new cisplatin-based study in NSCLC Co-primary endpoints Nephroprotection è Non-inferiority of survival or survival surrogate è 14 Med. Immune Oncology, Inc.
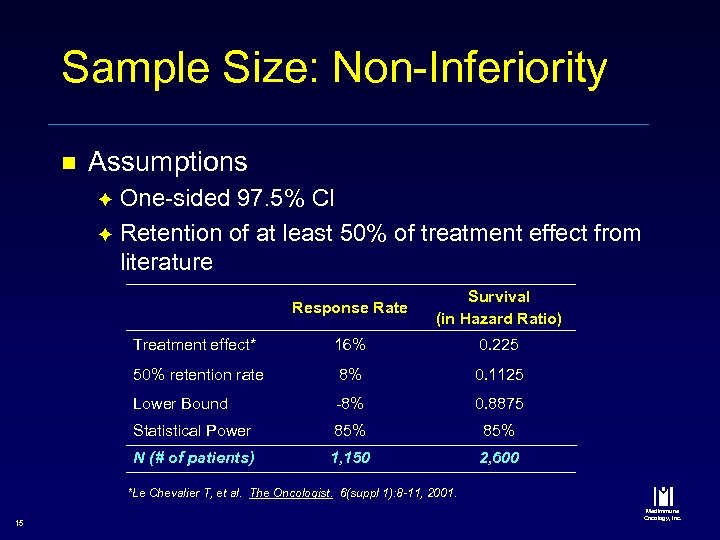 Sample Size: Non-Inferiority n Assumptions One-sided 97. 5% CI è Retention of at least 50% of treatment effect from literature è Response Rate Survival (in Hazard Ratio) Treatment effect* 16% 0. 225 50% retention rate 8% 0. 1125 Lower Bound -8% 0. 8875 Statistical Power 85% N (# of patients) 1, 150 2, 600 *Le Chevalier T, et al. The Oncologist. 6(suppl 1): 8 -11, 2001. 15 Med. Immune Oncology, Inc.
Sample Size: Non-Inferiority n Assumptions One-sided 97. 5% CI è Retention of at least 50% of treatment effect from literature è Response Rate Survival (in Hazard Ratio) Treatment effect* 16% 0. 225 50% retention rate 8% 0. 1125 Lower Bound -8% 0. 8875 Statistical Power 85% N (# of patients) 1, 150 2, 600 *Le Chevalier T, et al. The Oncologist. 6(suppl 1): 8 -11, 2001. 15 Med. Immune Oncology, Inc.
 Challenge n Accrual è Changing pattern of cisplatin utilization ü è n Decreased use of high-dose cisplatin regimens Competing higher priority protocols Trial length 6. 5 years Patient pool è Accrual = è Follow-up è 16 = 700 per year 240 patients per year = 2 years Med. Immune Oncology, Inc.
Challenge n Accrual è Changing pattern of cisplatin utilization ü è n Decreased use of high-dose cisplatin regimens Competing higher priority protocols Trial length 6. 5 years Patient pool è Accrual = è Follow-up è 16 = 700 per year 240 patients per year = 2 years Med. Immune Oncology, Inc.
 Summary n n n 17 Amifostine nephroprotection established Lack of tumor protection not established Additional definitive trial of tumor protection in cisplatin-treated NSCLC required Med. Immune Oncology, Inc.
Summary n n n 17 Amifostine nephroprotection established Lack of tumor protection not established Additional definitive trial of tumor protection in cisplatin-treated NSCLC required Med. Immune Oncology, Inc.


